Abstract
Hydrogels for the buccal application of the anesthetic drug lidocaine hydrochloride (LDC) were prepared using chitosan glutamate (CHG), a soluble salt of chitosan, or a binary mixture of CHG and glycerin, at different weight ratios. The in vitro drug release was studied at the pH value of saliva to assess the effect of the different formulations on drug delivery. The anesthetic activity of mucoadhesive LDC-CHG hydrogels was assessed in vivo after application on the buccal mucosa, compared to commercial semisolid formulations containing the same drug. LDC-loaded hydrogels can be proposed for the symptom relief of aphthosis or other painful mouth diseases.
Introduction
Several dosage forms are available or studied for the delivery of drugs at the level of buccal mucosa. Mouth cavity is an easily accessible site for local delivery of active compounds. Mouth tissues can be chosen for the application of conventional or modified drug release formulations (Citation1,Citation2). Solutions, tablets, gels, inserts, and devices fixed on the teeth are some examples. When a sustained drug release is required, polymer-based matrices or capsule-type delivery systems have been proposed.
Generally speaking, inflammatory diseases of the oral mucosa are called stomato-mucosites, including cheeks, lips, palate, and gingival inflammations. Mouth mucosa moreover hosts many residential or transitorily micro-organisms (bacteria, mycetes, viruses) that can cause or be involved actively in some mouth diseases.
Aphthae are among the more frequent ulcerations of the oral mucosa, specifically of tongue, lips, and palate. They are small lesions of the mucosa formed by little ulcers or abrasion areas surrounded by inflamed margins. The aphthous ulcer can be an isolated phenomenon, which spontaneously regresses in 10–14 days (minor aphthae), or appears cyclically (recurrent aphthosis). The etiology of these lesions is largely unknown, being associated to irritating foods, alcoholic drinks, smoke, anxious and stress conditions, minor traumas, and lowering of immunitary defences. Therapy is essentially directed to relieve the symptoms and favor ulcer healing. To this aim, antibacterial agents, corticosteroids, and non-steroidal anti-inflammatory drugs are used; supplements of vitamins B and ansiolytic drugs can be also useful. The application of local anesthetics helps in reducing the associated pain.
To prolong the activity of these drugs, bioadhesive tablets, gels, and ointments have been developed (Citation3–6). A bioadhesive patch (Dentipatch®, Noven Pharmaceuticals, USA) is commercially available (Citation2). Bioadhesion of locally or systemically-planned delivery systems can ensure a number of advantages, including a localized drug release and improved bioavailability, a longer duration of drug action with a concomitant reduction of the number of applications, and reduced or localized side-effects (Citation2,Citation7).
Most mucoadhesive formulations use high molecular weight natural or synthetic polymers; in particular, cationic polymers show a greater ability to compenetrate through and form hydrogen bonds with the negatively charged mucous layer and, among them, chitosan and chitosan salts have been described and even used in therapy (Citation8–11).
Chitosan (CH) belongs to the class of cationic bioadhesive polymers (Citation12). Biodegradability and absence of toxicity increase the use of CH and derivatives in the pharmaceutical technology (Citation13–16). Moreover, it can form hydrogels and films, is hydrophilic, and can be sterilized at high temperatures.
In the therapy of mouth mucosal diseases, a longer permanence of the drug in the target area is important to antagonize the washing-out effect of saliva. CH hydrogels have been shown to prolong both the retention times on the oral mucosa and the drug release (Citation17–19). The sustaining effect of CH on drug release time has been ascribed to its capacity of forming a gel at a low pH value, causing a time-dependent hydration and the diffusion of a dispersed drug (Citation20).
This paper describes the preparation and characterization of hydrogels made of chitosan glutamate (CHG). CHG is among the most investigated soluble salts of CH for drug delivery systems production, mainly microparticles (Citation21–25). It is characterized by high muco-adhesive and swelling properties, and a low pH-sensitivity (Citation25,Citation27). Recently, Maltese et al. (Citation28) described an ophthalmic formulation based on CHG as a viscosurgical device during cataract surgery. However, no study at present has investigated the use of CHG for the production of buccal formulations.
Lidocaine hydrochloride (LDC) was loaded in the hydrogels; this drug is a local anesthetic, topically used to relieve the pain or itch associated with systemic or skin pathologies. In particular, LDC-loaded hydrogels can be proposed for the treatment of aphthae or other painful mouth diseases.
To this aim, we tested the effects of the CHG hydrogel composition by means of in vitro drug release studies. In particular, addition of different percentages of glycerin in the formulation was evaluated as a way to modulate the viscosity and technological properties of hydrogels. The anesthetic activity was assessed in vivo after application on the buccal mucosa, compared to semi-solid commercial formulations containing the same concentration of LDC.
Material and methods
Materials
CHG (Protasan UP G 213) was purchased from FMC Biopolymer (Drammen, Norway). According to the supplier certificate of analysis, viscosity was 131 mPa·s (measured at room temperature); pH was 4.8; deacetylation degree was 85%. The content of glutamic acid was about 43%, that one of proteins was about 0.2%. LDC was purchased from Galeno s.r.l. (Comeana, Italy); Ph. Eur. grade glycerin was purchased from Carlo Erba (Milan, Italy). The commercial reference drug products were a lipophilic ointment containing 5.65% lidocaine HCl, corresponding to 5% free base (Ortodermina®; Sofar S.p.a., Milan, Italy) and a polyethylene glycol-based hydrogel containing 5% lidocaine (Xylocaina®; Astra Zeneca S.p.a., Basiglio, Italy).
Hydrogel preparation
After a preliminary screening, eight hydrogels were prepared () by varying the amount of polymer and glycerin and keeping constant the amount of drug (5% in weight), in order to allow an easier comparison with the reference commercial products. Deionized water was used as medium.
Table 1. Composition of CHG-LDC hydrogels.
The ingredients were weighed into small amber glass bottles, to reach the wished weight percent with respect to the volume of water ( 30 ml). Samples were mechanically stirred for 6 h at room temperature and the closed bottles were then stored in a refrigerator for 15 h, to allow the complete hydration of the system.
Each hydrogel was then split in two aliquots, one of which was kept at room temperature for viscosity measurements and the other one was stored at 4 ± 1°C and used for drug release tests. Both assays were performed immediately after the preparation of the hydrogels and then after 3, 7, and 12 months of respective storage.
Viscosity measurement
Viscosity was measured at room temperature by a Mettler RM 260 Rheomat viscosimeter and was expressed as mPa.s. Viscosity of the hydrogel samples stored at room temperature was measured at different times after preparation (see above), while in the samples stored in the refrigerator the viscosity was only checked after 1 year. Results are resumed in .
Table 2. Viscosity (mPas.s) of LDC-loaded CHG hydrogels stored at room temperature (23 ± 2°C) or in a refrigerator (4 ± 1°C) up to 12 months.
In vitro drug release studies
A dialysis system was used to monitor the diffusion of LDC from the prepared hydrogels as well as from the commercial drug products. A HD-PE cylinder (diameter 2. 5 cm, height 1 cm, capacity 4 ml) closed at one end and previously weighed was filled with each hydrogel and weighed again to know the exact amount of hydrogel (~ 4 g). The free end of the cylinder was wrapped with a Spectra-Por cellulose dialysis membrane (cut-off 3500 Da). The cylinder was then immersed in a glass beaker containing 30 ml of 0.2 M phosphate buffer solution, pH 6.6 (Italian Pharmacopoeia IX Ed.) under slow magnetic stirring (150 rpm) at 37 ± 1°C. At predetermined time intervals, 1-ml aliquots of the solution were withdrawn and immediately replaced with the same volume of fresh buffer solution. Each aqueous sample was analyzed by a Shimadzu UV-1601 spectrophotometer at 262 nm. Each release test was performed 3–4 times. Based on LDC aqueous solubility, the whole test was operated under steady-state conditions.
Hydrogel stability
All hydrogels were periodically submitted to a visual inspection to check their macroscopic stability during the different storage conditions.
Biocompatibility assessment of CHG hydrolgels
The potential damaging effects on mouth mucosal cells was tested by a microscopic examination of the cells after contact with the produced hydrogels (Citation27). Gels were incubated for 1 h at 37°C with buccal cells, obtained by scraping the mouth mucosa of both male and female healthy donors (n = 4; aged 28–35 years). Samples from the mucosa of the different volunteers were mixed before the test to avoid high individual variations. At the end, morphology of cells adhered to the hydrogels was evaluated by light microscopy.
In vivo anesthetic activity determination
The pharmacological activity of the hydrogels was assessed in healthy volunteers (n = 7 of both gender; aged 26–42 years) who signed a written consent to the test. The protocol received the approval by the ethical committee of the University of Catania.
Fifty milligrams of hydrogels or the commercial hydrogel (Xylocaina® gel) were applied by means of a little plastic spatula in the lower mouth vestibule. Each subject was then requested to fill in a form to register some parameters, like the diffusion of bitter taste from the site of application to other areas of the tongue or mouth; the rate of comparison of the local anesthetic activity and its duration; etc. Each volunteer assayed 4 or 5 among the prepared hydrogels and the reference commercial product; at least 6 h were left between two consecutive tests.
Statistical analysis of the above in vivo data was carried out using the Analysis of Variance test between the commercial formulation and each hydrogel. Student’s t-test was instead used to compare the various hydrogels.
Results and discussion
Hydrogel stability
The hydrogel samples stored at 4 ± 1°C did not undergo any macroscopic change after 1 year. Gels remained in fact transparent and uncolored, as soon after their preparation. Most samples stored at room temperature, on the contrary, were prone to alter, becoming turbid or forming solid particles in suspension. This phenomenon can of course in part be ascribed to the formation of yeasts and moulds, since no preserving agent was included in these formulations. However, the tendency to degradation for these samples was noted 3 months after the preparation.
In vitro drug release
The release profile of LDC from the prepared hydrogels was studied in vitro by a dialysis method, at 37°C and for 24 h, a time well above the potential time of contact of the hydrogels with the application site in vivo.
Among the formulation variables tested, increasing the polymer concentration from 1 to 5% linearly reduced the drug diffusion in the external medium. This was measured for both the glycerin percentages used, i.e. 5 and 10% ( and ).
Figure 1. LDC release profile from hydrogels prepared with different amounts of CHG (glycerin: 5% by weight) (n = 4).
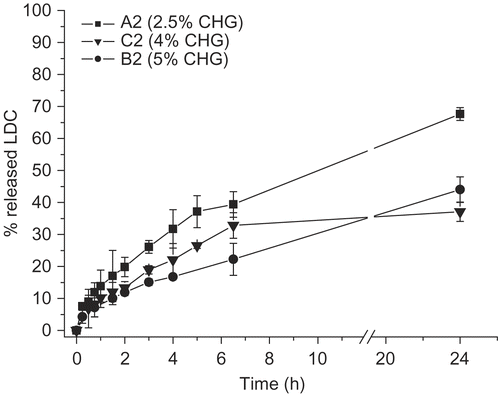
Figure 2. LDC release profile from hydrogels prepared with different amounts of CHG (glycerin: 10% by weight) (n = 3).
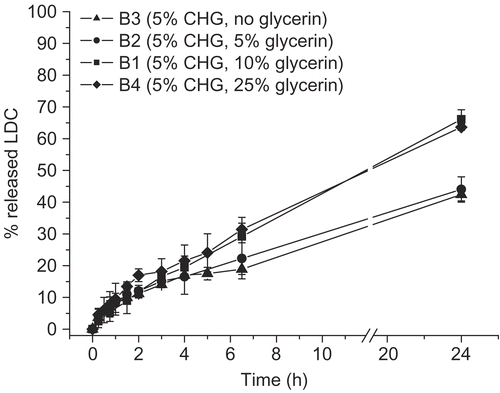
Conversely, a higher glycerin percent increased the drug release, particularly at a polymer concentration of 5 or 10% (w/w) (). A further increase of glycerin percent (at least up to 25% by weight) did not improve LDC release, whereas in the absence of glycerin the same release profile observed for the 5% CHG formulation was seen (cf. batches B2 vs B3) ().
Figure 3. LDC release profile from hydrogels prepared with a fixed percentage of CHG (5% by weight) and different glycerin amounts (n = 3 or 4).
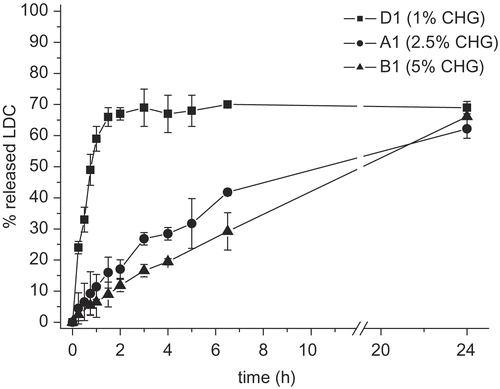
With respect to the two reference commercial products, the CHG-based hydrogels showed a drug release rate respectively lower than the gel (Xylocaina® gel Astra), but considerably more rapid than the ointment (Ortodermina®) ().
Figure 4. Comparison among LDC in vitro release profiles from two CHG hydrogels and the two reference commercial products (n = 3 or 4).
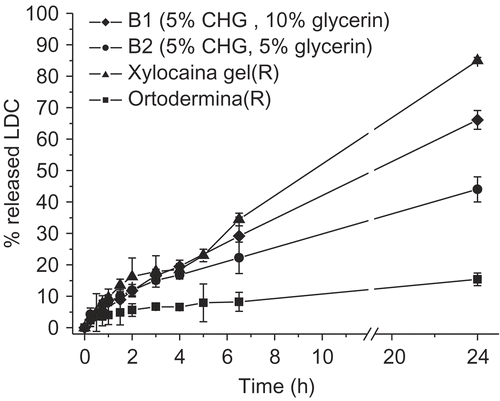
In particular, Xylocaina® gel gave an almost complete drug release after 24 h, whereas the CHG hydrogels (two of which, namely B1 and B2, are reported in as an example) displayed slower and only partial release profiles of the carried LDC. The behavior of the lipophilic ointment (Ortodermina®) was of course expected, because of its low affinity for the receiving aqueous medium.
The different release profiles observed for the CHG hydrogels, compared to the reference commercial gel, instead give interesting technological perspectives, suggesting the possibility of modulating the drug release rate by changing, for instance, simply the percentage of glycerin in the formulation.
The drug release curves did not seem to be directly correlated with the viscosity measured for the different hydrogels. For instance, shows a similar drug dissolution curve for two systems (B1 and B4) that have different viscosity values (respectively 173,000 and 100,100 mPa.s) ().
Also the amount of glycerin in the composition of the hydrogels did not give a linear trend in terms of viscosity. In fact, if in the hydrogels containing 5% CHG (B1–B3), the addition of glycerin caused a progressive increase of viscosity, for the corresponding hydrogels produced with 2.5% CHG (A1 and A2) changes in the percent of glycerin (5 or 10% in weight, respectively) did not affect the viscosity (cf. CitationTable 2). As a general trend, storage at room temperature led to a reduction of hydrogel viscosity; this could be due to a rearrangement of inter-polymeric chains in the systems, but also to the hydrolytic instability of the polymer with a progressive reduction of the molecular weight. Conversely, after storage at 4°C the viscosity showed slightly higher values than at the beginning, suggesting both a greater stability of the polymeric network and the formation of stronger interactions between the CHG chains.
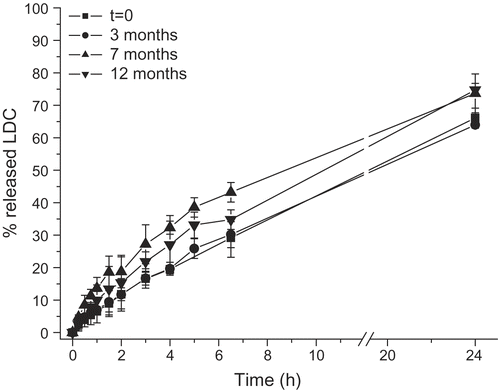
Drug release profiles from the hydrogels showed small changes, although statistically significant (p < 0.05), after several months of storage at 4°C. reports as an example the data relative to the hydrogels prepared with 5% CHG, but a similar behavior was observed also for the remaining formulations. Only the batch D1, containing 1% CHG and 10% glycerin, displayed different drug release values after storage, probably as a consequence of a reduced physical homogeneity among the ingredients.
Buccal biocompatibility of CHG hydrogels
Light microscopy examination of buccal cells, after contact with plain or LDC-loaded hydrogels, indicated no morphological change (not shown), suggesting a good local tolerability of these systems at least upon reduced contact times. Analogous results have been reported by Genta et al. (Citation27) for CHG films.
In vivo pharmacological activity
The local anesthetic activity of some prepared hydrogels was assessed in vivo on healthy volunteers, by applying 50 mg of each hydrogel in the mouth vestibule and registering the duration of the anesthetic activity and the feeling reported by each subject (diffusion and intensity of the bitter taste, tactile sensitivity, etc.). Results, reported in , were compared with those obtained applying the commercial gel formulation (Xylocaina® gel).
Table 3. Duration of the local anesthetic activity (minutes) in vivo. Numbers 1–7 refer to the evaluators. Analysis of Variance was applied between Xylocaina® gel and each hydrogel, while the Student’s t-test was used to compare the various hydrogels.
All the tested hydrogels produced a mean local anesthetic activity lasting 20–30 min. The ANOVA test suggested that variance was not statistically different (F > 0.1) from the results obtained with the commercial product Xylocaina® (≈ 19 min). These findings are not surprising considering the high random factor given by testing human data. The registered values were homogeneous, as shown by the low standard deviations (); this is an interesting finding for an in vivo assay performed on a limited number of subjects, thus allowing a good evaluation of the results.
It is noteworthy that, except for formulations B1 vs B3 (p < 0.5), the hydrogels gave significantly statistically different (p < 0.05 or < 0.01) in vivo activities between each other. The highly significant differences in the activity measured among the hydrogels indicate that the used production variables, namely the percentages of CHG and of glycerin, had a strong effect on the technological properties of these formulations.
The feelings reported by the subjects on the duration and diffusion of the anesthetic effect of the drug seemed to correlate with the viscosity of the hydrogels for formulations containing the same CHG percentage. Hydrogels B4 and D1, that contain the highest amount of glycerin (25% by weight) and the lowest concentration of polymer (1% by weight), respectively, along with a reduced viscosity (), displayed a shorter duration of the local anesthetic activity (). This peculiar composition could have caused a quicker flushing action by the salivary flow of the gel, and thus of the drug, from the application site.
Finally, it is noteworthy that the pharmacological data obtained in vivo are only in part related to the in vitro drug release curves. For instance, hydrogels B1 and B3 gave a similar duration of anesthetic activity (), and both were characterized by a similar drug release profile in the first 4 h of the test (); conversely, formulations B1 and B4, that showed super-imposable drug release curves (), gave a very dissimilar duration of the anesthetic activity. Of course, the experimental conditions used in the two in vitro release and in vivo pharmacological tests can justify such differences.
In conclusion, this study suggests the possibility of producing hydrogels for a controlled local release of LDC using chitosan glutamate, with the addition of a variable amount of a gelifying agent, such as glycerin. LDC-loaded muco-adhesive hydrogels can be of aid in reducing the pain symptoms that characterize aphthosis and other mouth diseases.
Acknowledgments
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Needleman IG, Smales FC. (1995). In vitro assessment of bioadhesion for periodontal and buccal drug-delivery. Biomaterials, 15, 617–24.
- Smart JD. (2004). Recent developments in the use of bioadhesive systems for delivery of drugs to the oral cavity. Crit Rev Ther Drug Carrier Syst, 21, 67–93.
- Carr MP, Horton JE. (2001). Evaluation of a transoral delivery system for topical anesthesia. J Am Dent Assoc, 132, 1714–9.
- Shin SC, Lee JW, Yang KH, Lee CH. (2003). Preparation and evaluation of bioadhesive benzocaine gels for enhanced local anesthetic effects. Int J Pharm, 260, 77–81.
- Shin SC, Cho CW, Choi JS. (2004). Development of the bioadhesive tetracaine gels for enhanced local anesthetic effects. Drug Dev Ind Pharm, 30, 931–6.
- Shin SC, Cho CW, Yang KH. (2004). Development of lidocaine gels for enhanced local anesthetic action. Int J Pharm, 287, 73–8.
- Lee JW, Park JH, Robinson JR. (2000). Bioadhesive-based dosage forms: the next generation. J Pharm Sci, 89, 850–66.
- Pignatello R, Stancampiano AH, Ventura CA, Puglisi G. (2007). Dexamethasone sodium phosphate-loaded chitosan-based delivery systems for buccal application. J Drug Target, 15, 603–10.
- Miyazaki S, Nakayama A, Oda M, Takada M, Attwood D. (1994). Chitosan and sodium alginate based bioadhesive tablets for intraoral drug delivery. Biol Pharm Bull, 17, 745–7.
- Illum L. (1998). Chitosan and its use as a pharmaceutical excipient. Pharm Res, 15, 1326–31.
- Langoth N, Kahlbacher H, Schöffmann G, Schmerold I, Schuh M, Franz S, Kurka P, Bernkop-Schnürch A. (2006). Thiolated chitosans: design and in vivo evaluation of a mucoadhesive buccal peptide drug delivery system. Pharm Res, 23, 573–9.
- Woodley J. (2001). Bioadhesion. New possibilities for drug administration? Ch Pharmacokinet, 40, 77–84.
- Felt O, Buri P, Gurny R. (1998). Chitosan: a unique polysaccharide for drug delivery. Drug Dev Ind Pharm, 24, 979–993.
- Paul W, Sharma CP. 2000. Chitosan, a drug carrier for the 21thcentury: a review. STP Pharma Sci, 10, 5–22.
- Chopra S, Mahdi S, Kaur J, Iqbal Z, Talegaonkar S, Ahmad FJ. (2006). Advances and potential applications of chitosan derivatives as mucoadhesive biomaterials in modern drug delivery. J Pharm Pharmacol, 58, 1021–32.
- Varshosaz J. 2007. The promise of chitosan microspheres in drug delivery systems. Expert Opin Drug Deliv, 4, 263–73.
- Needleman IG, Smales FC, Martin GP. (1997). An investigation of bioadhesion for periodontal and oral mucosal drug delivery. J Clin Periodontol, 24, 394–400.
- Senel S, Ikinci G, Kas S, Yousefi-Rad A, Sargon MF, Hincal AA. (2000). Chitosan films and hydrogels of chlorexidine gluconate for oral mucosal delivery. Int J Pharm, 193, 197–203.
- Senel S, Kremer MJ, Wertz PW, Hill JR, Kas S, Hincal AA, Squier CA. (2002). Chitosan for intraoral peptide delivery. In Muzzarelli RAA, Muzzarelli C, eds. Chitosan in Pharmacy and Chemistry. Ancona: Atec, 77–84.
- Fini A, Orienti I. (2003). The role of chitosan in drug delivery. Current and potential administrations. Am J Drug Deliv, 1, 43–59.
- Orienti I, Cerchiara T, Luppi B, Bigucci F, Zuccari G, Zecchi V. (2002). Influence of different chitosan salts on the release of sodium diclofenac in colon-specific delivery. Int J Pharm, 238, 51–59.
- Cerchiara T, Luppi B, Bigucci F, Zecchi V. (2003). Chitosan salts as nasal sustained delivery systems for peptidic drugs. J Pharm Pharmacol, 55, 1623–7.
- Gavini E, Hegge AB, Rassu G, Sanna V, Testa C, Pirisino G, Karlsen J, Giunchedi P. (2006). Nasal administration of carbamazepine using chitosan microspheres: in vitro/in vivo studies. Int J Pharm, 307, 9–15.
- Colonna C, Conti B, Perugini P, Pavanetto F, Modena T, Dorati R, Genta I. (2007). Chitosan glutamate nanoparticles for protein delivery: development and effect on prolidase stability. J Microencapsulation, 24, 553–64.
- Pignatello R, Mangiafico A, Pantó Y, Puglisi G, Furneri PM. (2008). Solid dispersions of chitosan glutamate for the local delivery of miconazole: characterization and in vitro activity. Open Drug Delivery J, 2, 44–51.
- Sandri G, Rossi S, Ferrari F, Bonferoni MC, Muzzarelli C, Caramella C. (2004). Assessment of chitosan derivatives as buccal and vaginal penetration enhancers. Eur J Pharm Sci, 21, 351–9.
- Genta I, Colonna C, Perugini P, Pavanetto F, Modena T, Valli M, Muzzarelli C, Conti B. (2005). Evaluation of bioadhesive performance of chitosan derivatives as films for buccal application. J Drug Deliv Sci Technol, 15, 459–63.
- Maltese A, Borzacchiello A, Mayol L, Bucolo C, Maugeri F, Nicolais L, Ambrosio L. (2006). Novel polysaccharides-based viscoelastic formulations for ophthalmic surgery: rheological characterization. Biomaterials, 27, 5134–42.