Abstract
Various enhancers, such as fatty acids (saturated, unsaturated), glycerides, propylene glycols, and non-ionic surfactants, have been incorporated in the loratadine-EVA matrix to increase the rate of skin permeation of loratadine from an EVA matrix. The enhancing effects of these enhancers on the skin permeation of loratadine were evaluated using a modified Keshary-Chien cell fitted with intact excised rat skin. The penetration enhancers showed a higher flux, probably due to the enhancing effect on the skin barrier, the stratum corneum. Among the enhancers used, such as the fatty acids, glycols, propylene glycols, and non-ionic surfactants, linoleic acid showed the best enhancement. For the enhanced transdermal delivery of loratadine, application of an EVA matrix containing a permeation enhancer might be useful in the development of a transdermal drug delivery system.
Introduction
The skin is an attractive route for drug administration because it can avoid the first-pass hepatic metabolism of drugs intended for systemic action, thereby offering potentially lower drug doses and reduced side effects (Citation1–4). Despite the many advantages of transdermal delivery systems over oral delivery, there are only a limited number of drugs administered using this type of system due to the excellent barrier function of the skin. The skin is a complex, dynamic layer organ with many functions that go far beyond its role as a barrier to the environment. The highly organized structure of the stratum corneum forms an effective barrier to the penetration of a wide range of substances. The transdermal route for systemic drug delivery has attracted considerable attention in recent years (Citation5–9). However, the low permeability of the skin is a major problem using this administration route. One way of reducing this problem and improving the bioavailability after the topical application of the drugs is to include a penetration enhancer in the formulation. Penetration enhancers, accelerants, or promoters are believed to interact with some components of the skin to cause increased fluidity in the intercellular lipid lamellae, swelling of the stratum corneum, and/or leach out some of the structural components and increase the level of drug penetration through the barrier membrane (Citation10–12).
In our previous report (Citation13), the usefulness of ethylene-vinyl acetate (EVA) polymer that is a heat processible, flexible, and inexpensive material (Citation14) as a controlling membrane was studied. The release of loratadine from the EVA matrix containing various plasticizers was measured, and the loratadine-EVA matrix system containing the best plasticizer was formulated.
In this study, to increase the skin permeation of loratadine, various penetration enhancers such as the fatty acids (saturated, unsaturated), the glycerides, the propylene glycols, and non-ionic surfactants were added to the EVA matrix system and the level of loratadine permeation through rat skin was evaluated. The purpose of this study was to develop the new loratadine-EVA matrix formulations containing a permeation enhancer that can provide enhanced transdermal delivery of loratadine for an extended period of time through skin.
Materials and methods
Materials
Loratadine was kindly supplied by Aju Pharm. Co., Ltd. (Korea). Ethylene vinyl acetate (EVA, 40%) was purchased from Aldrich Chemical Co., Inc. (USA). Lauric acid, oleic acid, and caprylic acid were obtained from Tokyo Kasei Kogyo Co., Ltd. 1-methyl-2-pyrrolidone and 2-pyrrolidone was acquired from Acros organics (USA). Myristic acid, linoleic acid, polyoxyethylene- 23-lauryl ether (Brij 35), polyoxyethylene-2-oleyl ether (Brij 92), and polyoxyethylene-23-lauryl ether (Brij 72), tetraethylene glycol (TEG), and diethylene glycol (DEG) were supplied by Sigma-Aldrich Co. (USA). Oleyl macrogol- 6 glycerides, caprylocaproyl mcarogol-8 glycerides, propylene glycol monocaprylate, propylene glycol laurate, and propylene glycol monolaurate were a kind gift from Gattefose (France). Acetonitrile was HPLC grade and purchased from J.T.Baker Inc. (USA). All other chemicals were of regent grade and used without further purification.
HPLC determination of loratadine
The level of loratadine that permeated was assayed by high performance liquid chromatography (HPLC). The HPLC system consisted of a pump (Knauer, DE/K-120, USA), ultraviolet detector (Waters 484, USA), C18 column (250 × 4. 6 mm, 5 μm), degaser, and an integrator (D520A, Youngin scientific Co., Ltd., Korea). The mobile phase contained a mixture (80:20, v/v) of acetonitrile and 0.025 M sodium dihydrogen phosphate buffer, adjusted to pH = 3.7 with H3PO4. A flow rate of 1. 0 ml/min yielded an operating pressure of ~ 1000 psi. The UV detector was operated at a wavelength of 248 nm. Under these conditions, the loratadine peak appeared at a retention time of 5.9 min.
Preparation of the loratadine-EVA matrix containing an enhancer
The loratadine-EVA matrix containing the enhancer was prepared using a casting process. The appropriate weight concentration of EVA copolymer beads was dissolved in 20 ml of chloroform in a beaker. The drug solution and an enhancer were then added with continuous stirring. This mixture was poured onto a glass plate and the solvent was allowed to evaporate off overnight at room temperature. The matrix was removed from the plate. A piece of the matrix was then cut properly before the experiment. The drug content was calculated from the weight ratio of the drug and copolymer used.
Skin preparation
Male Sprague Dawley (220–250 g) rats were obtained from Daehan Laboratory Animal Research Center Co. (Daejeon, Korea) and used for the skin preparation. The rats were given access to food and water ad libitum until used for the experiments. The rats were sacrificed in a CO2 chamber immediately before the experiments. The hair of the abdominal area was carefully removed with electric clippers. The full-thickness skin was surgically removed from each rat, and a square section of abdominal skin was excised. After the excision, the adhering fat and other visceral debris in the skin were removed carefully from the undersurface with tweezers (Citation15). The excised skin was used immediately.
Permeation of loratadine from the EVA matrix containing various enhancers
The in vitro permeation of loratadine from the EVA matrix through the rat skin was examined using a modified Keshary-Chien cell. The freshly excised full-thickness skin sample was mounted on the receptor site of the diffusion cell with the stratum corneum side facing upwards into the donor compartment and the dermal side facing downwards into the receptor compartment. An appropriate size of the matrix was placed on the stratum corneum side and covered with a round glass plate and clamped. The receptor medium was a 40% PEG 400 solution to achieve a sink condition. The cell was maintained at 37°C using a circulating water bath. The total samples were withdrawn at predetermined time intervals and replaced immediately with an equal volume of fresh medium. The permeation quantities of loratadine were analyzed by HPLC at 248 nm. Each data point represents an average of three determinations. Various types of enhancers were used to compare the enhancing effects at a 5% concentration. The enhancers used were a saturated fatty acid group, such as capric acid, myristic acid, and lauric acid, and an unsaturated fatty acid group, such as oleic acid and linoleic acid. Propylene glycols, such as propylene glycol monolaurate, propylene glycol laurate, and propylene glycol monocaprylate, were used. Non-ionic surfactants, such as polyoxyethylene-2-oleyl ether, polyethylene-2-stearyl ether, and polyoxyethylene-23-lauryl ether, and glycerides, such as oleyl macrogol-6 glycerides, and caprylocaproyl macrogol-8 glycerides, were also used.
Calculations
The enhancer might affect the fluidity of the stratum corneum structure, and drugs can permeate better through the rat skin. The cumulative amount of loratadine through the rat skin was plotted as a function of time (t). A linear profile was observed during the initial 12 h period and the slope of the linear portion of the curve was determined by linear regression. The effectiveness of the penetration enhancers was defined as the enhancement factor (EF), which was calculated using the following equation:
EF = (flux of EVA matrix containing enhancers) /(flux of control sample)
Results and discussion
Effects of fatty acids on the permeation of loratadine from the EVA matrix through the rat skin
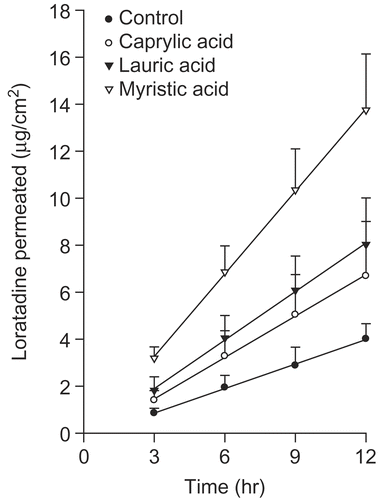
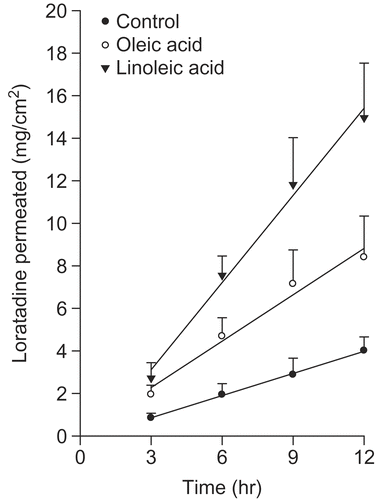
Fatty acids are attracting considerable attention as penetration enhancers (Citation16,Citation17). This class of enhancer has the advantage of being an endogenous component of human skin. Fatty acids can have a variety of chain lengths, double bonds characteristics (position, number, and configuration), branching schema, and substituents. These structural variations can affect their suitability as skin penetration enhancers (Citation18–21). Fatty acids can insert between the hydrophobic tails of the stratum corneum lipid bilayer, disturbing their packing, increasing their fluidity, and subsequently, decreasing the diffusional resistance to permeants (Citation22). Fatty acids have been shown to promote the skin permeation of drugs with a wide range of polarities through interactions with intercellular lipid domains (Citation23–25). The efficacy of fatty acids is intrinsically linked to their structure, with differences evident between the saturated and unsaturated forms and those of a different hydrocarbon chain length (Citation16,Citation26). Unsaturated fatty acids, particularly those with the cis conformation and C18 chain lengths, have been reported to be more effective enhancers than their saturated counterparts, promoting the permeation of such penetrants as naloxone (Citation27) and flurbiprofen (Citation28). When introduced into the predominantly saturated, straight-chained lipid environment of the stratum corneum, these kinked fatty acids tend to intercalate, disrupt the ordered lipid array (Citation29), and form separate fluid states that disorder the endogenous lipids (Citation30,Citation31). Saturated fatty acids with a linear shape and low solubility are less capable of disrupting the lipid packing of the stratum corneum and inserting themselves into the lipid bilayers than unsaturated fatty acids with high solubility.
shows the permeation profile of a loratadine from the EVA matrix with a saturated fatty acid. The loratadine-EVA matrix without the enhancer was tested as the control. The saturated fatty acid group showed a slightly higher permeation rate than the control. Among the saturated fatty acid groups, myristic acid showed the highest permeation rate. shows the permeation profile of the loratadine from the EVA matrix with an unsaturated fatty acid such as oleic acid and linoleic acid. In this test, linoleic acid showed the highest permeation rate of loratadine from the EVA matrix.
Effects of non-ionic surfactants on the permeation of loratadine from the EVA matrix through the rat skin
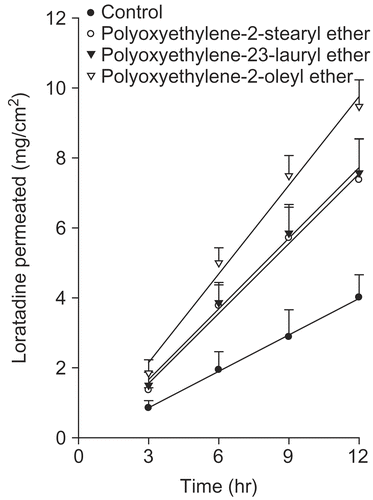
Surfactants have been reported to enhance the permeability of a variety of drugs (Citation32–37). They affect the permeability of several biological membranes, including skin (Citation35,Citation38). Therefore, they can enhance the rate of skin penetration of other compounds present in the formulation, and have recently been used to enhance the permeation rates of several drugs (Citation27,Citation39). Shin et al. (Citation36) examined the mechanism for the effect of non-ionic surfactants as permeation enhancers. Skin pre-treated with a non-ionic surfactant showed a loosely layered stratum corneum with wide intercellular spaces (Citation36). shows the permeation aspect of the loratadine from the EVA matrix with non-ionic surfactants. Brij 92 (polyoxyethylene 2-oleyl ether) showed the best enhancing effects. Brij 35 (polyoxyethylene 2-stearyl ether) and Brij 72 (polyoxyethylene 23-lauryl ether) showed a similar increase in the permeation rate.
Effects of the glycerides or the propylene glycols on the permeation of loratadine from the EVA matrix across the rat skin
The ability of glycerides and propylene glycol group as enhancers was examined. The results are shown in and , respectively. Caprylocaproyl macrogol-glyceride (Labrasol) increased the passive transport of drug molecules, and showed high tolerance and low toxicity. Moreover, Labrasol was included as a pharmaceutical excipient in the European Pharmacopoeia in 1998. Oleyl macrogo-6 glyceride (Labrafil) is a PEG derivative that is used as a co-surfactant in many pharmaceutical systems, such as microemulsions. This substance is biocompatible and biodegradable (Citation40). Among the glycerides, oleyl macrogo-6 glyceride produced a significant improvement in the rate of loratadine permeation.
Figure 4. Effects of glycerides on the permeation of loratadine from the EVA matrix through the excised rat skin.
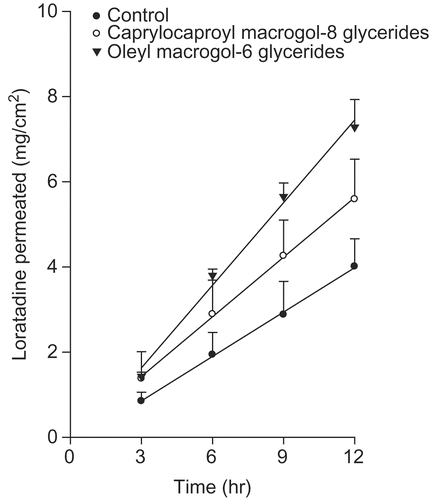
Figure 5. Effects of the propylene glycols on the permeation of loratadine from the EVA matrix through the excised rat skin.
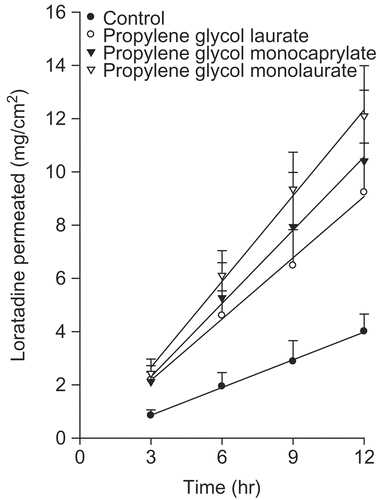
Propylene glycol (PG) is widely used as a vehicle for penetration enhancers, and permeates well through the human stratum corneum. PG readily permeates the skin, and, in so doing, can transport the drug molecules across the skin (Citation41). The permeation of PG through the tissue can alter thermodynamic activity of the drug in the vehicle, which can in turn modify the driving force for diffusion. PG may partition into the tissue, which can facilitate the uptake of the drug into skin, and there may be some minor disturbance to the intercellular lipid packing within the stratum corneum bilayers (Citation42).
shows the permeation data of loratadine according to the various enhancers showing enhancement. The rate of drug permeation through the rat skin was highest from the EVA matrix containing unsaturated fatty acids. Among the enhancers examined in this study, linoleic acid showed the highest level of enhancement.
Table 1. Enhancement factor according to the various enhancers.
Conclusion
Among the enhancers used, including the fatty acids (saturated, unsaturated), glycerides, propylene glycols, and non-ionic surfactants, linoleic acid showed the best enhancement. In conclusion, the application of an EVA membrane containing a permeation enhancer can be used in a transdermal drug delivery system to enhance the transdermal delivery of loratadine.
Acknowledgements
This work was supported by a research grant (No. R01-2007-000-20187-0) from Korea Science & Engineering Foundation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Barry BW. (1983). Properties that influence percutaneous absorption. In: Barry BW, ed. Dermatological Formulations; Percutaneous Absorption. New York: Marcel Dekker, 127–233.
- Guy RH, Hadgraft J. (1985). Transdermal drug delivery: the ground rules are emerging. Pharm Int, 6, 112.
- Smith EW, Maibach HI. (eds) (1995). Percutaneous Penetration Enhancers. Boca Raton, FL: CRC Press, 1–50.
- Roberts MS, Walters KA. (eds) (1998). Dermal Absorption and Toxicity Assessment. New York: Marcel Dekker, 1–785.
- Shaw J. (1984). Pharmacokinetics of nitroglycerine and clonidine delivered by transdermal route. Am Heart J, 108, 217–22.
- Banerjee P, Ritschal W. (1989). Transdermal permeation of vasopressin. II. Influence of Azone on in vitro and in vivo permeation. Int J Pharm, 49, 199–204.
- Kim C, Kim J, Chi S, Shim C. (1993). Effect of fatty acids and urea on the penetration of ketoprofen through rat skin. Int J Pharm, 99, 109–18.
- Fuhrman LC, Michniak BB, Behl CR, Malick AW. (1997). Effect of novel penetration enhancers on the transdermal delivery of hydrocortisone: an in vitro species comparison. J Contr Rel, 45, 199–206.
- Mura P, Faucci MT, Bramanti G, Corti P. (2000). Evaluation of transcutol as a clonazepam transdermal permeation enhancer from hydrophilic gel formulations. Eur J Pharm Sci, 9, 365–72.
- Elfbaum S, Laden K. (1968). The effect of dimethyl sulphoxide on percutaneous absorption. J Soc Cosmet Chem, 19, 119–27.
- Carelli V, Di Colo G, Nanoripieri E, Serafini M. 1993. Bile acids as enhancers of steroid penetration through excised hairless mouse skin. Int J Pharm, 89, 81–9.
- Magnusson BM, Runn P. (1999). Effect of penetration enhancers on the permeation of the thyrotropin releasing hormone analogue pGlu-3-methyl-His-Pro amide through human epidermis. Int J Pharm, 178, 149–59.
- Cho CW, Kim SJ, Yang KH, Song JH, Shin SC. (2008). Enhanced controlled release of loratadine from the ethylene-vinyl acetate matrix containing plasticizer. Drug Deliv, 15, 423–8.
- Miyazaki S, Ishii K, Sugibayashi K, Morimoto Y, Takada M. (1982). Antitumor effect of ethylene-vinyl acetate copolymer matrices containing 5-fluorouracil on Ehrlich Ascites carcinoma in mice. Chem Pharm Bull, 30, 3770.
- Durrhein H, Flynn GL, Higuchi WI, Behl CR. (1980). Permeation of hairless mouse skin I: experimental methods and comparison with human epidermis permeation by alkanols. J Pharm Sci, 69, 781.
- Tanojo H, Bouwstra JA, Junginger HE, Bodde HE. (1997). In vitro human skin barrier modulation by fatty acid: skin permeation and thermal analysis studies. Pharm Res, 14, 42–9.
- Oh SY, Jeong SY, Park TG, Lee JH. (1998). Enhanced transdermal delivery of AZT (Zidovudine) using iontophoresis and penetration enhancer. J Contr Rel, 51, 161–8.
- Elyan BM, Sidhom MB, Plakogiannis FM. (1996). Evaluation of the different fatty acids on the percutaneous absorption of metaproterenol sulfate. J Pharm Sci, 85, 101–5.
- Takeuchi Y, Yamaoka Y, Jukushima S, Miyawaki K, Taguchi K, Yasuka H, Kishimoto S, Suzuki M. (1998). Skin penetration enhancing action of cis-unsaturated fatty acids with ω-9 and ω-12 chain lengths. Biol Pharm Bull, 21, 462–9.
- Bhatia KS, Singh J. (1998). Synergistic effect of iontophoresis and a series of fatty acids on LHRH permeability through porcine skin. J Pharm Sci, 87, 462–9.
- Taguchi K, Fukushima S, Yamaoka Y, Takeuchi Y, Suzuki M. (1999). Enhancement of propylene glycol distribution in the skin by high purity cis-unsaturated fatty acids with different alkyl chain lengths having different double bond position. Biol Pharm Bull, 22, 407–11.
- Golden GM, Mckie JE, Potts RO. (1987). Role of stratum corneum lipid fluidity in transdermal drug flux. J Pharm Sci, 76, 25–8.
- Cooper ER. (1984). Increased skin permeability for lipophilic molecules. J Pharm Sci, 73, 1153–6.
- Barry BW, Bennett SL. (1987). Effect of penetration enhancers on the permeation of mannitol, hydrocortisone and progesterone through human-skin. J Pharm Pharmacol, 39, 535–46.
- Guy RH, Hadgraft J. (1987). The effect of penetration enhancers on the kinetics of percutaneous absorption. J Contr Rel, 5, 43–51.
- Kandimalla K, Kanikkannan N, Andega S, Singh M. (1999). Effect of fatty acids on the permeation of melatonin across rat and pig skin in-vitro and on the transepidermal water loss in rats in-vivo. J Pharm Pharmacol, 51, 783–90.
- Aungst BJ, Rogers NJ, Shefter E. (1986). Enhancement of naloxon penetration through human skin in vitro using fatty acidsfatty alcohols, surfactants, sulfoxides and amides. Int J Pharm, 33, 225–34.
- Chi SC, Park ES, Kim H. (1995). Effect of penetration enhancers on flurbiprofen permeation through rat skin. Int J Pharm, 126, 267–74.
- Green PG, Guy RH, Hadgraft J. (1988). In vitro and in vivo enhancement of skin permeation with oleic-acid and lauric acids. Int J Pharm, 37, 251–5.
- Ongpipattanakul B, Burnete RR, Potts RO, Francoeur ML. (1991). Evidence that oleic-acid exists in a separate phase within stratum-corneum lipids. Pharm Res, 8, 350–4.
- Naik A, Pechtold L, Potts RO, Guy RH. (1995). Mechanism of oleic acid-induced skin penetration enhancement in vivo in humans. J Contr Rel, 37, 299–306.
- Sarpotdar PP, Zatz JL. (1986). Percutaneous absorption enhancement by nonionic surfactants. Drug Dev Ind Pharm, 12, 1625–47.
- Cappel MJ, Kreuter J. (1991). Effect of nonionic surfactants on transdermal drug delivery: I. Polysorbates. Int J Pharm, 69, 143–53.
- Iwasa A, Irimoto K, Kasai S, Okuyama H, Nagai T. (1991). Effect of nonionic surfactants on percutaneous absorption of diclofenac sodium. Yakuzaigaaku, 51, 16–21.
- Lopez A, Llinares F, Cortell C, Herraez M. (2000). Comparative enhancer effects of span 20 with Tween 20 and Azone on the in vitro percutaneous penetration of compounds with different lipophilicities. Int J Pharm, 202, 133–40.
- Shin SC, Cho CW, Oh IJ. (2001). Effects of non-ionic surfactants as permeation enhancers towards piroxicam from the poloxamer gel through rat skins. Int J Pharm, 222, 199–203.
- Shokri J, Nokhodchi A, Kashbolaghi A, Hassan-Zadeh K, Ghafourian T, Barzegar-Jalali M. (2001). The effect of surfactants on the skin penetration of diazepam. Int J Pharm, 228, 99–107.
- Florence T, Tuker IG, Walters KA. (1994). Interaction of non-ionic alkyl and aryl ethers with membranes and other biological systems. In: Rosen MJ, ed. Structure Performance Relationships in Surfactants. ACS symposium Series, 253, 189–207.
- Chowan ZT, Pritchard R. (1978). Effect of surfactants on percutaneous absorption of naproxen. I: comparisons of rabbit, rat and human excised skin. J Pharm Sci, 67, 1272–4.
- Gao Z, Crowley WR, Shukla AJ, Johnson JR, Reger JF. 1995. Controlled release of contraceptive steroids from biodegradable and injectable gel formulations; in vivo evaluation. Pharm Res, 12, 864–8.
- Squillante E, Needham T, Maniar A, Kislaliglu S, Zia H. (1998). Codiffusion of propylene glycol and dimethyl isosorbide in hairless mouse skin. Eur J Pharm Biopharm, 46, 265–71.
- Adrian CW, Barry BW. (2004). Penetration enhancers. Adv Drug Deliv Rev, 56, 603–18.