Abstract
Previous studies in vitro had identified niaouli essential oil (NEO) as a valuable transdermal permeation promoter for estradiol (ES). Subsequent considerations on the complex issue of NEO provenance and composition stimulated the present investigation, which was aimed at defining the composition of NEOs obtained from four different sources, at evaluating their influence on transdermal permeation of ES through hairless mouse skin, and at formulating and evaluating simpler terpene mixtures mimicking the NEOs’ composition. While all oils contained 1,8-cineol (eucalyptol) as the main component, appreciable variations in composition could be evidenced, originating differences on the ES cutaneous permeation. Two artificial mixtures containing the same proportions of the main terpenes present in each oil (except the commercially unavailable γ-terpineol) proved equal or significantly superior in activity when compared with the original oils. It is felt that this study might contribute to the formulation of terpene mixtures acting more efficiently and reproducibly with respect to natural NEOs, whose complex and variable composition, depending on growing place, season, and extraction process, is well documented in the relevant literature.
Introduction
As testified by copious literature, the issue of absorption promoters in transcutaneous drug delivery continues to attract remarkable interest. Among a variety of promoters, many terpenes, alone or in combination with ethanol or propylene glycol, have been investigated for drugs such as e.g. fluorouracil (CitationWilliams & Barry, 2004), NSAIDs (CitationNegishi et al., 1995; CitationRhee et al., 2001; CitationBrain et al., 2006), propranolol hydrochloride and tamoxifen (CitationZhao & Singh, 1998, Citation1999), caffeine, hydrocortisone and triamcinolone acetonide (CitationGodwin & Michniak, 1999), hydrocortisone (CitationEl-Kattan, Asbill, & Michniak, 2000), haloperidol (CitationVaddi, Ho, & Chan, 2002), bupranolol (CitationBabu & Pandit, 2005), and zidovudine (CitationNarishetty & Panchagnula, 2004).
Several reports have also dealt with terpene-containing essential oils as penetration enhancers, as e.g. oils from chenopodium, eucalyptus, anise, ylang ylang (CitationWilliams & Barry, 2004), from melaleuca plants (CitationMonti et al., 2002, Citation2005, Citation2006; CitationReichling et al., 2006), from alpinia oxyphylla, magnolia fargesii, and ocimum basilicum (CitationFang et al., 2003; CitationFang, Tsai, et al., 2004; CitationFang, Leu, et al., 2004), etc. In particular, after performing tests on cajuput, cardamom, melissa, myrtle, niaouli, and orange oil, we evidenced niaouli essential oil (NEO) from Melaleuca viridiflora (Myrtaceae family) as the best permeation promoter for β-estradiol (ES) (CitationMonti et al., 2002).
Subsequent considerations on the complex issue of NEO provenance and composition stimulated the present investigation. This oil, characterized by a relatively high (>50%) 1,8-cineol content, reportedly originates from two different Melaleuca species: Melaleuca viridiflora, a small tree from northern Australia and New Caledonia (CitationHellyer & Lassak, 1968), and Melaleuca quinquenervia, a larger tree from eastern Australia (CitationLawrence, 1997). Each plant may secrete oils with different quali/quantitative composition, and the terpene components may vary depending on growing place, season, and extraction process (CitationLawrence, 1997). Furthermore, the foliar leaf oils of M. quinquenervia fall into two classes, or chemotypes, based on their chemical composition. One chemotype is rich in nerolidol (90%); the other in 1,8-cineol (30–70%) and sometimes viridiflorol (0–60%). It is the cineole-rich chemotype that is the source of niaouli oil produced in New Caledonia (CitationWorld Agoforestry Centre Database).
It is important mentioning here that a further Melaleuca species, Melaleuca alternifolia, is the most important source of Tea Tree oil (TTO, melaleuca oil), a topical antibacterial and antifungal distinctly differing in terpene composition from NEO (CitationCarson & Riley, 2001; CitationCarson, Hammer, & Riley, 2006). However, some suppliers indicate the latter species as another source of NEO. These discrepancies are indicative of the confusion surrounding the identity, origin, and composition of NEO.
The aim of the present study was to define the composition of NEOs obtained from different sources, and to evaluate their influence on transdermal permeation of estradiol in vitro through hairless mouse skin. The composition of each oil was determinated by gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS). The increase in percutaneous permeation of estradiol was evaluated at first using the oils as such; subsequent tests were performed with artificial mixtures containing the same proportions of the main terpenes present in each natural oil. It was thus hoped to provide information: (a) on the composition of NEOs of different provenance; (b) on their enhancing activity for β-estradiol in vitro; and (c) on the possibility to select an artificial mixture more effective than the natural one.
Materials and methods
Materials
The following products were used as received: β-estradiol (ES), d-limonene, and α-terpineol (Sigma Chemical Co., St. Louis, MO, USA); 1,8-cineole (ACEF SpA, Piacenza, Italy); α-pinene and p-cymene (Fluka Chemie AG, Buchs, Switzerland); propylene glycol (PG, ACEF SpA, Piacenza, Italy); ethyl alcohol (ethanol) and sodium azide (Carlo Erba, Milano, Italy). NEO samples of different origin were denominated as follows: NEO1 (from Melaleuca viridiflora, batch 00432235, ACEF SpA, Piacenza, Italy); NEO2 (from Melaleuca alternifolia, Vietnam, Cod. P1369), and NEO3 (from Melaleuca alternifolia, Australia, PAYS type, cod. P2805; both from Muller & Koster SpA, Milan, Italy); NEO4 (from Melaleuca quinquenervia, Madagascar, Lot MA-8, Appalachian Valley Natural Products, Friendsville, MD).
All other chemicals and solvents were of analytical grade.
Identification of the main components of NEO essential oils
The oil samples (NEO1, NEO2, NEO3, NEO4) were dissolved in n-hexane (HPLC solvent grade, 10%) and analyzed by GC-FID and GC-MS.
GC-FID analyses were preformed with a HP-5890 Series II instrument equipped with HP-WAX and HP-5 capillary columns (30 m × 0. 25 mm, 0.25 μm film thickness), using the following temperature program: 60°C for 10 min, ramp of 5°C/min up to 220°C; injector and detector temperatures 250°C; carrier gas nitrogen (2 ml/min); detector dual FID; split ratio 1:30; injection of 0.5 μl. The identification of the components was performed, for both columns, by comparison of their retention times with those of pure authentic samples and by means of their retention indices relative to a series of n-hydrocarbons. The relative proportions of the essential oil constituents were obtained by FID peak-area normalization.
GC-EI-MS analyses were performed using a Varian CP-3800 gas-chromatograph equipped with a DB-5 capillary column (30 m × 0. 25 mm; coating thickness 0.25 μm) and a Varian Saturn 2000 ion trap mass detector. Analytical conditions: injector and transfer line temperatures 220°C and 240°C, respectively; oven temperature programmed from 60°C to 240°C at 3°C/min; carrier gas helium at 1 ml/min; injection of 0.2 μl (10% hexane solution); split ratio 1:30. The identification of the constituents was based on comparison of their retention times and mass spectra with those of authentic samples of a home-made library built up from pure substances and/or components of known oils. Furthermore, computer matching by a commercial data base (NIST 2005) was used (CitationStenhagen, Abrahamsson, & McLafferty, 1974; CitationMassada, 1976; CitationJennings & Shibamoto, 1980; CitationSwigar & Silverstein, 1981; CitationDavies, 1990; CitationConnolly & Hill, 1991; CitationAdams, 1995).
In vitro skin permeation experiments
In vitro permeation tests through excised hairless mouse skin were carried out as previously described (CitationMonti et al., 1995) using horizontal cells. The volume of each half-cell was 8.5 ml, and the skin surface available for permeation was 2.0 cm2. Male hairless mice (Strain MF1-hr/hr/Ola, Nossan S.r.l., Correzzana, Milano) aged 7–10 weeks were used in all cases. The donor phase consisted of propylene glycol (PG) containing 1.0% w/w ES and 10.0% w/w NEO or terpenes mixture (cf. and ). The receiving phase was isotonic phosphate buffer saline (PBS, 66.7 mM, pH 7.4) containing 0.003% w/v sodium azide to prevent bacterial growth. Both donor and receiving solutions were stirred and thermostated at 37°C. At predetermined time intervals, 5.0 ml samples of the receiving phase were withdrawn for analysis, and replaced with an equal volume of fresh receptor phase. Sink conditions were always verified. The permeation experiment lasted 5 h and each permeation test was replicated at least four times.
Linear regression analysis of pseudo steady-state diffusion data allowed calculation of J, the steady-state flux (given by Q/A.t, where Q is the amount of permeant diffusing across the area A in time t). The permeation lag times (indicating the time taken by the drug to saturate the skin and to reach the receiving compartment) were calculated from the x-axis intercept values of the regression lines. Enhancement factors (EF), expressing the relative activity of each promoter, were calculated from the ratio Jb/Ja, where Jb and Ja are the average fluxes in presence and in absence of promoter, respectively.
HPLC analytical method
The concentration of the ES in the samples was determined by a validated HPLC method (liquid chromatograph with LC 6A pump and 20 μl Rheodyne injector, SPDM6A detector, and computer integrating system, Shimadzu Corp., Kyoto, Japan). The column ( 30 cm × 3. 9 mm) was packed with μ-Bondapack C18 (pore size 10 μm, Waters, USA-Milford, MA). The mobile phase consisted of 45% v/v methanol, 45% v/v acetonitrile, and 10% v/v water (flow rate 0.8 ml/min). The retention time and the detection wavelength were 6.4 min and 260 nm, respectively.
Statistical analysis
Statistical differences between means were assessed by GraphPad Prism software (GraphPad Software Inc., San Diego, CA). The evaluation included calculation of means and standard errors, and group comparisons using the Student’s two-tailed unpaired t-test. Differences were considered statistically significant at p < 0.05.
Results
Identification of the main components of NEO essential oils, and formulation of artificial mixtures
The terpene components of four different NEO samples, detected by GC-FID and GC-MS, are listed in ; depending on sample, the identified components account for > 97.8 to > 99.6% of the total composition. Six monoterpenes (1,8 cineol, α-pinene, α-terpineol, γ-terpineol, p-cymene, d-limonene) were present in substantial amounts in all oils; 1,8-cineol (eucalyptol) being the most abundant (53.0–70.4%). The highest content of this terpene (70.4%) was found in NEO4. The α-pinene range was 4–18.3%, NEO1 showing the highest content. α-terpineol was present in the 5.5–15.8% range, while the γ-terpineol range was 0.7–6.1%, both monoterpene alcohols being most abundant in NEO3. d-limonene and p-cymene were present in all oils in the 3.9–8.6% and 2.1–6.7% range, respectively. Comparatively smaller amounts of β-terpineol (0.2–2.2%) and β-pinene (0.4–1.8%) were present in all four oils, while the bicyclic monoterpene, sabinene, was present in a sizeable amount (4.1%) only in NEO3. Other terpenes (β-myrcene, (E)-β-ocimene, terpinolene, γ-terpinene, linalool, terpinen-4-ol, aromadendrene, and γ-cubebene) were found in minute amounts in some oils. These data appeared to exclude for any of the examined oils the qualification of Tea Tree Oil, since a terpinen-4-ol content in excess of 30% is required for this oil, although in practice this is often approximately 40% (CitationCarson & Riley, 2001).
Table 1. Terpene components (% w/w) of NEOs 1–4.
These analytical data allowed us to prepare the terpene artificial mixtures, whose composition is shown in . The mixtures, designed to mimic the main terpene composition of NEOs 1–4 and denominated MIX 1–4, contained varying proportions of 1,8-cineol, α-pinene, α-terpineol, d-limonene, and p-cymene, the most representative terpenes in the natural oils; γ-terpineol was not added to the mixtures on account of its commercial scarcity.
Table 2. Composition (% w/w ) of the terpene mixtures used as enhancers.
Effect of the four NEOs and of the artificial mixtures on mouse skin permeation in vitro
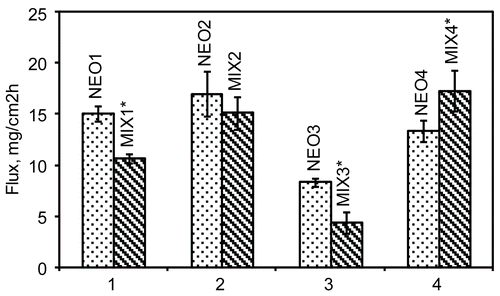
The permeation parameters (flux, lag time, percent permeated after 5 h and enhancement factor, E.F.) of estradiol (ES) across hairless mouse skin in absence and presence of the four different NEO samples are reported in , while reports the data related to the artificial mixtures.
Table 3. Skin permeation data of ES from vehicles containing NEOs from different sources (means ± standard error, SE).
Table 4. Skin permeation data of ES from vehicles containing artificial terpene mixtures (means ± SE).
The ES transdermal flux in the absence of an enhancer (V0) was 0.2 ± 0.041 μg/cm2.h. The addition of 10% NEO (NEOs 1–4, vehicles V1–V4) produced a 41.50- to 84.63-fold increase of ES transdermal flux with respect to vehicle V0. The permeation lag times ranged from 0.50 to 1.23 h. NEO1 (vehicle V1) and NEO2 (vehicle V2) provided the best enhancing effect for ES: the corresponding enhancement factors being 75.0 and 84.63, respectively.
Somewhat different results were observed when the terpene mixtures simulating the main terpene composition of NEOs 1–4 were used as enhancers (). The best enhancement factor over the enhancer-free vehicle V0 was shown by MIX4 (E.F. 78.73), followed by MIX2, MIX1 (E.F.s 75.23 and 53.13, respectively), and at the end MIX3 (E.F. 21.88).
The fluxes comparisons illustrated in clearly show that MIX4 produced permeation results not statistically different from NEO1 and NEO2, while it was significantly more efficient with respect to NEO3 and NEO4. NEO3 and the corresponding mixture MIX3 proved the poorest enhancement, showing the smallest E.F. values (41.50 and 21.88 for NEO3 and MIX3, respectively). In summary, the artificial compositions simulating NEO2 and NEO4 produced practically the same permeation-enhancing results shown by NEO2 (the best performer within the tested NEO series).
Discussion
The low percentage of terpinen-4-ol (< 0.1–0.8%) present in the original oil samples confirmed their qualification as Niaouli oil, even if derived (NEO2 and NEO3) from Melaleuca alternifolia, a typical source of Tea Tree Oil (TTO). In fact, as mentioned above, TTO is characterized by a terpinen-4-ol content in excess of 30%. A comparison of the composition of various Myrtaceous essential oils, reporting for NEO 1,8-cineol and limonene contents corresponding to 52 and 14%, respectively, vs 2.3 and 0.88 for TTO, further supports this attribution (CitationHarkenthal, Reichling, & Saller, 1999). As shown in , the four NEOs, although containing basically the same main terpenes, differed sensibly in quantitative composition, thus confirming the statements by various authors cited in the introduction, about variations depending on plant species, chemotype, growing place, season, and extraction process (CitationLawrence, 1997; CitationIreland et al., 2002).
Inspection of and clearly shows that three oils, NEO1, NEO2, and NEO4, while producing permeation fluxes not significantly different from each other, were significantly superior to NEO3. This finding should call for great attention when selecting a natural oil as a permeation enhancer: oils with the same denomination (Niaouli, in this case) do not necessarily produce the same results. Explaining the reasons for this different behavior, and for the sensibly lower enhancing activity of NEO3, presents serious difficulties when the composition of the oils is considered, as discussed below.
The four main terpene components of NIA, 1,8 cineole, α-pinene, α-terpineol, and d-limonene, are well known skin penetration enhancers (CitationBarry & Williams, 1993). The enhancing ability of a series of monoterpenes toward permeation of ES has been investigated in depth by Barry and co-workers (CitationWilliams & Barry, 1991a, Citation1991b; CitationMoghimi, Williams, & Barry, 1996). Among different chemical classes of terpenes studied, 1,8-cineole, a cyclic monoterpene from the ether class, and d-limonene, a cyclic hydrocarbon monoterpene, improved widely the permeation of ES across epidermal membrane (CitationWilliams & Barry, 1991a, Citation1991b). As reported by CitationFang et al. (2007) compounds such as 1,8 cineole have structures suitable for disrupting the lipid packing of the SC because of the presence of definitive hydrocarbon tails in addition to a polar head group.
These results were confirmed in a previous study performed in our laboratories (CitationMonti et al., 2002) where we found that 1,8 cineole provided the higher enhancement factor for ES (EF 33.0 ± 2.4), followed by d-limonene and α-pinene (EFs 9.4 ± 0.8 and 8.1 ± 0.6, respectively). α-terpineol didn’t improve significatively the drug permeation (EF 2.0 ± 0.6) probably due to its higher polarity (CitationYarita, Nomura, & Horimoto, 1994).
Many studies report that the effect of enhancers on the cutaneous permeation of a drug usually depends upon the physicochemical characteristics of both permeant as well as the enhancer molecule. It seems that hydrophilic terpenes are more effective in enhancing the permeation of hydrophilic drugs, whereas hydrocarbon terpenes (d-limonene and α-pinene) are more active towards lipophilic drugs (CitationMoghimi et al., 1996; CitationHori et al., 1991) such as ES.
For what concerns the present work, while 1,8 cineole was present in considerable quantities among all the vehicles (NEO 1–4) studied in this paper, NEO3 showed the lowest amount of hydrocarbons terpenes (16.8%) notwithstanding the presence of an appreciable amount of sabinene, not found in the other oils. Even if previous studies (data not published) demonstrated that sabinene was a good enhancer for ES cutaneous permeation, in this experiment it does not seem to play a significant role. Besides NEO3 presented the higher value of α-terpineol that we have seen to not contribute significatively to ES cutaneous permeation. If compared to the other oils NEO1 showed the lowest value of 1,8 cineole and d-limonene without considerable differences in drug permeation with respect to NEO2 and NEO4. This lack of differences could be ascribed at the higher amount of hydrocarbon terpenes found in NEO1 (26.9%) with respect to the other oils.
If one now considers the promotion effect of the four artificial mixtures, it is interesting to note that only in one case (MIX4) the transdermal flux of ES produced by the mixture was superior with statistically significant difference when compared to that of the original oil, NEO4. The other mixtures were not significantly different (MIX2) or significantly less active (MIX1 and MIX3) with respect to the corresponding natural oils (NEO1 and NEO3). It is noteworthy that some terpenes show a synergistic effect when mixed together (CitationYoung, 2006), therefore it can be assumed that the presence of a higher number of terpenes in each natural NEOs than in the corresponding artificial mixture may affect positively the permeation of ES through hairless mouse skin. An opposite trend was observed for the couple NEO4-MIX4: this could lead us to hypothize that some terpenes might act as inhibitors, but this is not supported from any evidence in the literature and should be further investigated.
The inferior performance of MIX1 and MIX3 with respect to MIX2 and MIX4 might be attributed to their lower 1,8-cineol and d-limonene contents, in the case of MIX3 not counterbalanced by the conspicuous presence of α-pinene as for MIX1.
This work pointed out that natural NEOs obtained from different sources presented different compositions. The presence of terpenes in different types and, expecially, different quantities might influence ES transdermal permeation leading to different fluxes and lag times when using one oil instead of another. While an elucidation of the role and interrelation of the individual components of the tested NEOs and mixtures is outside the scope of the present investigation, the present authors feel that this study might contribute to the formulation of terpene mixtures (i.e. MIX2 and MIX4) acting as efficiently as natural NEOs and more reproducibly with respect to natural oils, whose complex and variable composition, depending on so many factors, has been clearly evidenced.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adams, R.P. (1995). Identification of essential oil components by gas chromatography-mass spectroscopy. Stream, IL: Allured Publ. Corp, 469.
- Babu, R.J., Pandit, J.K. (2005). Effect of penetration enhancers on the transdermal delivery of bupranolol through rat skin. Drug Deliv, 12, 165–9.
- Barry, B.W., Williams, A.C. (1993). Terpenes as skin penetration enhancers. In: Walters KA, Hadgraft J, eds. Pharmaceutical skin penetration enhancement. New York: Marcel Dekker, 95–111.
- Brain, K.R., Green, D.M., Dykes, P.J., Marks, R., Bola, T.S. (2006). The role of menthol in skin penetration from topical formulations of ibuprofen 5% in vivo. Skin Pharmacol Physiol. 19, 17–21.
- Carson, C.F., Riley, T.V. (2001). Safety, efficacy and provenance of tea tree oil (Melaleuca alternifolia) oil. Contact Dermatitis. 45, 65–7.
- Carson, C.F., Hammer, K.A., Riley, T.V. (2006). Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Revs. 19, 50–62.
- Connolly, J.D., Hill, R.A. (1991). Dictionary of terpenoids. London: Chapman & Hall.
- Davies, N.W. (1990). Gas chromatographic retention of monoterpenes and sesquiterpenes on methyl silicone and Carbowax 20M phases. J Chromatogr. 503, 1–24.
- El-Kattan, A.E., Asbill, C.S., Michniak, B.B. (2000). The effect of terpene enhancer lipophylicity on the percutaneous permeation of hydrocortisone formulated in HPMC gel systems. Int J Pharm. 198, 179–89.
- Fang, J.Y., Leu, Y.L., Hwang, T.L., Cheng, H.C., Hung, C.F. (2003). Development of sesquiterpenes from Alpinia oxiphylla as novel skin permeation enhancers. Eur J Pharm Sci. 19, 253–62.
- Fang, J.Y., Tsai, T.H., Hung, C.F., Wong, W.W. (2004). Development and evaluation of essential oil from Magnolia Fargesii for enhancing the transdermal absorption of theophylline and cianidanol. J Pharm Pharmacol. 56, 1493–500.
- Fang, J.Y., Leu, Y.L., Hwang, T.L., Cheng, H.C. (2004). Essential oils from sweet basil (Ocimum basilicum) as novel enhancers to accelerate transdermal drug delivery. Biol Pharm Bull. 27, 1819–25.
- Fang, J.Y., Tsai, T.H., Lin, Y.Y., Wong, W.W., Wang, M.N., Huang, J.F. (2007). Transdermal delivery of tea catechins and theophylline enhanced by terpenes: a mechanistic study. Biol Pharm Bull. 30, 343–9.
- Godwin, D.A., Michniak, B.B. (1999). Influence of drug lipophilicity on terpenes as transdermal penetration enhancers. Drug Dev Ind Pharm. 25, 905–15.
- Harkenthal, M., Reichling, H-K., Saller, R. (1999). Comparative study on the in vitro antibacterial activity of Australian tea tree oil, cajuput oil, niaouli oil, manuka oil, kanuka oil, and eucalyptus oil. Pharmazie. 54, 460–3.
- Hellyer, R.O., Lassak, E.V. (1968). The steam-volatile constituents of Melaleuca viridiflora Sol. ex Gaertn. Aust J Chem. 21, 2585–7.
- Hori, M., Satoh, S., Maibach, H.I., Gug, R.H. (1991). Enhancement of propranolol hydrochloride and diazepam skin absorption in vitro: effect of enhancer lipophilicity. J Pharm Sci. 80, 32–5.
- Ireland, B.F., Hibbet, D.B., Goldsack, R.J., Doran, J.C., Brophy, J.J. (2002). Chemical variation in the leaf essential oil of Melaleuca quinquenervia (Cav.)ST Blake Biochem System Ecol. 30, 457–70.
- Jennings, W., Shibamoto, T. (1980). Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography. New York: Academic Press, 472.
- Lawrence, B.M. (1997). Progress in essential oils. Perfum Flavor. 22, 49–56.
- Leung, A.Y., Foster, S. (2000). In: Enciclopedia delle piante medicinali. Aporie. 117–9.
- Massada, Y. (1976). Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry. New York: J. Wiley & Sons.
- Moghimi, H., Williams, A., Barry, B. (1996). A lamellar matrix for stratum corneum intercellular lipids. IV. Effects of terpene penetration enhancers on the permeation of 5-fluorouracil and oestradiol through the matrix. Int J Pharm. 145, 49–59.
- Monti, D., Saettone, M.F., Giannaccini, B., Galli-Angeli, D. (1995). Enhancement of transdermal penetration of dapiprazole through hairless mouse skin. J Contr Rel. 33, 71–7.
- Monti, D., Chetoni, P., Burgalassi, S., Najarro, M., Saettone, M.F., Boldrini, E. (2002). Effect of different terpene-containing essential oils on permeation of estradiol through hairless mouse skin. Int J Pharm. 237, 209–14.
- Monti, D., Najarro, M., Chetoni, P., Burgalassi, S., Saettone, M.F., Boldrini, E. (2005). Vehicle and enhancer effects on transdermal permeation of estradiol from gel formulations: evaluation in vitro. J Drug Del Sci Tech. 15, 469–73.
- Monti, D., Najarro, M., Chetoni, P., Burgalassi, S., Saettone, M.F., Boldrini, E. (2006). Niaouli oil as enhancer for transdermal permeation of estradiol. Evaluation of gel formulations on hairless rats in vivo. J Drug Del Sci Tech. 16, 473–6.
- Narishetty, S.T.K., Panchagnula, R. (2004). Transdermal delivery of zidovudine: effect of terpenes and their mechanism of action. J Contr Rel. 95, 367–79.
- Negishi, J., Takayama, K., Higashiyama, K., Chida, Y., Isowa, K., Nagai, T. (1995). Promoting effect of O-alkylmenthol and O-acylmenthol derivates on the percutaneous absorption of ketoprofen in rats. STP Pharma Sci. 5, 156–61.
- Reichling, J., Landvatter, U., Wagner, H., Kostka, K.H., Schaefer, U.F. (2006). In vitro studies on release and human skin permeation of Australian tea tree oil (TTO) from topical formulations. Eur J Pharm Biopharm. 64, 222–8.
- Rhee, Y.S., Choi, J.G., Park, E.S., Chi, S.C. (2001). Transdermal delivery of ketoprofen using microemulsions. Int J Pharm. 228, 161–70.
- Stenhagen, E., Abrahamsson, S., McLafferty, F.W. (1974). Registry of Mass spectral data. New York: Wiley & Sons, 3358.
- Swigar, A.A., Silverstein, R.M. (1981). Monoterpenes. Milwaukee: Aldrich Chem. Comp.
- Vaddi, H.K., Ho, P.C., Chan, S.Y. (2002). Terpenes in propylene glycol as skin-penetration enhancers: permeation and partition of haloperidol, Fourier transform infrared spectroscopy, and differential scanning calorimetry. J Pharm Sci. 91, 1639–51.
- Williams, A.C., Barry, B.W. (1991a). Terpenes and the lipidprotein-partitioning theory of skin penetration enhancement. Pharm Res. 8, 17–24.
- Williams, A.C., Barry, B.W. (1991b). The enhancement index concept applied to terpene penetration enhancers for human skin and model lipophilic (oestradiol) and hydrophilic (5-fluorouracil) drugs. Int J Pharm. 74, 157–68.
- Williams, A.C., Barry, B.W. (2004). Penetration enhancers. Adv Drug Deliv Rev. 56, 603–18.
- World Agoforestry Centre Database. Available online at: http://www.worldagroforestrycentre.org/sites/Treedbs/aft/speciesprinterfriendly.asp?Id=1138. Accessed 01.12.2008
- Yarita, T., Nomura, A., Horimoto, Y. (1994). Type analysis of citrus essential oils by multidimensional supercritical fluid chromatography/gas chromatography. Anal Sci. 10, 20–9.
- Young, G. (2006). “The fundamentals of longevity” Young living CD#81, 2006; http://www.essentialoiltherapies.com/training_tapes.html. Accessed 10.12.2008
- Zhao, K., Singh, J. (1998). Mechanisms of percutaneous absorption of tamoxifen by terpenes: eugenol, D-limonene and menthone. J Contr Rel. 55, 253–60.
- Zhao, K., Singh, J. (1999). In vitro percutaneous absorption enhancement of propranolol hydrochloride through porcine epidermis by terpenes/ethanol. J Contr Rel. 62, 359–66.