Abstract
Pharmacologic chelators do not effectively penetrate cell membranes and blood–brain barrier. This study assesses methylsulfonylmethane (MSM) as a permeability enhancer and an excipient to facilitate EDTA transport across biologic membranes, and to make possible localized, regional chelation. Topical application of MSM with C14EDTA onto the rat cornea led to uptake of the C14EDTA in all tested ocular tissues. Without MSM, EDTA did not penetrate the eye. The ability of MSM to deliver EDTA into an eye provides an opportunity for regional chelation therapy. Additionally, these studies suggest that MSM could also be an adjuvant for delivering ciprofloxacin and other chemical compounds to specific, local tissue sites.
Introduction
A limitation of chelation therapy is chelators poorly penetrate tissue (CitationLiu et al., 2006; CitationHe et al., 2007; CitationJayasena et al., 2007). Pharmacologic chelators used for heavy metal poisonings are poorly absorbed through the gastrointestinal tract and need to be administered parenterally. A topically applied chelator, such as sodium ethylenediaminetetraacetic acid (EDTA), does not penetrate well through layers of epithelial cells. When calcium deposits are removed from the cornea, as in the treatment of band keratopathy, the five-cell layer of corneal epithelium is removed to allow EDTA to reach and chelate the aggregates of calcium salts in the underlying corneal stroma (CitationNajjar et al., 2004).
Most pharmacologic chelators act in the intravascular space. In iron toxicity, desferrioxamine chelates the free iron in the blood (CitationBlanusa et al., 2005; CitationKalinowsky & Richardson, 2005). In hypercalcemia, EDTA chelates the large pool of freely diffusible calcium ions in the plasma. Once a chelator combines with metal ions in blood, the complexes are then excreted in the urine (CitationAndersen & Aaseth, 2002; CitationAndersen, 2004). Chelation therapy may be more effective and used more widely if the pharmacologic chelators can penetrate through biological membranes such as cell membranes, blood–brain barrier, and blood–retina barriers. One approach is to add a permeability enhancing agent to facilitate transport of chelators across biologic membranes and increase the amount of drug that gets into tissue (CitationMeaney & O’Driscoll, 2000; CitationBarrow et al., 2005). Permeability enhancers may allow molecules to move between cells or through cells. They may modulate the tight junctions between cells, allowing molecules to pass between adjacent cells. Or they may transport through the cell by active or passive diffusion, endocytosis, or transcytosis. Non-ionic surfactants such as polysorbates increase permeability by solubilizing membrane and membranes components which could be deleterious to the cell (CitationDimitrijevic, Shaw, & Florence, 2000). Several other studies as well suggest the limitation of the permeability enhancers (CitationUchiyama et al., 1996; CitationQuan et al., 1998; CitationSakai et al., 1998; CitationEley & Triumalashetty, 2001; CitationOuyang et al., 2002; CitationSmith, Wood, & Dornish, 2004) and therefore the hunt for safer molecules is of immense clinical relevance.
Methylsulfonylmethane (MSM) has been described as a permeability enhancer, but there is no experimental evidence to support this claim. MSM is structurally related to dimethyl sulfoxide (DMSO), which is capable of increasing the absorption rate of some compounds into skin and other tissues (CitationChandrasekaran & Shaw, 1978). MSM is more polar and less reactive than the organic solvent DMSO and could serve to be an efficient non-toxic permeability enhancer. The aim of this study was to determine if topically administered methylsulfonylmethane is a permeability enhancer capable of increasing the transport of drugs into tissue. We studied the ability of MSM to transport methylene blue and ciprofloxacin across tissue membranes in vitro. Then we studied, in vivo, the ability of MSM to transport a chelator, sodium ethylenediaminetetraacetic acid (EDTA), into the eye. The eye presents unique opportunities to drug delivery and is most accessible to topical drug application. Because the cornea, lens, and vitreous are avascular, their uptake of EDTA provides a means to examine drug penetration in avascular cellular tissues. If a chelating agent can permeate tissues at potentially therapeutic concentration with the use of a permeability enhancer, opportunities are possible for new use of chelation therapy. Also development of an effective permeability enhancer offers new means to deliver other drugs.
Materials and methods
Animals
Male Sprague-Dawley rats (Harlan, Indianapolis, Indiana), weighing 200–250 g, were used to study the transport of EDTA into the eye. The NIH guidelines and ARVO statement for the Use of Animals in Ophthalmic and Vision Research were strictly followed for the welfare of the animals.
Effect of MSM on methylene blue dye transport across porcine intestinal membrane
To demonstrate MSM (Sigma-Aldrich, St. Louis, MO) ability to enhance permeability, in vitro experiments were conducted using a two-chamber apparatus for measuring permeability of molecules through porcine intestinal membrane. Samples of MSM at various concentrations (2.7, 27, and 54 mg/ml) and methylene blue 1. 0 mg/ml were placed in one chamber. At time zero, 30, 60, 90, and 120 min, 1 ml aliquots were removed from the other chamber. For the control methylene blue 1. 0 mg/ml without MSM was placed in the first chamber. The absorbance of aliquots from the second chamber was measured with a spectrometer at 668 nm.
To determine if MSM had a post-treatment or memory effect on tissue permeability, porcine intestinal membrane was soaked in MSM ( 27 mg/ml) for 24 h, rinsed, and then soaked in water for another 24 h. The membrane permeability to methylene blue was then tested with the two-chamber membrane apparatus. Another membrane soaked in water for 48 h served as a control and its permeability after the soaking was tested with methylene blue and no MSM. A positive control membrane was soaked in water for 24 h then tested with methylene blue and MSM.
Effect of MSM on ciprofloxacin transport across porcine intestinal membrane
To demonstrate the ability of MSM to enhance permeation of a drug across a tissue membrane, ciprofloxacin, a broad spectrum antibiotic, was studied with the porcine intestinal membrane two-chamber apparatus. Ciprofloxacin (Bayer AG) and MSM were added to the first chamber. For the control, ciprofloxacin without MSM was used. Aliquots from the second chamber were removed at various time points and absorbance measured at 275 nm.
Effect of MSM on transport of EDTA into the eye
To determine if topically applied MSM can enhance the penetration of sodium EDTA through the cornea into the aqueous, two study formulations of EDTA eye drops were prepared by combining 2.6% 14C-EDTA tetra sodium salt (American Radio labeled Chemicals, St. Louis, MO) with and without 5.4% MSM. Ten microliters of one of the solutions was topically administered to an eye. The rats were sacrificed and aqueous humor was removed from the anterior chamber 30, 60, and 120 min after receiving the eye drops and radioactivity determined as described below for the ocular tissues.
To determine the uptake and distribution of topically administered C14 EDTA with MSM in ocular tissue, 10 μl eye drops were given to each eye. The rats were sacrificed and their eyes were enucleated at 5, 10, 20, 40, and 60 min later. The aqueous humor was drawn and the tissues were divided into cornea, lens, vitreous, and retinochoroidal tissue. The tissues were lysed with tissue solubilizer (Biosol™; National Diagnostics, Atlanta, GA) incubated at 50°C for 3 h and mixed with scintillation solution (Bioscient™; National Diagnostics, Atlanta, GA). The samples were counted in a liquid scintillation counter.
Data analysis
The data are expressed as the mean ± SE. Statistical comparisons were made using the Mann-Whitney U-test followed by an evaluation of the treated vs untreated groups with the Bonferroni procedure (SigmaStat; Systat Software, San Jose, CA). Radiation from the radio labeled C14 EDTA measured in counts per minute was converted to micromoles of EDTA.
Results
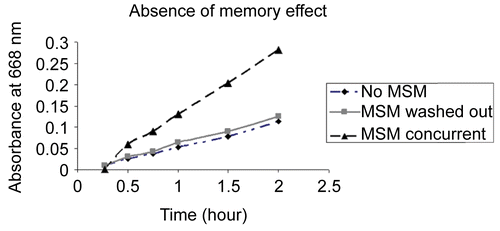
MSM enhanced the transport of methylene blue through the porcine intestinal membrane at a linear rate. The passage rate of the dye increased with higher concentrations of MSM (27 and 54 mg/ml) (). Without MSM or at 2. 7 mg/ml much less methylene blue diffused across the membrane. Similarly, MSM enhanced the passage of ciprofloxacin across the porcine membrane as compared to not using MSM. The amount of ciprofloxacin with MSM increased at a linear rate, resulting in twice as much ciprofloxacin in the second chamber after 2 h ().
Figure 1. Higher concentrations of methylenesulfonylmethane increased the rate of transport of methylene blue 1 mg/ml across a porcine intestinal membrane as measured with a spectrometer set at 668 nm over a 2-h time period.
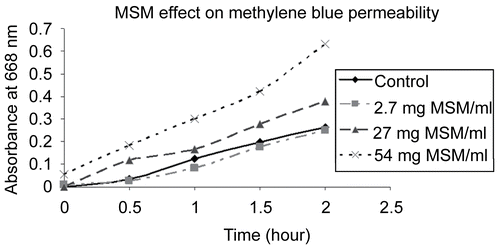
Figure 2. Methylenesulfonylmethane (MSM) increased the rate of transport of ciprofloxacin 0. 5 mg/ml better than ciprofloxacin alone across a porcine intestinal membrane as measured with a spectrometer set at 275 nm over a 2-h time period.
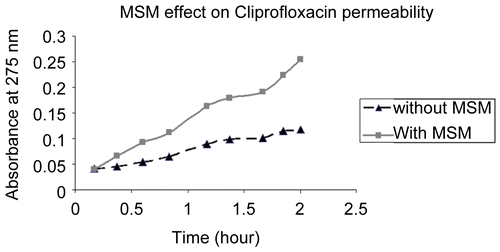
The study to determine MSM effect on porcine intestinal membrane permeability after removing MSM showed that removing MSM led to recovery of the membrane relative impermeability. Membrane soaked in MSM for 24 h and washed was no more permeable to methylene blue than control solution of methylene blue without MSM (). Concurrent MSM increased the permeability of methylene blue through the membrane.
Figure 3. Study determined if the porcine intestinal membrane impermeability recovered after exposure and removal of MSM. Membrane soaked in MSM for 24 h then washed was no more permeable to methylene blue than control solution of methylene blue without MSM. Concurrent MSM increased the permeability of methylene blue through the membrane.
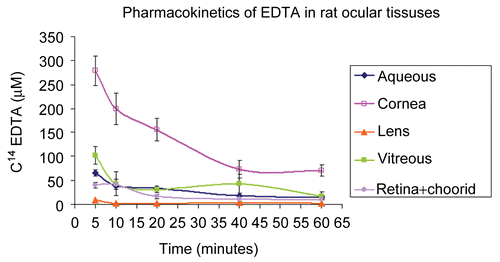
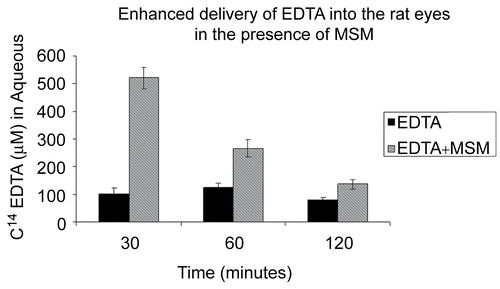
The in vivo experiments demonstrated that MSM greatly enhanced the transport of EDTA through the rat cornea into the aqueous humor (). Without MSM, EDTA permeation through the cornea into the aqueous was negligible. With MSM, levels of C14 EDTA in the aqueous were five times greater than without MSM at 30 min (p < 0.001) and twice as great at 60 min (p < 0.01). Furthermore, without MSM, EDTA permeation appeared to follow zero-order kinetics.
Figure 4. C14-EDTA combined with and without the carrier MSM applied topically to rat eyes. MSM facilitated the delivery of EDTA into the aqueous.
Topical application of C14 EDTA with MSM rapidly permeated the rat corneal epithelium into corneal stroma. As shown in , corneal concentration of C14 EDTA (280 μM) was highest at the first measurement point at 5 min and then after gradually declined. From 40 min to the last measurement at 60 min, corneal concentration of C14 EDTA persisted at levels of ∼ 70 μM. Once transported through the cornea, C14 EDTA with MSM permeated all examined intraocular tissues (). Of the examined intraocular tissues, aqueous contained the highest concentration of C14 EDTA (65 μM). The gradual decline of aqueous C14 EDTA over the first 40 min paralleled the decline in corneal C14 EDTA, but at about 25% of the cornea level. Peak vitreous C14 EDTA concentration measured 51 μM. The very cellular retinochoroidal tissue had peak levels of C14 EDTA of ∼ 40 μM at the first measurement time of 5 min and gradually declined, so by 20 min it was half its peak concentration and at 1 h still had 11 μM. The less cellular but thick and dense rat lens had peak levels of 9 μM C14 EDTA at 5 min, which declined to 5 μM by 10 min and persisted to the last time measurement of 1 h.
Discussion
New methods are needed to enhance drug absorption across biologic barriers of the skin, mucosa, and cornea and the blood–brain barrier to improve efficacy of treatment and to reduce toxicities associated with systemic therapy. Transport of certain drugs across biologic membranes is challenging. Proteins and peptide drugs including some of the new biologic agents must be administered by the parenteral route or in the case of the retinal disorders by repeatedly injecting directly into the eye. One solution is to find a suitable permeability enhancing agent to serve as an excipient to facilitate transport of the drugs across membranes.
One category of permeability enhancer uses a formulation containing surfactants or bile salts as a permeability enhancer. These adjuvants disrupt the cell membrane and actually solubilize the membrane and membrane components (CitationSwenson, Milisen, & Curatolo, 1994; CitationMeaney & O’Driscoll, 2000). Other chemicals such as deoxycholate, glyococholate, or taurocholate required high concentrations which caused local irritation and cell toxicity (CitationUchiyama et al., 1996; CitationQuan et al., 1998; CitationSakai et al., 1998). Alkylglycosides have been observed to open a cellular tight junction without solubilizing cell membranes (CitationEley & Triumalashetty, 2001; CitationOuyang et al., 2002; CitationSmith, Wood, & Dornish, 2004). It is prudent to seek for efficient and safe permeability enhancers.
This study is the first experimental study of MSM as a permeation enhancer. MSM has been used for a variety of purposes unrelated to its permeability enhancing property. The organosulfone compound has been used as a polar solvent for both organic and inorganic substances, an intermediate for synthesis of drugs and a dietary supplement. MSM, which is found widely in fruits, vegetables, and grains in small amount, is considered safe at recommended oral dosages for humans (CitationHorváth et al., 2002). This study showed that MSM enhanced the transport of an indicator dye, methylene blue, and the transport of a commonly used antibiotic, ciprofloxacin, across a membrane. Its ability to enhance the permeability of other drugs is under study, with focus upon the physical properties of drugs that can be transported and its barriers.
This study showed that methylsulfonylmethane greatly enhances the transport of EDTA into the intraocular tissues. A drop of MSM and EDTA (MSM+EDTA) on the cornea led to a significant amount of EDTA in the retinochoroidal tissue. In contrast, topically administered EDTA alone did not reach the intraocular structures in significant amounts. The pharmacokinetic profile and distribution of the MSM+EDTA showed that its entry and clearance were different for different ocular tissues. Cornea tissue had high and persistent levels of C14 EDTA. At 5 and 10 min after instillation, significant amounts of C14 EDTA accumulated in the retinochoroidal layers. The route to the retinochoroidal tissue is unclear. The amounts of C14 EDTA absorbed by the lens and vitreous were much less than more cellular ocular tissues. EDTA may be absorbed primarily by cells as oppose to metabolically inert material. Less than 1% of the vitreous contains hyalocytes, the primary living cell of the vitreous. The lens, similarly, has few metabolically active lens epithelial cells which reside in the outer cortical layer inside the inert lens capsule. After 20 min, C14 EDTA uptake in the vitreous and retinochoroidal tissue were similar and may indicate diffusion from the retinochoroidal tissue to the cellular vitreous cortex.
MSM+EDTA as an eye drop have the potential of chelating abnormal accumulations of metals from ocular tissue which could not be achieved solely with EDTA. Potential ophthalmic treatments include the topical application of MSM+EDTA for removal of calcium salts in band keratopathy, removal of calcification that occurs with certain intraocular lens implant materials, and dissolution of asteroid bodies. Potentially iron accumulations in blood staining of the cornea and ocular siderosis could be treated with regional chelation therapy.
Earlier, it was suggested EDTA may act as a permeation enhancer (CitationGrass, Wood, & Robinson, 1985). EDTA penetration into ocular tissue may be due to its effect on calcium ion-dependent cell adhesion molecules formed at the cellular junction between the individual epithelial cells. Reducing the calcium ion may increase diffusion between the corneal epithelial cells. In the present study small amounts of C14 EDTA passed into the aqueous in the absence of MSM. The aqueous C14 EDTA was one-fifth the amount of C14 EDTA measured when MSM was present. CitationGrass et al. (1985), using topical EDTA 0.5% without MSM, found peak levels in the aqueous of 19. 9 nM. Using a 5-fold higher EDTA concentration along with MSM, aqueous EDTA level in this study peaked at 60 μM, a 3000-fold difference.
MSM is necessary for significant amounts of EDTA to penetrate intraocular tissues. The two-chamber permeability studies revealed that methylene blue does not cross a permeable membrane such as corneal tissue in the absence of MSM. To the best of our knowledge, this is the first experimental study of MSM to demonstrate its permeation enhancing capabilities. The mechanism explaining how MSM increases permeability is not known. MSM (C2H6O2S) is a small molecule with a molecular weight of 94.13. Its size and polarity may allow it to pass through a cells lipid bilayer. Based upon the membrane recovery experiments (), we speculate that MSM does not disrupt membranes like a detergent. MSM effect is transient and membrane permeability recovers once MSM is gone. MSM must be present for another molecule to pass through biologic barriers.
MSM does not require metabolically active cells to transport agents. In the in vitro study porcine intestinal membrane was non-living tissue used in water instead of a growth medium. Active transport of an agent mediated by membrane transport proteins coupled to an energy source is not possible in non-living membrane. Also transport mediated by endocytosis and transcytosis requires a living cell. MSM is able to penetrate acellular structures such as Descemet membrane of the cornea and the lens capsule, which do not have tight intercellular junctions. MSM ability to deliver other chemicals is promising. Whether MSM modifies the biologic barrier to permit passage of a drug in an unaltered form or whether MSM modifies a drug physiochemically to facilitates its transport across the membrane and possibly effect the drug’s pharmacologic action is not known. We will later report that MSM does not alter the chelation ability of the transported EDTA.
The amount of MSM in the eye drop absorbed systemically is insignificant and not toxic. Radio labeled MSM in amounts larger than used in this study was fed to rats. The study showed that MSM is rapidly absorbed (mean tmax of 2.1 h and the t1/2 was 12.2 h) and was safe. Soft tissue distribution of radioactivity indicated a fairly homogenous distribution throughout the body, with relatively lower concentrations in skin and bone. Approximately 85.8% of the dose was recovered in the urine after 120 h and 3% was found in the feces. No quantifiable level of MSM was found at 120 h (CitationMagnuson, Appleton, & Ames, 2007).
Our present study demonstrates that EDTA can be delivered into the eye. We will later report that MSM does not alter the transported EDTA ability to chelate metal ions in the eye by demonstrating experimentally and clinically the removal of metal ions. The ability to chelate metals ions such as calcium from a specific body region opens the possibility to treat a variety of degenerative and inflammatory processes. Calcium often accumulates as a consequence of these processes and, in turn, these accumulations affect physiologic function. Removal of abnormal calcium accumulations may improve physiologic function. Regional drug treatment aided by a permeation enhancer holds the hope for more efficacious treatment without exposing the other body systems to unwanted side effects. Localized application of MSM + EDTA has potential therapeutic benefits for regional treatment of degenerative and inflammatory disorders.
Acknowledgements
Funds from Chakshu Research Inc. are greatly appreciated.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. Ira G. Wong and Jerry B. Gin are consultant and employee, respectively, of Chakshu Research Inc.
References
- Andersen, O. (2004). Chemical and biological considerations in the treatment of metal intoxication by chelating agents. Mini Rev Med Chem. 4, 11–21.
- Andersen, O., Aaseth, J. (2002). Molecular mechanisms of in vivo metal chelation: implications for clinical treatment of metal intoxications. Environ Health Persp. 110 (suppl 5), 887–90.
- Barrow, D.J. Jr., Chandrasekaran, S., Heerklotz, H.H., Henary, M.M., Michniak, B.B., Nguyen, P.M., Song, Y., Smith, J.C., Strekowski, L. (2005). Mechanistic studies on percutaneous penetration enhancement by N-(4-halobenzoyl)-S,S-dimethyliminosulfuranes. J Lipid Res. 46, 2192–201.
- Blanusa, M., Varnai, V.M., Piasek, M., Kostial, K. (2005). Chelators as antidotes of metal toxicity: therapeutic and experimental aspects. Curr Med Chem. 12, 2771–94.
- Chandrasekaran, S.K., Shaw, J.E. (1978). Factors influencing the percutaneous absorption of drugs. Curr Probl Dermatol. 7, 142–55.
- Dimitrijevic, D., Shaw, A.J., Florence, A.T. (2000). Effects of some non-ionic surfactants on transepithelial permeability in Caco-2 cells. J Pharm Pharmacol. 52, 157–62.
- Eley, J.G., Triumalashetty, P. (2001). In vitro assessment of alkyglycosides as permeability enhancers. AAPS PharmsciTech. 2, 1–7.
- Grass, G.M., Wood, R.W., Robinson, J.R. (1985). Effect of calcium chelating agents on corneal permeability. IOVS. 26, 110–3.
- He, X., Hahn, P., Iacovelli, J., Wong, R, King, C, Bhisitkul, R, Massaro-Giordano, M, Dunaief, JL. (2007). Iron homeostasis and toxicity in retinal degeneration. Prog Retin Eye Res. 26, 649–73.
- Horváth, K., Noker, P.E., Somfai-Relle, S., Glávits, R., Financsek, I., Schauss, A.G. (2002). Toxicology of methylsulfonylmethane in rats. Food Chem Toxicol. 40, 1459–62.
- Jayasena, T., Grant, R.S., Keerthisinghe, N., Solaja, I., Smythe, G.A. (2007). Membrane permeability of redox active metal chelators: an important element in reducing hydroxyl radical induced NAD+ depletion in neuronal cells. Neurosci Res. 57, 454–61.
- Kalinowsky, D.S., Richardson, D.R. (2005). The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol Rev. 57, 547–83.
- Liu, G., Men, P., Harris, P.L., Rolston, R.K., Perry, G., Smith, M.A. (2006). Nanoparticle iron chelators: a new therapeutic approach in Alzheimer disease and other neurological disorders associated with trace metal imbalance. Neurosci Lett. 406, 189–93.
- Magnuson, B.A., Appleton, J., Ames, G.B. (2007). Pharmacokinetics and distribution of (35S) methylsulfonylmethane follow oral administration to rats. J Agric Food Chem. 55, 1033–8.
- Meaney, C.M., O’Driscoll, C.M. (2000). A comparison of the permeation enhancement potential of simple bile salt and mixed bile salt:fatty acid micellar systems using the CaCo-2 cell culture model. Int J Pharm. 207, 21–30.
- Najjar, D.M., Cohen, E.J., Rauan, C.J., Laaibson, P.R. (2004). EDTA chelation for calcific band keratopathy: results and long-term follow-up. Am J Ophthalmol. 137, 1056–64.
- Ouyang, H., Morris-Natschke, S.L., Ishaq, K.S., Ward, P., Liu, D., Leonard, S., Thakker, D.R. (2002). Structure-activity relationship for enhancement of paracellular permeability across Caco-2 cell monolayers by 3-alkylamido-2-alkoxypropylphosphocholines. J Med Chem. 45, 2857–66.
- Quan, Y.S., Hattori, K., Lundborg, E., Fujita, T., Murakami, M., Muranishi, S., Yamamoto, A. (1998). Effectiveness and toxicitiy screening of various absorption enhancers using Caco-2 cell monolayers. Biol Pharm Bull. 21, 615–20.
- Sakai, M., Imai, T., Ohtake, H., Otagiri, M. (1998). Cytotoxicity of absorption enhancers in Caco-2 monolayers. J Pharm Pharmacol. 50, 1101–8.
- Smith, J., Wood, E., Dornish, M. (2004). Effect of chitosan on epithelial cell tight junctions. Pharm Res. 21, 43–9.
- Swenson, E.S., Milisen, W.B., Curatolo, W. (1994). Intestinal permeability enhancement: efficacy, acute local toxicity, and reversibility. Pharm Res. 11, 1132–42.
- Uchiyama, T., Yamamoto, A., Hatano, H., Fujita, T., Muranishi, S. (1996). Effectiveness and toxicity screening of various absorption enhancers in the large intestine: intestinal absorption of phenol red and protein and phospholipid release from the intestinal membrane. Biol Pharm Bull. 19, 1618–21.