Abstract
The aim of this research is to reduce the frequency of taking therapeutic drugs. Thus, anti-cancer drug [5-fluorourical (5-FU)] loaded chitosan/polyethylene glycol microparticles were prepared by a phase-inversion technique with tripolyphosphate (TPP) used as a cross-linking agent. The relationships between 5-FU release behavior/encapsulation efficiencies and chitosan concentrations, TPP concentrations, as well as cross-linking time were studied to identify better/superior conditions (3.5 wt% chitosan, 3 wt% TPP, and cross-linking time = 4 h) for preparing 5-FU-loaded microparticles. Furthermore, in order to ascertain the influence of their physical properties on 5-FU release performance, 5-FU-loaded microparticles were evaluated by swelling tests and scanning electron microscopy.
Introduction
5-fluorourical (5-FU) is one of the most commonly used drugs in the chemotherapy of human cancers (head, neck, breast, etc.) (CitationAckland, Garg, & Dunstun, 1997; CitationDafeng et al., 2003). In this study, 5-FU-loaded chitosan/polyethylene glycol (PEG) microparticles were prepared by a phase-inversion process, because of its easily obtained porous microparticles with desired morphology and pore size (CitationMi et al., 2002).
Chitosan is a biopolymer, which has been developed for controlled drug release due to its excellent biodegradable and biocompatible characteristics (CitationShu & Zhu, 2000; CitationMi et al., 2002). It is the deacetylated form of chitin, which is a natural polymer extracted from economic crustacean shells, such as prawns, crabs, shrimps, and insects. However, the mechanical strength of chitosan is poor, and it is therefore necessary to modify chitosan to improve its mechanical properties and chemical stability, etc. In order to improve its mechanical strength and increase porosity, polymers such as polyethylene glycol (PEG) can be added to chitosan (CitationJiang & Han, 1999; Zhang et al., Citation2002; CitationKolhe & kannan, 2003). In addition, several blends of chitosan with other synthetic polymers have been studied. However, PEG is of particular interest because of its water-soluble, biocompatible, and non-toxic nature (CitationJiang & Han, 1999).
Drug release from chitosan microparticles could be controlled by reversibly physical cross-linking instead of chemical cross-linking to avoid possibly undesirable effects, such as toxicity. For example, tripolyphosphate (TPP) is non-toxic and its multivalent anions can interact with positively charged amino groups in chitosan via electrostatic forces (CitationShu & Zhu, 2000; CitationKo et al., 2002). Furthermore, TPP with more negative charges showed a significant ability to ionically cross-link chitosan (CitationShu & Zhu, 2002).
In this research, 5-FU-loaded microparticles were prepared by a phase-inversion technique. In addition, TPP was used as a cross-linking agent and NaOH was used to consolidate/harden for 5-FU-loaded microparticles. The objective of this research was to identify the better/superior conditions (chitosan concentrations, TPP concentrations, and cross-linking time) for the release behavior/encapsulation efficiencies of 5-FU from these 5-FU-loaded microparticles. Furthermore, in order to ascertain the effect of their physical properties on 5-FU release performance, 5-FU-loaded microparticles were also characterized by swelling tests and scanning electron microscopy (SEM).
Materials and methods
Chitosan with 300 kDa molecular weight and a deacetylation percentage of ~ 95% was supplied by Primex Enterprises Company (Iceland). PEG 6000 was supplied by Fluka Enterprises Company (Switzerland). TPP was supplied by Sigma Enterprises Company (USA), who also supplied the 5-FU. Various concentrations (2.1, 2.8, and 3.5 wt%) of chitosan were blended with PEG (chitosan/PEG = 70/30) through the attractive intermolecular interaction between chitosan and PEG (CitationKolhe & Kannan, 2003). A citosan/PEG mixture was prepared by dissolving chitosan and PEG into 2 wt% acetic acid solutions and 5-FU was dissolved in CH3OH to prepare 100 ppm 5-FU solution. Next, 50 g of the chitosan/PEG mixture and 25 ml of 100 ppm 5-FU solution were mixed for 30 min and subsequently shaken ultrasonically for 30 min. Finally, they were dropped into different concentrations (1, 3, and 5 wt%) of TPP solutions (pH = 8.5) for different cross-linking times (1, 2, 4, and 8 h) and after 10 min, then 5-FU-loaded microparticles were put into solutions ( 100 ml of 2 N NaOH) for 1 h. After separating the 5-FU-loaded microparticles from solutions, they were rinsed with deionized water repeatedly and then freeze-dried (−53°C) under vacuum for 3 days.
Encapsulation efficiency was investigated by dispersing 0.1 g of 5-FU-loaded microparticles in 20 ml of CH3OH for 30 min. Then the drug content was determined by HPLC.
Swelling ratios were studied by putting 5-FU-loaded microparticles into 50 ml of PBS (phosphate buffered saline, pH = 7.4) for 36 h.
5-FU-loaded microparticles (0.5 g) were suspended in 100 ml of PBS (phosphate buffered saline, pH = 1.2 and 7.4) and incubated in a shaking water bath at 37°C, 70 rpm. Aliquots of 1 ml were withdrawn at appropriate intervals and an equal volume of fresh medium was added to maintain a constant volume. The amount of released 5-FU was analyzed using HPLC. All the experiments were carried out in triplicate.
The surface morphology of 5-FU free and loaded microparticles were examined by SEM (HITACHI S3000N, Japan).
Results and discussion
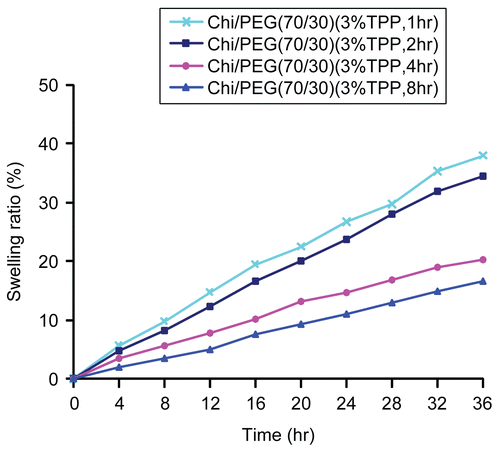
shows the influence of cross-linking time on 5-FU encapsulation efficiency in 5-FU-loaded microparticles. In general, the encapsulation efficiencies decreased with increasing cross-linking time. The reason behind this may be that 5-FU is soluble in water and dissolved in solution during the cross-linking process. shows the influence of cross-linking time on swelling ratio of 5-FU-loaded microparticles. Generally, there was an inverse relationship between swelling ratios and cross-linking time. However, the swelling ratios showed only a small difference between 4 and 8 h of cross-linking time. In addition, the encapsulation efficiencies clearly decreased from 4 to 8 h of cross-linking time. Therefore, 4 h was chosen as a better/superior cross-linking time for further parts of this research.
Figure 1. Influence of cross-linking time on 5-FU encapsulation efficiency in 5-FU-loaded microparticles.
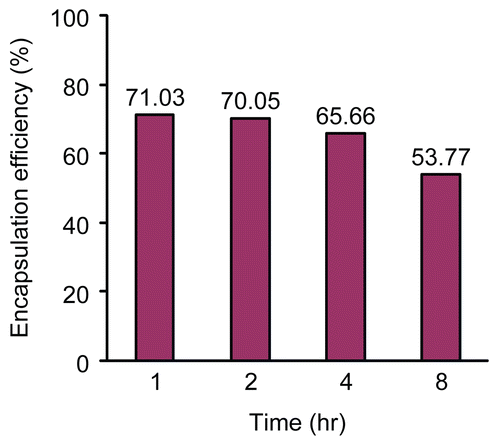
Figure 2. Influence of cross-linking time on swelling ratio of 5-FU-loaded microparticles (3.5 wt% chitosan).
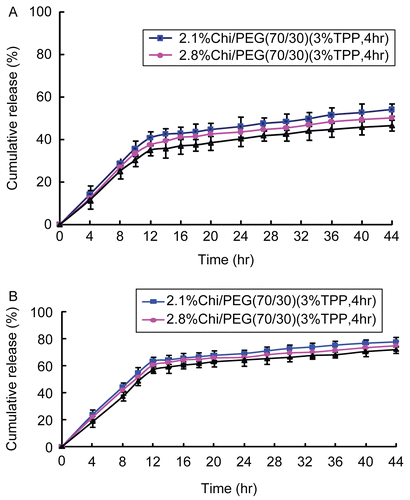
and show the influence of chitosan concentrations and pH of PBS on cumulative release of 5-FU from 5-FU-loaded microparticles (chitosan/PEG = 70/30, 3 wt% TPP, and cross-linking time = 4 h). The higher the chitosan concentration, the lower the cumulative release. This behavior is confirmed in the literature (CitationKo et al., 2002). The reason behind this behavior may be that the higher the chitosan concentration, the higher the viscosity of chitosan solution/the greater the ionization of amine groups, and the increased viscosity of chitosan solution/ionization of amine groups form relatively strong walls of 5-FU-loaded microparticles upon interaction with TPP. Thus, the diffusion of 5-FU through the relatively strong walls of 5-FU-loaded microparticles into the PBS was retarded and thus the cumulative release of 5-FU decreased for higher chitosan concentrations. Therefore, 3.5 wt% chitosan was chosen as a better/superior chitosan concentration for other parts of this study. The results also demonstrated that the higher the pH of PBS, the lower the cumulative release, since the structure of 5-FU-loaded chitosan micropartices was destroyed at lower pH of PBS and thus the cumulative release of 5-FU increased at lower pH of PBS.
Figure 3. Influence of chitosan concentrations on cumulative release of 5-FU from 5-FU-loaded microparticles (chitosan/PEG = 70/30, 3 wt% TPP, and cross-linking time = 4 h) in (A) pH = 7.4 and (B) pH = 1.2 of PBS (mean ± SD, n = 3).
and show the effect of TPP concentrations and pH of PBS on the 5-FU release behavior from 5-FU-loaded microparticles (3.5 wt% chitosan, chitosan/PEG = 70/30, and cross-linking time = 4 h). Generally, the cumulative release of 5-FU from 5-FU-loaded microparticles decreased with increasing TPP concentration. This behavior also is confirmed in the literature (CitationKo et al., 2002). The reason behind this behavior may be that the higher the TPP concentration, the higher the cross-linking density of 5-FU-loaded microparticles, and thus leading to more compact structure of 5-FU-loaded microparticles and then decreasing the diffusion as well as cumulative release of 5-FU from 5-FU-loaded microparticles. However, the cumulative releases showed only a small difference between 3 and 5 wt% TPP. Therefore, 3 wt% TPP was chosen as a better/superior TPP concentration due to economical considerations.
Figure 4. Effect of TPP concentrations on the 5-FU release behavior from 5-FU loaded microparticles (3.5 wt% chitosan, chitosan/PEG = 70/30, and cross-linking time = 4 h) in (A) pH = 7.4 and (B) pH = 1.2 of PBS (mean ± SD, n = 3).
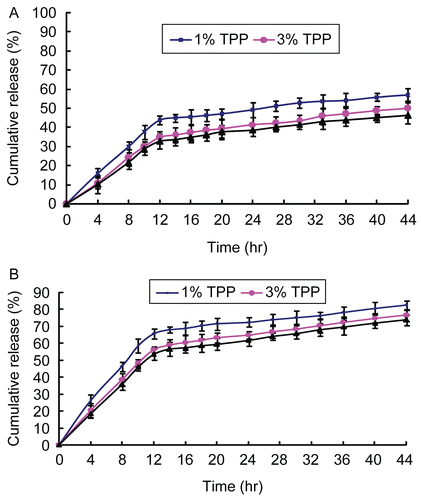
When cross-linking time was increased from 1 to 8 h, the release of 5-FU from 5-FU-loaded microparticles (3.5 wt% chitosan, chitosan/PEG = 70/30, and 3 wt% TPP) decreased (see ). The reason behind this behavior may lie in the short cross-linking time, which might not give sufficient interaction time for 5-FU-loaded microparticles. Thus, the release of 5-FU from 5-FU-loaded microparticles decreased as the cross-linking time increased. However, the cumulative releases showed only a small difference between 4 and 8 h of cross-linking time. These results reflected those of the swelling ratio tests (see ).
Figure 5. Effect of cross-linking time on the release of 5-FU from 5-FU-loaded microparticles (3.5 wt% chitosan, chitosan/PEG = 70/30, and 3 wt% TPP) in (A) pH = 7.4 and (B) pH = 1.2 of PBS (mean ± SD, n = 3).
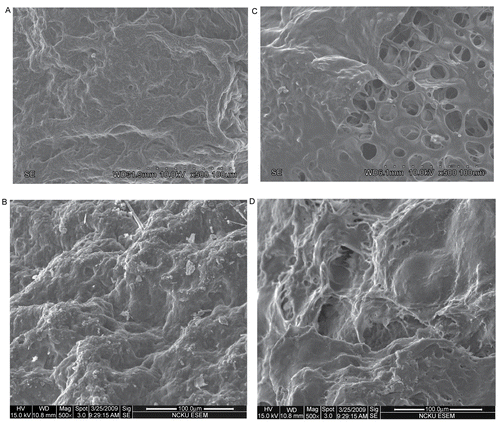
The surface morphologies of 5-FU free and loaded microparticles are shown in and , respectively. White 5-FU particles are identified in . In addition, the surface morphologies of 5-FU-loaded microparticles prepared with different conditions are also shown in . The 5-FU-loaded microparticles prepared with 2.1 wt% chitosan and 0.9 wt% PEG (see ) had larger wrinkles and holes than those of 5-FU-loaded microparticles prepared with 3.5 wt% chitosan and 1.5 wt% PEG (see ). These surface morphologies provide supportive evidence for the cumulative release of 5-FU being smaller for 3.5 wt% chitosan in . Furthermore, the 5-FU-loaded microparticles prepared with 1 wt% TPP (see ) had larger wrinkles and holes than those of 5-FU-loaded microparticles prepared with 3 wt% TPP (see ). These surface morphologies are consistent with the cumulative release of 5-FU being smaller for 3 wt% TPP in . Moreover, the 5-FU-loaded microparticles prepared with 2 h of cross-linking time (see ) had larger wrinkles and holes than those of 5-FU-loaded microparticles prepared with 4 h of cross-linking time (see ). These surface morphologies provide supportive evidence for the cumulative release of 5-FU being smaller for 4 h of cross-linking time in .
Figure 6. The surface morphology of (A) the microparticle and (B) the 5-FU-loaded microparticle prepared with 3.5 wt.% chitosan, chitosan/PEG = 70/30, 3 wt.% TPP, and cross-linking time = 4 hr.

Figure 7. The surface morphology of the 5-FU-loaded microparticle prepared with (A) 2.1 wt.% chitosan, chitosan/PEG = 70/30, 3 wt% TPP, (B) 3.5 wt% chitosan, chitosan/PEG = 70/30, 3 wt% TPP, (C) 3.5 wt% chitosan, chitosan/PEG = 70/30, 1 wt% TPP, and (D) 3.5 wt% chitosan, chitosan/PEG = 70/30, 3 wt% TPP, and cross-linking time = 4 h.
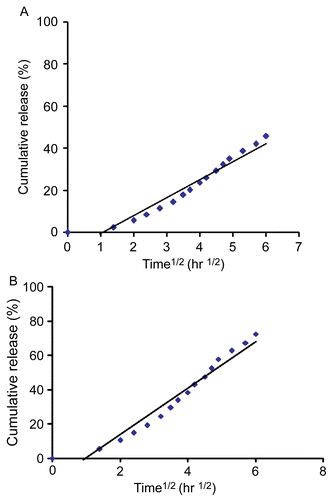
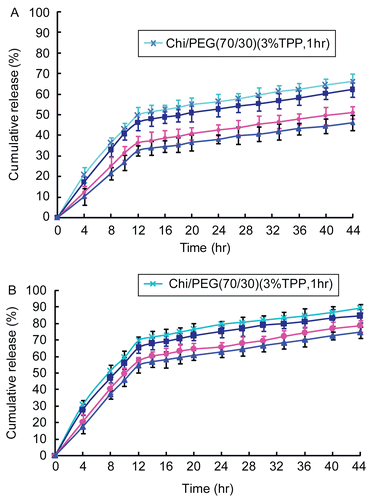
and of the release of 5-FU from 5-FU-loaded microparticles (3.5 wt% chitosan, chitosan/PEG = 70/30, 3 wt% TPP, and cross-linking time = 4 h) vs square root time were found to be linear, indicating that the 5-FU release mechanism from these 5-FU-loaded microparticles might be of a diffusion control type, an observation which is confirmed in the literature (CitationRao & Murthy, 2002).
Conclusions
Swelling ratios decreased with increasing cross-linking time, but encapsulation efficiencies decreased with increasing cross-linking time, thus 4 h was chosen as a better/superior cross-linking time. In general, the higher the chitosan concentration, the lower the cumulative release and the higher pH of PBS, the lower the cumulative release. Generally, the cumulative release of 5-FU from 5-FU-loaded microparticles decreased with increasing TPP concentration and cross-linking time. In conclusion, the better/superior conditions for preparing 5-FU-loaded microparticles were identified to be 3.5 wt% chitosan, 3 wt% TPP, and cross-linking time = 4 h. In addition, surface morphologies confirmed that the cumulative release of 5-FU was smaller for 3.5 wt% chitosan and 3 wt% TPP. Furthermore, we suggest that the 5-FU release mechanism from these 5-FU-loaded microparticles might be of the diffusion control type.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ackland, S.P., Garg, M.B., Dunstan, R.H. (1997). Simultaneous determination of dihydrofluorouracil and 5-fluorouracil in plasma by high-performance liquid chromatography. Anal Biochem. 246, 79–85.
- Dafeng, C., Gua, J., Liu, W., Fawcett, J.P., Dong, O. (2003). Sensitive liquid chromatographic assay for the simultaneous determination of 5-fluorouracil and its prodrug, tegafur, in beagle dog plasma. J Chromatogr B. 795, 377–82.
- Jiang, W.H., Han, S.J. (1999). The interactions of chitosan-poly(ethylene glycol) in the presence of added salt in water viscosity effect. Eur Polym J. 35, 2079–85.
- Ko, J.A., Park, H.J., Hwang, S.J., Park, J.B., Lee, J.S. (2002). Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int J Pharm. 249, 165–74.
- Kolhe, P., Kannan, R.M. (2003). Improvement in ductility of chitosan through blending and copolymerization with PEG: FTIR investigation of molecular interations. Biomacromolecules. 4, 173–80.
- Mi, F.L., Shyu, S.S., Chen, C.T., Lai, J.Y. (2002). Adsorption of indomethacin onto chemically modified chitosan beads. Polymer. 43, 757–65.
- Rao, B.S., Murthy, K.V.R. (2002). Studies on rifampicin release from ethylcellulose coated nonpareil beads. Int J Pharm. 231, 97–106.
- Shu, X.Z., Zhu, K.J. (2000). A novel approach to prepare tripolyphosphate/chitosan complex beads for controlled release drug delivery. Int J Pharm. 201, 51–8.
- Shu, X.Z., Zhu, K.J. (2002). The influence of multivalent phosphate structure on the properties of ionically cross-linked chitosan films for controlled drug release. Eur J Pharm Biopharm. 54, 235–43.
- Zhang, M., Li, X.H., Gong, Y.D., Zhao, N.M., Zhang, X.F. 2002. Properties and biocompatibility of chitosan films modified by blending with PEG. Biomaterials. 23, 2641–8.