Abstract
Local tumor recurrence after cervical cancer surgery remains a clinical problem. Vaginal delivery of thermosensitive hydrogel may be suited to reduce tumor relapse rate with more efficacy and safety. A pilot study was carried out to evaluate the efficacy of carboplatin-loaded poloxamer hydrogel to prevent local recurrence of cervical cancer after surgery. In vivo vaginal retention evaluation of 27% poloxamer hydrogel in mice was proven to be a suitable vaginal drug delivery formulation due to its low gelation temperature. A mimic orthotopic cervical/vaginal cancer recurrence model after surgery was established by injecting murine cervical cancer cell line U14 into the vaginal submucosa to simulate the residual tumor cells infiltrated in the surgical site, followed by drug administration 24 h later to interfere with the formation/recurrence of the tumor. By infusing fluorescein sodium-loaded hydrogel into the vagina of mice, a maximized accumulation of fluorescein sodium (Flu) in the vagina was achieved and few signals were observed in other organs. When used in the prevention of the cervical cancer formation/recurrence in mice, the carboplatin-loaded poloxamer hydrogel exhibited great efficacy and systemic safety. In conclusion, thermosensitive hydrogel presents a simple, practical approach for the local drug delivery via vagina against cervical cancer recurrence.
Introduction
Although radical hysterectomy with pelvic lymphadenectomy has been widely accepted as the treatment for patients with cervical cancer, 10–20% of the patients recurred after radical surgery, and 5-year survival rate is less than 5%. To reduce the recurrences and improve survival in patients with high-risk factors, concurrent chemoradiation (CTRT) has long been held as the optimum postoperative adjuvant therapy (Favero et al., Citation2014; Sun et al., Citation2014).
As far as chemotherapy is concerned, cisplatin has long been the first-line chemotherapy drug against cervical cancer by systemic administration as an adjuvant to radiotherapy. However, it has been widely recognized that cisplatin-based combinations have significant toxicity and usually lead to many complications and side effects (Lebrun & Frenay, Citation2003; Ziebarth et al., Citation2012).
In pursuit of better efficacy and lower systemic toxicity, local chemotherapy has also long been utilized in clinical practice as a means of achieving high therapeutic concentrations of chemotherapy to the site of malignant disease and avoiding the entrance of drug into the blood circulation and other normal organs (Martinez et al., Citation2009; Hack et al., Citation2015). Furthermore, to increase drug solubility and deliver drug to the disease site in a controlled manner, polymer delivery vehicles for implantation intra-tumorally or adjacent to the cancerous tissue has been studied extensively, such as gels, nanoparticles, polymeric films, rots, wafers, nanofibers and so on (Beppu et al., Citation2012; Liu et al., Citation2012; Nagpal, Citation2012; Liu et al., Citation2013; Cho et al., Citation2015; Liu et al., Citation2015; Zhang et al., Citation2016).
Poxolamer-based thermosensitive hydrogel, which is in liquid state at room temperature but in gel form at body temperature, has long been used as gynecological conventional dosage form for the treatment of vaginal infections, vaginal health, hygiene maintenance and contraception (Zhou et al., Citation2013; Rossi et al., Citation2014; Patel et al., Citation2015; Tugcu-Demiroz et al., Citation2015). Compared to conventional hydrogels for vaginal drug delivery, thermosensitive hydrogels are more successful in the aspects of minimizing the possibility of gel leakage, sustained drug delivery and easy operation (Almomen et al., Citation2015). To the best of our knowledge, however, there have been no reports on the prevention of cervical cancer recurrence with drug-loaded thermosensitive hydrogel up to now. The purpose of our study is to seek the possibility and feasibility of thermosensitive hydrogel-based local drug delivery system as a new, reasonable, easy-to-use and low-cost therapeutic option supplementary to minimize the risk of cervical cancer recurrence.
In the present study, carboplatin-loaded poloxamer hydrogel was used because of its relatively better water solubility than cisplatin, offering a possibility of higher drug loading rate. Then, the carboplatin-loaded poloxamer hydrogel was delivered by vaginal route to interfere the formation of the orthotopic cervical cancer in mice, thereby mimicking its possible application in the prevention of local tumor recurrence after cervical cancer surgery in clinic.
Materials and methods
Materials and animals
Poloxamer 407 and fluorescein sodium salt (coded as “Flu”) was purchased from Aladdin Chemistry Co. Ltd. (Shanghai, China). Carboplatin (>99.0%, coded as “Car”) was purchased from Hisun Pharmaceutical Co. Ltd. (Zhejiang, China).
Female Kunming mice with body weight ranging from 25 to 40 g (5–8 weeks old) were provided by the Experimental Animal Center of Jilin University.
Preparation of poloxamer hydrogel
Vaginal thermosensitive hydrogel formulations were prepared using the cold method (Park et al., Citation2010). Distilled water was cooled to 4 °C. Poloxamer (20, 24 and 27% w/v) were then added to the distilled water with continuous agitation. The hydrogels were left at 4 °C until a clear solution was obtained.
The fluorescein sodium-loaded poloxamer hydrogel was prepared similarly but Flu (1% in weight percent of the polymer used) was added to the cooled water first before the addition of poloxamer (20, 24 and 27% w/v). The obtained hydrogel was coded as “Flu/gel”.
The carboplatin-loaded poloxamer hydrogel was prepared by the mixture of carboplatin (2% or 5% in weight percent of the polymer used) and poloxamer (only 27% w/v), which was coded as “Car2/gel” or “Car5/gel”, respectively.
Thermogelling properties and measurement of retention of hydrogel in vagina
The sol-to-gel transition was first determined by the test tube inverting method (Li et al., Citation2012). The 4 ml vials (diameter 1.1 cm) containing 1 ml of hydrogel solutions were kept in a water bath ranging from 20 °C to 36 °C, respectively. The gelation point was determined by flow or no flow criterion over 30 s with the vial inverted. Each point of temperature represented an average of three time repeated measurements with an accuracy of ±0.5 °C.
Dynamic rheological experiments were performed on a US 302 rheometer (Anton Paar (Shanghai) Trading Co., Ltd, Shanghai, China). The poloxamer solution (20%, 24% and 27%) was placed between parallel plates of 25 mm diameter and a gap of 0.5 mm. G′ is an elastic component of the complex modulus for measuring the gel-like behavior of a system, whereas G″ is a viscous component of the complex modulus and is a measure of the sol-like behavior of the system. The gelation temperature was defined as the intersection point between elasticity modulus and viscous modulus.
Nine female KM mice were used for the study of the retention of Flu/gel in the vagina. About 25 μl of Flu/gel (20%, 24% and 27%) was injected into the mouse vagina by a syringe. After administration, the mice were kept in an upside down position for 3 min and then were imprisoned in an overturned beaker with a black plate below them to collect possible leakage. The black plate was withdrawn and imaged at 1 h after hydrogel infusion by fluorescent imaging system (CRI Maestro 500FL) to detect leaked Flu signals.
In vitro drug release studies
Approximately 0.5 ml Car2/gel or Car5/gel was placed in the dialysis membrane (molecular weight cutoff 3.5 kDa) and incubated in a thermostated shaker. (preheated to 37 °C) until the hydrogel was formed. Sodium acetate buffer (2.0 ml, pH 5.0) was then added slowly down the wall of the bottle onto the top of the hydrogel. Afterwards, the samples were shaken horizontally at a constant rate in the water bath at 37 °C. At predetermined time intervals, 1.0 ml of the released solution was withdrawn and analyzed by inductively coupled plasma mass spectrometry (ICP-MS) to determine the amount of released carboplatin in buffer. Equal amount of fresh buffer solution was added back.
Biodistribution studies by fluorescence imaging
Twelve KM mice were used for the study of the biodistribution of Flu from Flu/gel in the vagina. About 25 μl of Flu/gel(27%) was injected into the mouse vagina and the animals were sacrificed at 1 h, 6 h, 12 h and 24 h after hydrogel infusion with three mice at each time point. After repeated vaginal douching to remove the residual hydrogel in the vagina, samples of reproductive tracts (RTs), rectum, liver, kidneys, spleen, lung and heart were harvested and imaged by fluorescent imaging system to detect the biodistribution of Flu released from Flu/gel in mice. Fixed exposure time was adopted at all time points. Semi-quantitative comparison between different organs and different time intervals was also made by means of commercial software (MaestroTM2.4).
Prevention of cervical cancer local recurrence in vivo
Mouse uterine cervical cancer cell line U14 was purchased from the Medical Department of Jilin University in China. Female KM mice were used to prepare the cervical cancer models. U14 cells (4 × 105/ml) in PBS (25 μl) were injected into submucosa nearby the cervix in situ. Twenty-four hours after cell injection, the tumor-inoculated mice were randomly divided into five groups with seven mice in each group: (1) infusion of Car2/gel (50 μl, 0.27 mg carboplatin for each mouse); (2) infusion of Car5/gel (50 μl, 0.675 mg carboplatin for each mouse); (3) intravenous injection of carboplatin (0.675 mg carboplatin for each mouse); (4) infusion of blank gel(50 μl); (6) control group without any treatment. The time of tumor inoculation was designated as day0. The gel infusion was performed for consecutive five days (day1–5) and injection for three times every other day (day1, 3 and 5), with equivalent total dose of carboplatin in the two groups.
Mice were monitored every three days for weight loss and evidence of tumor formation (local recurrence). Time of local recurrence was defined as the detection of a slight bulge in the perineal area of mice.
On the 15th day after carboplatin treatment, animals in each group were sacrificed and the excised RT/tumor tissues were immediately weighed, macroscopically observed and histologically analyzed. Immunohistochemical staining of paraffin sections of the livers was done with PCNA antibody at a dilution of 1:100. Staining was done using an Envision kit (Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol.
The tumor inhibition rate was calculated with the following Formula (1):
(1)
where Wc means the average tumor weight in Group 5 (control) and Wx means the average tumor weight in group 1, 2, 3, or 4.
Statistical analysis
All data are expressed as mean ± SE. Pair-wise comparisons (Student’s t-test) or nonparametric (Mantel–Cox) tests were applied where appropriate.
Result
Thermogelling properties and retention of poloxamer gel in the vagina
The thermogelling properties of the poloxamer gel were evaluated by tube inverting method. As shown in , the gelation of poloxamer solution was dependent on the poloxamer concentration as previously reported. The gelation temperature of 27% poloxamer solution was as low as 24 °C with the gelation time being 2.5 min. When temperature reached 36 °C, it took only 0.25 min for 27% poloxamer solution to be transferred from solution to gel.
Table 1. Thermogelation behaviors of different aqueous compositions.
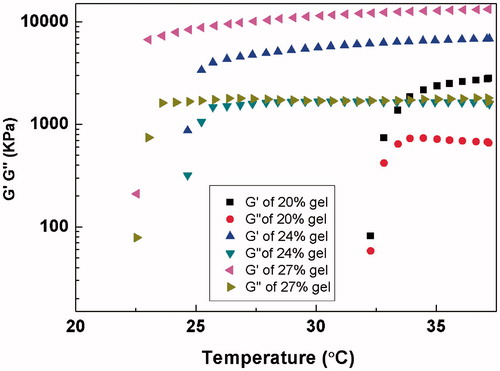
The gelation property of gels was also evaluated by rheological measurements. The sol/gel transition temperature was defined by a drastic change of the rheological behavior and the elasticity modulus which is low at solution stage but increases drastically at the gelation temperature. As shown in , the elasticity modulus and mechanical strength was in proportion to the poloxamer concentration. The gelation temperatures were about 22.5, 25 and 32.5 °C for 20%, 24% and 27% poloxamer solution, respectively, which was consistent with the results tested by tube inverting method.
Figure 1. Measurements of the viscoelastic properties of 20%, 24% and 27% poloxamer gels as a function of temperature. G′=elastic component, G″= viscous component.
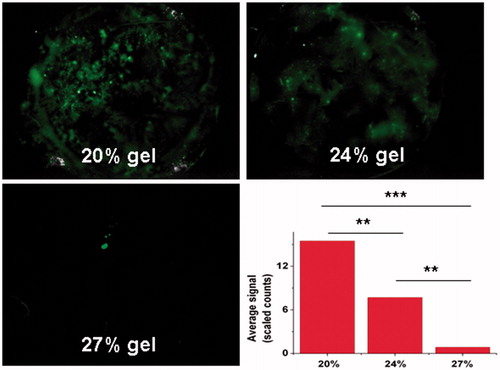
To evaluate the retention ability of poloxamer hydrogel of different concentrations, 25 μl of Flu/gel (20%, 24% and 27% of poloxamer) was infused into the mice vagina without the procedure of animal anesthesia. As shown in , 20% and 24% Flu/gel rapidly leaked from the vagina, resulting in splashy Flu signals in the black plate within 1 h. On the contrary, little Flu signals were detected for the mice receiving 27% Flu/gel. According to the result of semi-quantitative fluorescence from the leaked gel, average signals were 17.68 ± 5.62, 9.32 ± 3.25 and 0.74 ± 0.08 for 20%, 24% and 27% Flu/gel, respectively. There was a significant difference between 27% Flu/gel and the others (p < 0.001). The leaked gel of 27% gel was only about 4.19% and 7.94% of 20% gel and 24% gel, respectively, suggesting that the high poloxamer concentration was beneficial for hydrogel gelation in the mice vagina, resulting in long vaginal retention. Based on the requirements of gel infusion in mice, therefore, 27% poloxamer gel was adopted in the rest of all experiments in the present study.
Figure 2. Vaginal retention evaluation of 20%, 24% and 27% poloxamer gel loaded with Flu in mice. Fluorescence imaging was used to detect the leakage on the black plate at 1 h after gel infusion. Semiquantitative fluorescence intensities were summarized as a histogram. The results were given as mean value ± SD, over three mice in a group.
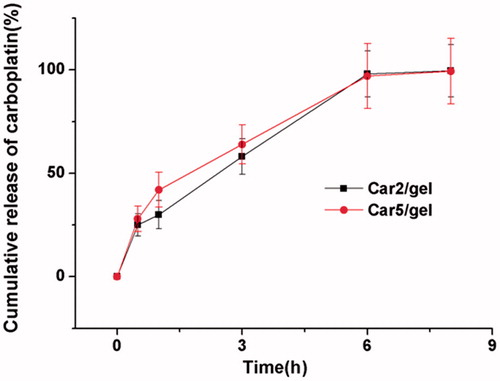
In vitro release
The in vitro release profile of carboplatin-loaded poloxamer gel was studied in the acetate sodium buffer (pH5.0) to mimic the acidic environment of vagina. As shown in , about 25 and 28% carboplatin were released within the first 0.5 h from Car2/gel and Car5/gel, and the residual drug was completely released within 6 h for both, indicating that the structure of the gel functioned as a resistant barrier to the release of carboplatin which is sparingly soluble in water.
Biodistribution
To visually trace the drug released from the poloxamer hydrogel in mice vagina, Flu was chosen as model drug and 27% poloxamer hydrogel loaded with 1% (wt/wt) Flu was prepared and infused into the mice vagina. Then, animals were sacrificed at different time points and the main organs were imaged by fluorescent imaging system to observe the release and biodistribution behavior of Flu in vivo.
As shown in , Flu was rapidly released from the hydrogel at 1 h after the infusion and completely coated the vaginal tract. With time, the fluorescence intensity in the vagina gradually became weaker. At 24 h, the Flu signals in the RT have completely disappeared. No obvious signals were observed in the heart, liver, spleen, kidneys and lung at all time points.
Figure 4. (A) Typical ex vivo images of the excised organs(one vagina, two rectum, three heart, four liver, five spleen, six lung, seven kidneys) examined by CRI Maestro 500FL at 1, 6,12 and 24 h after Flu/gel infusion into the mice vagina. (B) Semi-quantitative fluorescence intensities of various organs determined at different time points. The results were given as mean value ± SD, over three mice in a group.
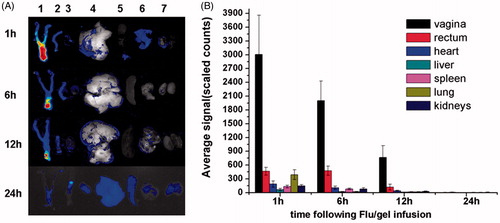
Semiquantitative determination of the fluorescence intensities showed that Flu was preferably distributed in the vagina rather than in the other organs within 24 h. At 1 h, the average signal of Flu in the vagina was as high as 3006 ± 853, compared with 465 ± 86, 185 ± 72, 65 ± 25, 130 ± 29, 392 ± 106 and 145 ± 29 in the rectum, heart, liver, spleen, lung and kidneys, respectively. At 6 h, the average signal of Flu in the vagina was about 100 and 25 times that in liver and kidneys. At all the time points, there was significant difference between the fluorescence intensities of vagina and that of other organs (p = 0.000).
Prevention of cervical cancer recurrence in vivo
To mimic the post-surgery cervical/vaginal environment with residual tumor cells, U14 cells were inoculated into the mice vagina. Twenty-four hours after tumor inoculation, the mice were treated with carboplatin-loaded hydrogel or intravenous injection of carboplatin, followed by the observation of the time of the tumor formation and the change of body weight in mice.
As shown in , on the 7th day after tumor inoculation, the tumor formation/recurrence rates in the Car2/gel, Car 5/gel, Car5/i.v, blank gel and control group were 28.6, 0, 14.3, 57.1 and 57.1%, respectively. On the16th day, the formation/recurrence rates of the Car 5/gel was 57.1% while the rest of groups were all 100%. Median times of recurrence were approximately 10, 10, 10, 7, and 7 days in Car2/gel, Car 5/gel, Car5/i.v, blank gel and control group, respectively. (no statistic difference among all groups. p > 0.05, log-rank test)
Figure 5. (A) The tumor formation/recurrence rate, (B) relative body weight, (C) average tumor weight and (D) tumor inhibition rate in various groups after drug administration. The data were given as mean value ± SD over seven mice in each group.
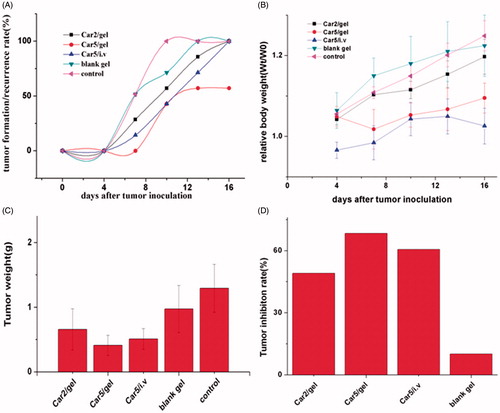
As shown in , vaginal infusion of neither carboplatin-loaded hydrogel nor blank gel caused any obvious weight loss whereas there was a slight decline in the body weight of mice receiving carboplatin injection at the initial period of the experiment.
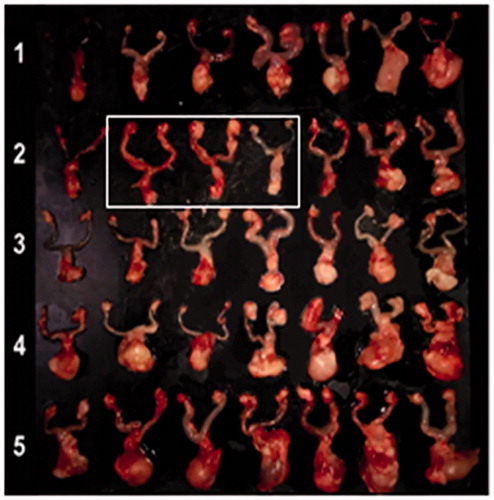
On day 15, all animals were sacrificed and their RT (including tumor) were harvested. Macroscopic observation showed a big difference in tumor size between all carboplatin-treated groups and control groups (). The tumor weights in the blank gel group and control group were much higher than that in the carboplatin-treated groups (). The average tumor weights were 0.66 ± 0.22 , 0.41 ± 0.16 , 0.51 ± 0.16 , 1.16 ± 0.19 and 1.3 ± 0.37 g in Car2/gel (group1), Car5/gel (group2), Car5/i.v (group3), blank gel (group4) and control group(group5), respectively(group1, 2 or 3 versus group5 p < 0.001; versus group4 p < 0.01; no statistic difference among group 1, 2 and 3).
Figure 6. Macroscopic observation of ex vivo RTs (including tumor) 16 days after tumor inoculation. (1) Infusion of Car2/gel; (2) infusion of Car5/gel; (3) intravenous injection of carboplatin; (4) infusion of blank gel; (5) control group. White box indicates the three RTs without tumor formation/recurrence in mice of Car5/group.
The tumor inhibition rate was calculated according to the Formula (1) in Materials and Methods section. As shown in , the highest rate of 68.3% in Car5/gel group was found, and then followed by 60.6% in Car5/i.v, 49.1% in Car2/gel group and 10% in blank gel group.
Finally, tumor slices from the mice of the experimental groups 15 days post drug administration were prepared and stained with hematoxylin and eosin (H&E). The sample in the Car5/group was taken from the mouse with or without visible tumor recurrence. As shown in , regardless of being treated with drug or not in different groups, once tumor recurrence/formation occurred, large amount of living cells distributed in the tumors were observed. For only three samples without visible tumor recurrence in Car5/group, no visible tumor nests were detected in the vagina of mouse and an integral structure of the vaginal epithelium tissue was clearly visible without obvious abnormality such as edema and congestion.
Figure 7. H&E staining and PCNA immunostaining of ex vivo RTs (including tumor) 16 days after tumor inoculation. Bar = 100 μm. T = viable tumor tissue, V = normal vaginal tissue. Car5/gel # = sample without tumor recurrence, Car5/gel & = sample with tumor recurrence.
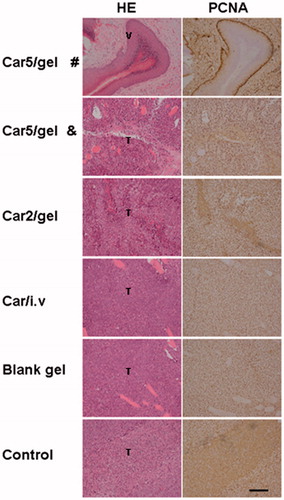
PCNA immunohistochemical analysis was used to assess the proliferation activity in all carboplatin-treated groups and control groups. PCNA, proliferating cell nuclear antigen associated with S phase of DNA replication, serves as a proliferation marker and is standard analysis with many tumors. Evaluated in a blinded fashion by two pathologists, once tumor recurrence occurred, there was no statistical difference in the number of PCNA positive cells among all groups except for the three samples without tumor recurrence in Car5/gel group ().
Discussion
Local tumor recurrence after cervical cancer surgery remains a clinical problem. Both cervix uteri and upper part of vagina is the most common site of tumor recurrence (Kraima et al., Citation2014). Although the vaginal drug delivery system (VDDS), includes tablets, tampons, films, sponges, foams, creams, gels, solutions, ointments, ovules, soft gelatin capsules, pessaries, douches, suppositories and vaginal rings, has long been used for the treatment of usual gynecological diseases (Yoo et al., Citation2006; Khutoryanskiy, Citation2011; Pereira & Bruschi, Citation2012; Tugcu-Demiroz et al., Citation2013). To date, however, there were few studies on the use of VDDS in the application of preventing cervical cancer recurrence. So in the present study, poloxamer hydrogel, a dosage form has been extensively studied by numerous researchers, was selected as VDDS because of its thermosensitive property allowing long vaginal retention time and persistent drug release profile. We expect this seemingly dated formulation to create new vitality in the prevention of cervical cancer recurrence.
The thermosensitivity of poloxamer hydrogel makes the drug administration by vagina convenient and fast, and avoid the drug leakage to the fullest extent which is often occurred in the use of nonthermosensitive hydrogel. It should be noted that the high concentration of poloxamer (27% w/v) in the present study is just to meet the requirement of vaginal retention in the active mice, which is much unlikely to fit the postoperative patients. According to our study, the vaginal temperature in the human and mouse is alike at about 37.2 °C. No doubt, the fast gelation will be favorable for the rention of gel in the vagina, but too low phase transition temperature (<25 °C) may cause trouble to the manufacturing, handling and administering of the gel especially in tropical areas. Therefore, a reasonable balance is desirable. The present study of thermogelling properties and retention of poloxamer gel in the mouse vagina has a certain reference value to the future experiment in human being.
Although we have successfully established the local cervical cancer recurrence model in mice by surgically remove the solid tumor subcutaneously inoculated in mice, the technical complexity involved in the resection of orthotopic cervical cancer in mice is obviously beyond our capabilities (Zong et al., Citation2015). Thus, we adopted a simulated tumor recurrence model by injecting a small number of U14 cells into the mouse vagina to mimic the residual cervical cancer cells distributed within the resection region after surgery. The drug intervention to tumor formation/recurrence was carried out at 24 h after the tumor inoculation to ensure the full infiltration of cells into the vaginal tissue.
At the same carboplatin dose (a total of 3.38 mg carboplatin for each mouse), both hydrogel infusion and intravenous injection showed great efficacy, but the former was slightly better than the latter from the aspects of reduced tumor formation/recurrence rate, inhibited tumor proliferation and attenuated systemic toxicity. We once found that the cisplatin concentration in vaginal tissue at 1 h after intravenous injection was comparatively high being second only to kidneys and liver among all organs and tissues in mouse, probably due to the rich blood supply in vagina. This may explain why thermosensitive hydrogel-based local chemotherapy didn’t show obvious advantage over systemic chemotherapy in the prevention of local tumor formation/recurrence. However, this new protocol undoubtedly provides more choice for some patients who can’t endure the systemic chemotherapy via intravenous injection due to severe side effects or phlebitis induced by repeated chemotherapy.
Conclusion
This study indicated that it is feasible to use carboplatin-loaded poloxamer hydrogel for the vaginal drug delivery and its thermosensitivity offers the advantage of little drug leakage and local drug distribution in the vagina. The maximized drug accumulation in the vagina ensured great systemic safety and efficacy against the formation/recurrence of orthotopic cervical/vaginal tumor in mice by the vaginal infusion of drug-loaded hydrogel. Therefore, thermosensitive hydrogel-based drug delivery system was very suitable for the local drug delivery via vagina and for the prevention of cervical cancer recurrence after surgery.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Reference
- Almomen A, Cho S, Yang CH, et al. (2015). Thermosensitive progesterone hydrogel: a safe and effective new formulation for vaginal application. Pharmaceut Res 32:2266–79
- Beppu T, Sugimoto K, Shiraki K, et al. (2012). Clinical utility of transarterial infusion chemotherapy using cisplatin-lipiodol emulsion for unresectable hepatocellular carcinoma. Anticancer Res 32:4923–30
- Cho EJ, Sun B, Doh KO, et al. (2015). Intraperitoneal delivery of platinum with in-situ crosslinkable hyaluronic acid gel for local therapy of ovarian cancer. Biomaterials 37:312–19
- Favero G, Pierobon J, Genta ML, et al. (2014). Laparoscopic extrafascial hysterectomy (completion surgery) after primary chemoradiation in patients with locally advanced cervical cancer. Int J Gynecol Cancer 24:608–14
- Hack CC, Voiss P, Lange S, et al. (2015). Local and systemic therapies for breast cancer patients: reducing short-term symptoms with the methods of integrative medicine. Geburtshilfe Und Frauenheilkunde 75:675–82
- Khutoryanskiy VV. (2011). Advances in mucoadhesion and mucoadhesive polymers. Macromol Biosci 11:748–64
- Kraima AC, Derks M, Smit NN, et al. (2014). Lymphatic drainage pathways from the cervix uteri: implications for radical hysterectomy? Gynecol Oncol 132:107–13
- Lebrun C, Frenay M. (2003). [Chemotherapeutic neurotoxicity]. Revue Neurologique 159:741–54
- Li N, Yu MH, Deng LD, et al. (2012). Thermosensitive hydrogel of hydrophobically-modified methylcellulose for intravaginal drug delivery. J Mater Sci Mater Med 23:1913–19
- Liu R, Wolinsky JB, Catalano PJ, et al. (2012). Paclitaxel-eluting polymer film reduces locoregional recurrence and improves survival in a recurrent sarcoma model: a novel investigational therapy. Ann Surg Oncol 19:199–206
- Liu S, Wang X, Zhang ZY, et al. (2015). Use of asymmetric multilayer polylactide nanofiber mats in controlled release of drugs and prevention of liver cancer recurrence after surgery in mice. Nanomed-Nanotechnol Biol Med 11:1047–56
- Liu S, Zhou GY, Liu DX, et al. (2013). Inhibition of orthotopic secondary hepatic carcinoma in mice by doxorubicin-loaded electrospun polylactide nanofibers. J Mater Chem B 1:101–9
- Martinez G, Costantino G, Clementi A, et al. (2009). Cisplatin-induced kidney injury in the rat: L-carnitine modulates the relationship between MMP-9 and TIMP-3. Exp Toxicol Pathol 61:183–8
- Nagpal S. (2012). The role of BCNU polymer wafers (Gliadel) in the treatment of malignant glioma. Neurosurg Clin N Am 23:289
- Park TH, Kim ST, Park JS, et al. (2010). Effect of zinc oxide on the rheological and mucoadhesive properties of poloxamer 407-based mucoadhesive thermosensitive gel. Drug Develop Indus Pharm 36:1436–43
- Patel N, Thakkar V, Moradiya P, et al. (2015). Optimization of curcumin loaded vaginal in-situ hydrogel by box-behnken statistical design for contraception. J Drug Deliv Sci Technol 29:55–69
- Pereira RRD, Bruschi ML. (2012). Vaginal mucoadhesive drug delivery systems. Drug Develop Indus Pharm 38:643–52
- Rossi S, Ferrari F, Bonferoni MC, et al. (2014). Comparison of poloxamer- and chitosan-based thermally sensitive gels for the treatment of vaginal mucositis. Drug Develop Indus Pharm 40:352–60
- Sun L, Sheng XG, Jiang JY, et al. (2014). Surgical morbidity and oncologic results after concurrent chemoradiation therapy for advanced cervical cancer. Int J Gynecol Obstetr 125:111–15
- Tugcu-Demiroz F, Acarturk F, Erdogan D. (2013). Development of long-acting bioadhesive vaginal gels of oxybutynin: Formulation, in vitro and in vivo evaluations. Int J Pharm 457:25–39
- Tugcu-Demiroz F, Acarturk F, Ozkul A. (2015). Preparation and characterization of bioadhesive controlled-release gels of cidofovir for vaginal delivery. J Biomater Sci-Polymer Ed 26:1237–55
- Yoo JW, Dharmala K, Lee CH. (2006). The physicodynamic properties of mucoadhesive polymeric films developed as female controlled drug delivery system. Int J Pharm 309:139–45
- Zhang JY, Wang X, Liu TJ, et al. (2016). Antitumor activity of electrospun polylactide nanofibers loaded with 5-fluorouracil and oxaliplatin against colorectal cancer. Drug Deliv 23:794–800
- Zhou QN, Zhong L, Wei XH, et al. (2013). Baicalein and hydroxypropyl-gamma-cyclodextrin complex in poloxamer thermal sensitive hydrogel for vaginal administration. Int J Pharm 454:125–34
- Ziebarth AJ, Smith H, Killian ME, et al. (2012). Completed versus aborted radical hysterectomy for node-positive stage IB cervical cancer in the modern era of chemoradiation therapy. Gynecol Oncol 126:69–72
- Zong S, Wang X, Yang YP, et al. (2015). The use of cisplatin-loaded mucoadhesive nanofibers for local chemotherapy of cervical cancers in mice. Eur J Pharm Biopharm 93:127–35