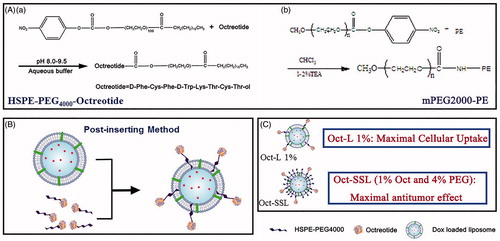
Abstract
Octreotide had been exploited as a targeting ligand for nanoparticle tumor localization overexpressing somatostatin receptor. In addition to particle size and other physiochemical properties, ligand density had great influence on the delivery of active targeted nanoparticles. Herein, octreotide-targeted liposomal doxorubicin was constructed with different ligand density by post-inserting HSPE-PEG4000-Octreotide into pre-formed liposome. The octreotide ligand insertion was confirmed by activity detection of octreotide in HSPE-PEG4000-Octreotide with synchronous fluorescence. 1% octreotide density could achieve the best uptake efficiency on NCI-H-446 and SMMC-7721 cell lines among all liposomes. Octreotide-grafted long-circulating liposome (Oct-SSL) was prepared based on 1% octreotide density. The results showed that the insertion of PEG reduced cellular uptake and cytotoxicity. However, Oct-SSL showed a higher MRT compared with Oct-L showing the relative higher long circulating effect in pharmacokinetic study. Oct-SSL also showed tumor growth inhibition of twofold compared with other groups in Heps xenograft model mice. Oct-SSL with suitable ligand density and PEG modification exhibited significant effective tumor targeting for antitumor drug delivery.
Introduction
Long-circulating immunoliposome has become an ideal nanocarrier for anticancer agents in recent years(Carrion et al., Citation2001, Kirpotin et al., Citation2006, Jakoby et al., Citation2015, Shi et al., Citation2015, Doddapaneni et al., Citation2016). Enhanced permeation and retention effect (EPR effects) and long-circulation time resulting from surface-grafted flexible and hydrophilic PEG chains enhance the accumulation of drug payloads at the site of tumor(Klibanov et al., Citation1990, Gref et al., Citation2000, Maeda et al., Citation2000, Iyer et al., Citation2006, Greish, Citation2007, Wang et al., Citation2016). Meanwhile, the exposed ligands coupled to the terminal end of PEG chains induce receptor-mediated internalization and increase affinity between liposome and cell. As a result, the distribution and efficiency of drug encapsulated by long-circulating immunoliposome are improved. Moreover, the therapy effect also has been improved due to its specifically discernment function for target tissues (Zalipsky, Citation1995, Mercadal et al., Citation1999, Torchilin et al., Citation2001, Susumu et al., Citation2007, Bansal et al., Citation2016, Yuan et al., Citation2016).
In recent years, numerous detailed investigations have been focused on the varieties, amount, molecular weight and configuration of long-circulating hydrophilic nanoparticles (Gavia and Shon, Citation2012, Pozzi et al., Citation2014, Stoffelen et al., Citation2015, Li et al., Citation2016). Among these studies, few particular investigation focus on the amount and distribution of ligands on the surface of liposome. However, with respect to membrane chemistry, Paul S. Cremer (Jung et al., Citation2009) and Esmaiel Jabbari (Sarvestani & Jabbari, Citation2008) studied the effect of ligand density on ligand–receptor binding in controlled models. The results showed that ligand–receptor binding increases with increase in ligand density till a critical point. More ligand density inhibits ligand–receptor binding in view of interaction among ligands, which means the lack of positive correlation between ligand–receptor binding and ligand density.
For ligands-modified liposome, it is still inconclusive about the influence of ligands density on the targeting effect of delivery system. However, it is of great importance to maximize drug delivery across the cell membrane or differentiate uptake of cells expressing different levels of receptors (Saul et al., Citation2003). As far as we know, Justin M. Saul (Saul et al., Citation2003) focuses on folate-grafted liposomal doxorubicin and demonstrated that cellular uptake would increase and then decline. Two possible explanations for the decline are provided, one is the combination of slow liposomal uptake rate and downregulation or “shut-off” of the folate receptors, similar to Cremer’s (Jung et al., Citation2009); The other one is attributed to the competitive binding of remaining folate nanoparticles which are not separated from folate-grafted liposome (Shiokawa et al., Citation2005, Yamada et al., Citation2008).
Octreotide is a synthetic biological somatostatin analog consisting of eight amino acids (D-Phe-Cys-Phe-D-Trp-Lys-Thr-Cys-Thr-ol). The anti-tumor effect of octreotide is mainly mediated through somatostatin receptor subtype 2 and subtype 5 (SSTR-2,-5) (Feindt et al., Citation1997, Guillermet-Guibert et al., Citation2005). Although widely existing in many normal tissues, SSTRs, predominately SSTR-2, and then SSTR-5 are overexpressing in most neuroendocrine tumors (Huang et al., Citation2000, Guillermet-Guibert et al., Citation2005). The therapeutic potential of the pair of SSTR-2 and SSTR-5 has been fully demonstrated by clinical use of radioactive derivatives of octreotide (Huang et al., Citation2000) for diagnostic visualization of tumors and conjugate of octreotide with antitumor agents, such as paclitaxel (Huang et al., Citation2000, Shen et al., Citation2008), doxorubicin (Nagy et al., Citation1998) and CPT-11 (Iwase and Maitani, Citation2011) by taking advantage of targeting effect of octreotide. Our previous study proves the targeting effect of octreotide-modified carrier to SSTR-2 (Shiokawa et al., Citation2005, Sun et al., Citation2010), which has also been proved by Zhang et al. (Citation2010).
This study aimed to optimize a critical point for ligand density in octreotide-targeted liposome for tumors overexpressing SSTR-2, then octreotide-grafted long-circulating liposome (Oct-SSL) based on the optimized octreotide density was prepared to examine antitumor activity in vivo. In this study, post-insertion method rather than the thin-film method (Zhang et al., Citation2010, Sun et al., Citation2010) was applied for the construction of octreotide-targeted liposome in order to strictly ensure the octreotide density at the surface of liposome. Cytotoxicity and cellular uptake of different octreotide density doxorubicin-loaded liposomes were investigated on NCI-H-446, SMMC 7721 and CHO cell lines, which expressed high, low and no level of SSTR-2, respectively. Moreover, the effect of PEG on cell uptake and in vivo anti-tumor efficacy of Oct-SSL, also named sterically stabilized liposome, was investigated. Oct-SSL with suitable ligand density and PEG modification exhibited significant effective tumor targeting for antitumor drug delivery.
Experiments
Materials
Phosphatidylcholine (PC) derived from soybean was a gift from Evonik Degussa (Germany) and cholesterol was purchased from Sinopharm Chemical Reagent Co. Ltd (China). Doxorubicin was purchased from RPG Life Sciences (Mumbai, India). RPMI-1640 medium, heat-inactivated fetal bovine serum, streptomycin and penicillin were purchased from HyClone (Logan, UT). 3-(4, 5-methylthiazol-2-yl)-2, 5-diphenyltetrazoliumbromide (MTT) was purchased from Ameresco (Solon, OH). BCA protein assay kit was gained from KeyGEN (Nanjing, China).
Activity determination of HSPE-PEG-octreotide by synchronous fluorescence spectra (SFS)
The HSPE-PEG-Octreotide was synthesized by conjugation of p-nitrophenylcarbonyl-PEG-HSPE and octreotide according our previous work (Sun et al., Citation2010). To investigate whether the conjugation interfered in the activity of octreotide or not, SFS was conducted to detect the microenvironment change of amino acid residues tryptophan in the active site of octreotide. It could prove that no change of tryptophan microenvironment, if there was no significant shift observed in the maximum emission wavelength before and after modification. Equal molar concentration of octreotide and HSPE-PEG4000-Octreotide were prepared, respectively, and their SFS were recorded at Δλ = 60 nm to determine the characteristics of Trp residue in the active site of octreotide by spectrofluorophotometer (Shimadzu RF-5310 PC, Shimadzu Co. Ltd, Japan).
Preparation and characterization of DOX-loaded plain liposome (P-L) and octreotide-modified targeting liposome
Liposome was constructed by ethanol injection method. Briefly, a molar ratio of 5:1 PC to cholesterol dissolving in 5 mL ethanol was slowly dropped into 10 mL 300 mM (NH4)2SO4 solution, then stirred steadily at 35 °C. After evaporating ethanol from the solution by using rotary evaporator, 300 mM (NH4)2SO4 solution was added to total 10 mL. Blank liposome was obtained after incubation at 35 °C for 30 min and ultrasonication under 400 W in ice bath for 250 s. Blank liposome was dialyzed in 8 K MWCO dialysis bag against 0.9% NaCl solution to remove leaked ammonium sulfate.
Various density of Octreotide-targeted liposomes were obtained by post-insertion method (Paul S. Uster Citation1996, Ka-yun Ng (Ng et al., Citation2000), Debbie L. Iden, (Iden & Allen, Citation2001), Justin M. Saul (Saul et al., Citation2003), and Jian Lu (Lu et al., Citation2006). In brief, separate aliquots of blank liposome from a single batch were equilibrated at 40 °C water bath and then different aliquot of concentrated HSPE-PEG-Octreotide solution was added at defined molar lipid–ligand ratio. After incubated at 40 °C for 4 h, these mixtures were dialyzed in 8K MWCO dialysis bag against 0.9% NaCl solution, respectively, for 2 days to remove the unincorporated HSPE-PEG-Octreotide and leaked ammonium sulfate. Oct-L stood for Octreotide-targeted liposome and the following percentage was the molar ratio of octreotide to phospholipids.
Ammonium sulfate gradient method was used to loaded drug DOX and liposome (plain liposome or octreotide-targeting liposome) were incubated at 45 °C for 30 min at a molar lipid to drug ratio of 100:1, and free DOX was separated from DOX-loaded liposome through a Sephadex G-50 column equilibrated with 0.9% NaCl solution. DOX concentration was determined by UV spectrophotometry at 480 nm after liposome lysed with acidic isopropanol. The size distribution of liposome was determined by Zetasizer (3000HS, Malvern Instruments, UK), and H-600 Transmission electron microscopy instrument (Hitachi, Japan) was used to visualize the morphology and size distribution of octreotide-modified liposomes.
Octreotide ligand insertion percentage and efficiency
To test the amount of octreotide ligand and its insertion percentage, HSPE-PEG-Octreotide was covalently labeled with FITC (molar ratio 5%) at the D-Phe residue. FITC-Octreotide-PEG4000-HSPE was purified by Sepharose CL-4B column and incorporated to liposome similar to HSPE-PEG4000-Octreotide. After removing free FITC-Octreotide-PEG4000-HSPE from octreotide-targeted liposome by dialysis, an aliquot of liposome was lysed with 0.5% Triton X-100. The amount of octreotide ligand was determined by spectrofluorophotometer (EX 495 nm, EM 517 nm). Another aliquot of liposome was lysed with methanol, evaporated and dissolved in chloroform. Then, the amount of lipid was determined by the Stewart method (Stewart, Citation1980). The octreotide ligand insertion percentage was defined as molar ligand–lipid ratio and the insertion efficiency was defined as the ratio of defined octreotide ligand insertion percentage to actual insertion percentage.
Drug release in vitro
To investigate the DOX release profile from liposome, 1 mL of 50 μg/mL DOX or DOX-loaded liposome (plain or octreotide targeted DOX concentration adjusted with pH 7.4 PBS) was added to a MWCO 8 K dialysis bag and incubated in 30 mL pH7.4 PBS at 37 °C with gentle stir. At defined time, 0.5 mL aliquot of external pH 7.4 PBS was removed and replaced with equal volume of fresh pH 7.4 PBS. DOX concentration of external pH 7.4 PBS was quantified using GloMax-Multi Jr Single Tube Multimode Reader (Promega, Madison, WI) in blue mode.
Cytotoxicity and cellular uptake studies of octreotide-targeted liposome
Cell cultures
CHO (Chinese hamster ovary cells), NCI-H-446 (human lung carcinoma cell line) and SMMC 7721 (human hepatocarcinoma cell line) were cultured in RPMI 1640 medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 100 U/mL penicillin and 100 mg/mL streptomycin at 37 °C under an atmosphere of 5% CO2 and 90% relative humidity.
Expression level of SSTRs in different cell lines
Reverse transcriptase-polymerase chain reaction (RT-PCR) was utilized to detect the gene expression of SSTR-2 and SSTR-5 on NCI-H-446, SMMC-7721 and CHO. Total RNA was abstracted from cells by using Trizol solution following invitrogen protocol. Reverse transcription was carried out at 42 °C for 45 min in a volume of 50 μL containing 2 μg total RNA, using Oligo (Feindt et al., Citation1997) 18 as primer and the super Moloney murine leukemia virus reverse transcriptase as catalyst. The sample was then heated at 70 °C for 15 min to terminate the reaction. cDNA amplification was performed by polymerase chain reaction (PCR). Aliquot of 2 μL of the reverse transcription reaction sample was added to the Hot start Taq Mix(Biouniquer) in a volume of 20 μL and then heated to 94 °C for 10 min. Thirty-five amplification cycles were undertaken at 94 °C for 30 s, at 58 °C for 30 s and 72 °C for 1 min, followed by an incubation at 72 °C for 7 min. The reaction sample was stored at 4 °C until analysis (Tanon, Shanghai Co. Ltd, China). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was chosen as a control and heated to 94 °C for 10 min followed by 22 amplification cycles performed at 94 °C for 30 s, at 58 °C for 30 s and 72 °C for 1 min, followed by an incubation at 72 °C for 7 min. The reaction sample for control was stored at 4 °C until analysis. All reaction samples were analyzed on 1.8% agarose gel.
In vitro cytotoxicity study
The cytotoxicity of free DOX, DOX-loaded liposome (plain liposome or various density of octreotide-targeted liposomes) was demonstrated as inhibition rate to the proliferation of cells by using MTT assay. In brief, 8 × 103 cells per well were seeded in 96-well plates (Costar, IL) and incubated with complete medium for 24 h, and were then treated with a series of DOX concentration of different octreotide density liposomes diluted with RPMI 1640 for another 24 h. At given time intervals, 20 μL/well of MTT (5 mg/mL in pH 7.4 PBS) was added and after 4 h incubation, the medium was replaced with 150 μL DMSO per well. The formazan crystals produced by viable cells were dissolved in DMSO and assessed as UV absorbance intensity by Microplate Reader (Thermo Electron Corporation) at λ = 570 nm. A well without cell was treated the same process to access the absorbance intensity of remaining solvent. The inhibition ratio was defined as following equation:
where ODS referred to the absorbance intensity of cells exposed to DOX treatment, ODcontrol represents the absorbance intensity of cells treated only with culture medium and ODblank was the absorbance intensity of remaining solvent.
Quantitative study of cellular uptake
To illustrate the influence of ligand density on cellular uptake, quantitative investigation of the cellular uptake on different density of octreotide-targeted liposomal doxorubicin was conducted as following. The 24-well plates were seeded with 4 × 104 cells per well and incubated in complete medium for 36 h. Then, the medium was removed and the cells were washed three times with PBS, followed by incubation with 0.4 mL liposomal DOX (plain or octreotide-targeted liposome) at a DOX concentration of 5 μg/mL (diluted with incomplete RPMI 1640) for 8 h at 37 °C. After incubation, the cells were washed with PBS three times and lysed with 1% SDS solution. The samples were frozen repeatedly and then centrifuged to remove the cellular debris. Subsequently, protein content was tested by BCA assay, while DOX concentration was determined by spectrofluorophotometer.
Fluorescent microscopy of cellular uptake
Qualitative study of cellular uptake was undertaken as following. 4 × 104 cells per well were seeded in 24-well plates and incubated in complete medium for 24 h. Then, the cells were treated with 0.4 mL liposomal DOX (plain or octreotide-targeted liposome) at a DOX concentration of 10 μg/mL (diluted with incomplete RPMI 1640) for 8 h at 37 °C. After treatment, the cells were washed three times with PBS and taken photos (Inverted fluorescent microscopy, Olympus, Tokyo, Japan).
Cytotoxicity and cellular uptake studies of octreotide-grafted long-circulating liposome
Preparation and characterization of octreotide-grafted long-circulating liposome
mPEG2000-PE was synthesized as the method described in “Activity determination of HSPE-PEG-octreotide by synchronous fluorescence spectra (SFS)” section using mPEG2000 and PE according to the same principle, and its structure was verified by NMR. Oct-SSL was constructed using ethanol injection method. mPEG2000-PE was decorated to the surface of the liposome as described in “Preparation and characterization of DOX-loaded plain liposome (P-L) and octreotide-modified targeting liposome” section. The concentration of mPEG2000-PE used was 4%.
In vitro cytotoxicity study
The 96-well plates were seeded with 8 × 103 cells per well (Costar, IL) and incubated with complete medium for 24 h, Liposomes with DOX concentration of 0.3125, 0.625, 1.25, 2.5, 5, 10 μg/mL diluted with incomplete RPMI 1640 were added and treated for another 24 h. Then, 20 μL/well of MTT (5 mg/mL in pH 7.4 PBS) was added to P-L , Oct-L (liposome with 1% octreotide), SSL (DOX long-circulating liposome), Oct-SSL (DOX octreotide-grafted long-circulating liposome) solution (their structures were shown in ) for further 4 h incubation. After that the medium was replaced with 150 μL DMSO per well, the formazan crystals produced by viable cells were dissolved in DMSO and assessed as UV absorbance intensity by Microplate Reader at λ = 570 nm. A well without cells was treated the same process to access the absorbance intensity of remaining solvent. The inhibition rate was defined in “In vitro cytotoxicity study” section.
Fluorescent microscopy of cellular uptake
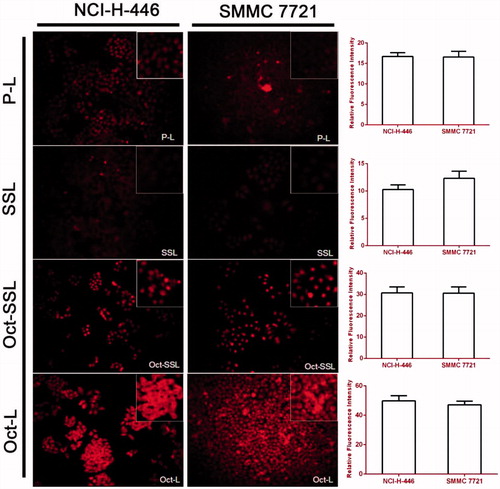
Qualitative study of cellular uptake was investigated as following. The 24-well plates were seeded with 4 × 104 cells per well and incubated in complete medium for 24 h. Then, the cells were treated with P-L, Oct-L, SSL and Oct-SSL at DOX concentration of 10 μg/mL (diluted with incomplete RPMI 1640) for 8 h at 37 °C. After treatment, the cells were washed three times with PBS and taken photos. The semiquantitative analysis for cell uptake was done by image J software (National Institutes of Health, Bethesda, MD).
Pharmacokinetic study
Twelve male rats (180–220 g) were randomly divided into four groups (n = 3). P-L, SSL, Oct-L and Oct-SSL, a single oral dose 5 mg/kg were administrated by tail-vein injection. Blood samples of 0.2 mL were collected from retro-orbital plexus into heparinized tubes at 0.083, 0.25, 0.5, 0.75, 1, 2, 4, 8, 12, 24, 36 and 48 h, then centrifuged at 8000 rpm for 10 min to collect the supernatant plasma. A double volume of acetonitrile and methanol (v/v = 1:1) was added into supernatant plasma for deproteinization. The mixture was vortexed for 10 min and then centrifuged at 12 000 rpm for 10 min, DOX concentration in blood was measured by HPLC. The plot of C6 concentration in plasma versus time was obtained and the pharmacokinetic parameters were analyzed using two-compartment model.
In vivo antitumor efficacy assay
In vivo anti-tumor efficacy of Oct-SSL was evaluated on hepatoma xenograft model to investigate the potential antitumor efficiency. All tumor-bearing mice were weighed and randomly divided into four groups (n = 5): (1) saline as control; (2) DOX; (3) P-L; (4) Oct-L and (5) Oct-SSL. All the formulations were administrated via tail vein at a dose of 5 mg/kg/mouse. The initial day of i.v. administration was defined as Day 0, and administration was then repeated once in every 2 days over a 6-day therapeutic period. The body weight of mice and tumor size were also measured every other day. Tumor volumes were calculated as follows:
where V is the volume of tumor, L is the longer diameter of tumor and S is the shorter diameter of tumor.
Statistical analysis
All the experiments were conducted in triplicates and the results were shown as mean ± SD unless particularly outlined. Student’s two sample t-test was used in statistical evaluation. p < 0.05 was considered statistically significant.
Results and discussion
Activity confirmation of Octreotide-PEG-HSPE by SFS
To make sure the conjugation between p-nitrophenylcarbonyl (pNP)-PEG-HSPE and octreotide did not compromise the activity of octreotide, it was necessary to confirm that the microenvironment of amino acid residues in the active site of octreotide did not change after modification. SFS had been utilized usually to investigate the change of microenvironment of amino acid residues of protein (Wang et al., Citation2010), an apparent shift in SFS would be observed if the microenvironment changed. The modification was realized by the easy conjugation between pNP group and primary amino group of D-Phe residue at the end of octreotide[22]. The conjugation did not happen in the active site of octreotide consisting of Cys-Phe-D-Trp-Lys-Thr-Cys, however, octreotide was an octopeptide and it was reasonable that the modification might affect the microenvironment of the active site. Fortunately, the SFS results showed overlapped spectra of octreotide and HSPE-PEG-Octreotide with coincidental EM 418 nm at △λ = 60 nm (spectra did not show), which meant the microenvironment of Trp at the center of active site did not change. It was probably concluded that octreotide remains active after covalent conjugate with pNP–PEG-HSPE. This conclusion could also be confirmed by the cytotoxicity and cellular uptake studies as following.
Characterization of octreotide-targeted liposomal DOX
According to method 2.3, DOX-loaded liposome bearing 0.5%, 1%, 2%, 3%, or 4% molar ratio of octreotide ligand (to lipid) was prepared, respectively. The insertion efficiency of all the insertion percentages used above were 100% as measured by method 2.4. Mean size of these liposomes were shown in . The mean sizes of octreotide-targeted DOX-loaded liposomes were around 100 nm and increased slightly with the increasing different density of octreotide ligand, which was similar to the study reported(Uster et al., Citation1996). But, this slight difference in size would not literarily account for the difference in cellular cytotoxicity and uptake. The size and morphology of liposomes were visualized directly by TEM. The observed size of liposome (1% octreotide ligand decoration as example) was approximately 100 nm (), which was similar to hydrodynamic diameter obtained from the DLS.
Figure 1. (A) Transmission electron microscopy images of octreotide-modified liposomes. The scale bar indicates 100 nm. (B) Mean size and PDI of different density of octreotide targeted DOX-loaded liposomes (n = 3). And (C) In vitro release profiles of DOX and liposomes with different octreotide ligands densities (n = 3).
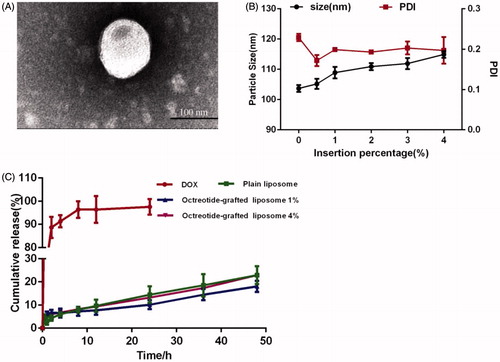
Post-insertion method had been proved to be an efficient method for liposomal modification(Uster et al., Citation1996, Iden & Allen, Citation2001, Lu et al., Citation2006). Not only because it showed excellent insertion efficiency as demonstrated here, but also due to the various density of octreotide-targeted liposomes came from a single preparation of P-L. In this case, the slight increase in mean size might illustrate the feasibility of post-insertion method. Most importantly, compared to the method that including ligands to a thin layer of lipid prior to liposome preparation[19], post-insertion method made most targeted ligands incorporate into the exterior layer of liposome, making all the ligands available for targeting (Saul et al., Citation2003).
Drug release in vitro
The drug release profile of free DOX and different formulations of liposomal DOX was shown in . As illustrated in , free DOX released fast and totally released after 24 h incubation, while only about 20% of DOX released from liposomes with different octreotide ligands. The slow release of DOX might result from its encapsulation mechanism (Zhang et al., Citation2010) and could not be affected by the modification of octreotide, suggesting the modification of targeted ligand would not result in drug leakage(Uster et al., Citation1996). It also could be proved that during the time of cellular uptake experiment, DOX did not leak from liposome.
Expression of SSTR in different cell lines
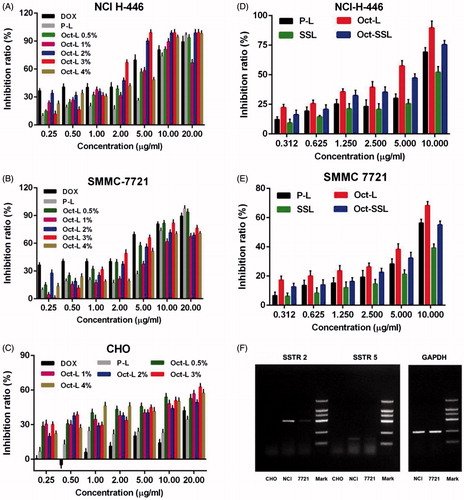
Expression levels of SSTR-2 and SSTR-5 of NCI-H-446, SMMC 7721 and CHO cells were accessed by RT-PCR. As shown in , NCI-H-446 and SMMC 7721 cells expressing high and low level of SSTR-2, respectively, could be used as positive-cell lines, while CHO cells expressed no detective level of SSTR-2 served as negative-cell lines. All three of them didn’t express significant level of SSTR-5.
Figure 2. (A) Cytotoxicity of DOX and liposomal DOX on NCI-H-446, (C) SMMC-7721(B) and CHO cells. (D) Cytotoxicity of P-L, Oct-L (liposome with 1% octreotide), SSL , Oct-SSL on NCI-H-446, (E) SMMC-7721 (n = 3). (F) RT-PCR result of cDNA amplification of SSTR 2 and SSTR 5 in CHO, NCI-H-446 and SMMC 7721 cells.
In vitro cytotoxicity study, quantitative and fluorescent study of cellular uptake of octreotide-targeted liposome
MTT assay was performed on three cell lines to evaluate the inhibitive effect of DOX and various liposomal DOX on cellular proliferation (). The result showed that octreotide modification obviously enhanced cytotoxicity of liposomal DOX on NCI-H-446 and SMMC-7721 cells. It might be explained by the incensement of receptor-mediated internalization in situation of NCI-H-446 and SMMC-7721 cells. In case of CHO cells, octreotide modification also showed increased cytotoxicity, However, the cytotoxicity of octreotide-targeted liposomes to CHO cells at high concentration of DOX was relatively low (about 60%) in comparison with SSTR-2 positive cells. For SSTR-2 positive tumors cells (NCI-H-446 and SMMC-7721), the IC50 of DOX solution, P-L, Oct-L 0.5%, Oct-L 1%, Oct-L 2%, Oct-L 3% and Oct-L 4% in NCI H-446 cells was 7.753, 49.25, 6.720, 3.584, 3.593, 1.068 and 6.717, respectively, as well as 7.789, 51.19, 6.456, 7.350, 4.208, 1.304 and 12.11, respectively, in SMMC 7721 cells. DOX-loaded liposomes with 3% octreotide ligands showed the maximal cytotoxicity, and higher ligand density did not enhance cytotoxicity. On the contrary, in case of CHO cells expressing no detectable level of SSTR-2, liposomes with various density of octreotide ligands had similar cytotoxicity. All of them showed less than 60% inhabitation ratio, suggesting the uptake of CHO cells might have little bearing on receptor-mediated internalization as shown in a previous work by Sun et al., (Citation2010).
Respectively, were quantitative results and fluorescent images for cellular uptake of Oct-L with different octreotide density in two SSTR-2 positive cells, NCI-H-446 and SMMC-7721. For cellular uptake after 8 h incubation with Oct-L, the optimal octreotide density for both cell lines was 1%, which differed from 3% (the optimal density concluded by 24 h MTT assay). In quantitative study, Oct-L with 1% density could be taken in more than P-L and 0.5% density of Oct-L, and then decreased and remained same with increase in density. In conclusion, octreotide-targeted liposome with ligand density more than 1% elicited less cellular uptake, resembling the work of Justin M. Saul (Saul et al., Citation2003), both demonstrated the importance of optimizing ligand density for cell uptake.
Figure 3. DOX uptake of NCI-H-446 and SMMC 7721 cell lines after 8 h incubation with P-L and Oct-L 0.5% ∼ 4% at DOX concentration of 5 μg/mL at 37 °C (n = 3).
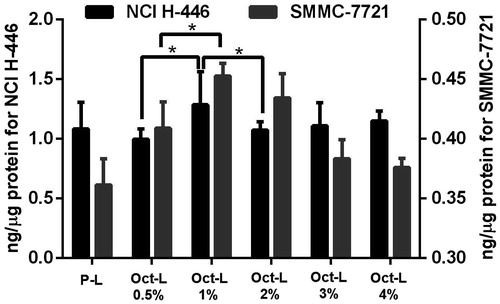
Figure 4. Fluorescence image of NCI-H-446 and SMMC 7721 cells after 8 h incubation with P-L and Oct-L 0.5% ∼ 4% at a DOX concentration of 10 μg/mL. Original magnification 200×.
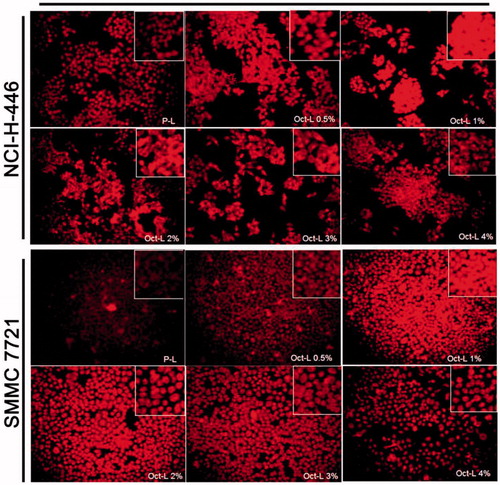
The decline of cellular uptake of liposomes with more than 1% ligand density might be attributed to the downregulation or deactivation of somatostatin receptor (Saul et al., Citation2003). Besides, high density of ligand on the surface of liposome might resulted in sterical disturbance to the affinity of ligand to receptor, as demonstrated by Zhiyong Poon(Poon et al., Citation2010) whose study constructed nanocarriers capable of optimizing the density of ligand cluster and proved the negative impact of dense ligand density.
Difference in incubation time might be the confirmed reason for the discrepancy between the two optimal ligand densities formed in MTT and cell uptake. To test this hypothesis, MTT assay with drug treatment time of 8 h was performed and illustrated that 1% was the best ligand density for cytotoxicity. (data was not shown). In 24 h MTT assay, increased critical point in ligand density might be attributed to downregulation or deactivation of somatostatin receptor in longer incubation. When incubated for 24 h, 3% ligand density just achieved the same binding level as 1% ligand density at 8 h incubation. In addition, a matter of cellular uptake mediated by lipid membrane fusion might account for this difference. Lipid membrane fusion is common mechanism for liposome cellular uptake. Generally, the fluidity of liposome membrane was improved due to increasing PEG-PE inserting into PE bilayer of vesicles. Meanwhile, better fluidity of liposome membrane could cause vesicle aggregation or enhance the fusion once the concentration of PEG-PE reaches 3%(Yang et al., Citation1997). Therefore, the contribution of cellular uptake from membrane fusion might be another reason of increased critical ligand density in 24 h incubation.
Cytotoxicity and cellular uptake studies of Octreotide-grafted long-circulating liposome
It noted that the nanoparticles shell of PEG2000 or PEG5000 could transform from mushroom-like into a brush-like, and could achieve long-circulating effect when the ratio of PEG versus phospholipid was 5%(Mori et al., Citation1991, Woodle, Citation1998). Due to 1% octreotide modification, the amount of mPEG2000-PE was set at 4%. The particle size of SSL and Oct-SSL was 87.8 nm (PDI = 0.244) and 95.5 nm (PDI = 0.233), respectively, which was similar to P-L and Oct-L. At the same time, SSL and Oct-SSL exhibited high DOX encapsulation efficiency, which was 84.6% and 89.9%, respectively.
MTT assay was applied to investigate whether the increase in octreotide long-circulating material influenced the targeting effect of octreotide (). The result showed that the cellular uptake of liposomes was inhibited after modifying long-circulating material for NCI-H-446 and SMMC-7721 cells. Long circulating PEG decoration might generally decrease cell cytotoxicity due to steric hindrance and the shielding effect of octreotide.
The fluorescent images of cellular uptake were shown in . In both NCI H-446 and SMMC-7721 cell lines, the total amount of uptake was SSL < PL < Oct-SSL < Oct-L, indicating that long-circulating modification significantly impeded the uptake of octreotide-targeted liposomes. However, the uptake of Oct-SSL was much more than SSL, indicating that octreotide-targeted modification still played a role in cell uptake, resembling the results of MTT assay.
Pharmacokinetic study
The mean pharmacokinetic parameters in rats after i.v. injection of P-L, Oct-L, Oct-SSL, SSL are presented in . Oct-SSL was absorbed into the blood circulation with the largest AUC. While the MRT of SSL was higher than P-L, it indicated the effect of PEG long-cycle modification. It worth noting that after octreotide modification (Oct -SSL), the in vivo long-circulation reduced with the decreased MRT, indicating that long circulating and targeting effect restricted each other. However, Oct -SSL showed a higher MRT compared with Oct-L showing the relative higher long circulating effect.
Table 1. Pharmacokinetic parameters in rats after i.v. injection of P-L, Oct-L, Oct-SSL, SSL (Mean ± SD, n = 3).
In vivo anti-tumor efficacy
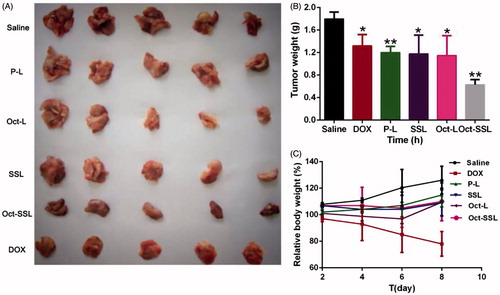
After 48 h of last administration, mice in each group were sacrificed to investigate tumor size and tumor weight. As shown in , Oct-SSL exhibited the best antitumor effect compared with Oct-L, SSL and P-L groups. Students t-test was used to demonstrate the tumor weight difference in these groups. It was shown that Oct-SSL improved antitumor effect significantly in all treatments (p < 0.05). The antitumor effect of Oct-L was better than P-L, indicating octreotide modification could play an important role in the increased uptake of doxorubicin liposomes in tumor cells. Furthermore, antitumor effect of SSL was better than P-L while Oct-SSL was better than Oct-L. It indicated that PEG modification with long-circulating effect could increase the residence time in vivo, which contributed to the antitumor effects in vivo. showed a significant weight reduction of mice in DOX group while the weight of other groups kept the plain level. It might be resulted from the toxicity of DOX, on the other hand it demonstrated the biological safety of our formulations.
Figure 6. In vivo antitumor effects of different liposomes on hepatoma xenograft tumor-bearing mice. (A) The representative images of the xenograft tumors collected from the mice after treatment with different liposome. (B) The weight of tumor and(C) and changes of body weight of mice after treated with different formulations for a total four doses. Results were expressed as the mean ± SD (n = 5). *p < 0.05, **p < 0.01.
Conclusion
Octreotide-targeted doxorubicin liposome with different ligand density was fabricated by post-insertion method to illustrate the influence of ligand density on the effect of drug in vivo cellular studies, including cytotoxicity and cellular uptake. It was easy to imagine a saturation point for the binding of ligands to cell surface receptor when all the receptors were occupied by ligands. Besides, high-ligand density would shorten the distance between liposome and surface-grafted ligands, leading to multivalent interaction (Sapra & Allen, Citation2003). As a result, increasing avidity of liposome to cells kept along with cellular uptake of liposomal payload. However, beyond a critical ligand density, cellular uptake and cytotoxicity would instead decline as demonstrated by the work described earlier and previous study (Saul et al., Citation2003). This decline might have to do with the downregulation or shut-off of receptors or sterical disturbance among ligands pointed by us.
An interesting phenomenon found in our work was that the optimal ligand density concluded from 24 h MTT assay differed from that drawn from 8 h cellular uptake study. Although no specific reasons were confirmed, we believed this discrepancy had bearing on durance of incubation time. In this case, based on the optimal octreotide (1% octreotide density) we got earlier, DOX Oct-SSL was prepared to investigate the effect of PEG on cell uptake and in vivo antitumor efficacy. Oct-SSL showed low cell uptake while exhibited the best anti-tumor efficacy compared with Oct-L in vivo in our study. Oct-SSL also showed a higher MRT compared with Oct-L showing the relative higher long-circulating effect in pharmacokinetic study. Our research located an optimal octreotide ligand density and effect of PEG decoration for SSTR-2 positive tumor; it would be expected to play a guiding role for other octreotide-modified drug-delivery system.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This work was financially supported by the National Natural Science Foundation of China (No. 81373983 and 81573377), Natural Science Foundation (No. 20141352) and Six Talent Peaks Project (No. SWYY-011) of Jiangsu Province, and the Fundamental Research Funds for the Central Universities (No. 2016PT067).
References
- Bansal D, Yadav K, Pandey V, et al. (2016). Lactobionic acid coupled liposomes: an innovative strategy for targeting hepatocellular carcinoma. Drug Deliv 23:140–6
- Carrion C, Domingo JC, De Madariaga MA. (2001). Preparation of long-circulating immunoliposomes using PEG-cholesterol conjugates: effect of the spacer arm between PEG and cholesterol on liposomal characteristics. Chem Phys Lipids 113:97–110
- Debbie L, Iden TMA. (2001). In vitro and in vivo comparison of immunoliposomes made by conventional coupling techniques with those made by a new post-insertion approach. Biochimica Et Biophysica Acta 1513:207–16
- Doddapaneni R, Patel K, Owaid IH, Singh M. (2016). Tumor neovasculature-targeted cationic PEGylated liposomes of gambogic acid for the treatment of triple-negative breast cancer. Drug Deliv 23:1232–41
- Feindt J, Mentlein R, Krisch B. (1997). Time-dependent influence of the somatostatin analogue octreotide on the proliferation of rat astrocytes and glioma cells. Brain Res 746:309–13
- Gavia DJ, Shon YS. (2012). Controlling surface ligand density and core size of alkanethiolate-capped Pd nanoparticles and their effects on catalysis. Langmuir 28:14502–8
- Gref R, Luck M, Quellec P, et al. (2000). 'Stealth' corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces 18:301–13
- Greish K. (2007). Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J Drug Target 15:457–64
- Guillermet-Guibert J, Lahlou H, Pyronnet S, et al. (2005). Somatostatin receptors as tools for diagnosis and therapy: Molecular aspects. Best Pract Res Clin Gastroenterol 19:535–51
- Huang CM, Wu YT, Chen ST. (2000). Targeting delivery of paclitaxel into tumor cells via somatostatin receptor endocytosis. Chem Biol 7:453–61
- Iden DL, Allen TM. (2001). In vitro and in vivo comparison of immunoliposomes made by conventional coupling techniques with those made by a new post-insertion approach. Biochimica Et Biophysica Acta-Biomembranes 1513:207–16
- Iwase Y, Maitani Y. (2011). Octreotide-targeted liposomes loaded with CPT-11 enhanced cytotoxicity for the treatment of medullary thyroid carcinoma. Mol Pharm 8:330–7
- Iyer AK, Khaled G, Fang J, Maeda H. (2006). Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today 11:812–18
- Jakoby J, Beuschlein F, Mentz S, et al. (2015). Liposomal doxorubicin for active targeting: surface modification of the nanocarrier evaluated in vitro and in vivo: challenges and prospects. Oncotarget 6:43698–711
- Jung H, Robison AD, Cremer PS. (2009). Multivalent ligand-receptor binding on supported lipid bilayers. J Struct Biol 168:90–4
- Kirpotin DB, Drummond DC, Shao Y, et al. (2006). Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res 66:6732–40
- Klibanov AL, Maruyama K, Torchilin VP, Huang L. (1990). Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett 268:235–7
- Li T, Xu K, Fu Y, Cai K. (2016). Inducing the migration behavior of endothelial cells by tuning the ligand density on a density-gradient poly(ethylene glycol) surface. Colloids Surf B Biointerfaces 143:557–64
- Lu J, Jeon E, Lee B-S, et al. (2006). Targeted drug delivery crossing cytoplasmic membranes of intended cells via ligand-grafted sterically stabilized liposomes. J Controll Release 110:505–13
- Maeda H, Wu J, Sawa T, et al. (2000). Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 65:271–84
- Mercadal M, Domingo JC, Petriz J, et al. (1999). A novel strategy affords high-yield coupling of antibody to extremities of liposomal surface-grafted PEG chains. Biochim Biophys Acta 1418:232–8
- Mori A, Klibanov AL, Torchilin VP, Huang L. (1991). Influence of the steric barrier activity of amphipathic poly(ethyleneglycol) and ganglioside GM1 on the circulation time of liposomes and on the target binding of immunoliposomes in vivo. FEBS Lett 284:263–6
- Nagy A, Schally AV, Halmos G, et al. (1998). Synthesis and biological evaluation of cytotoxic analogs of somatostatin containing doxorubicin or its intensely potent derivative, 2-pyrrolinodoxorubicin. Proc Natl Acad Sci USA 95:1794–9
- Ng K-Y, Zhao L, Liu Y, Mahapatro M. (2000). The effects of polyethyleneglycol (PEG)-derived lipid on the activity of target-sensitive immunoliposome. Int J Pharm 193:157–66
- Poon Z, Chen S, Engler AC, et al. (2010). Ligand-clustered “and in vivo tumor targeting” nanoparticles for modulated cellular uptake and in vivo tumor. Angew Chem Int Ed Engl 49:7266–70
- Pozzi D, Colapicchioni V, Caracciolo G, et al. (2014). Effect of polyethyleneglycol (PEG) chain length on the bio-nano-interactions between PEGylated lipid nanoparticles and biological fluids: from nanostructure to uptake in cancer cells. Nanoscale 6:2782–92
- Sapra P, Allen TM. (2003). Ligand-targeted liposomal anticancer drugs. Progress Lipid Res 42:439–62
- Sarvestani A.S, Jabbari E. (2008). Modeling the kinetics of cell membrane spreading on substrates with ligand density gradient. J Biomech 41:921–5
- Saul JM, Annapragada A, Natarajan JV, Bellamkonda RV. (2003). Controlled targeting of liposomal doxorubicin via the folate receptor in vitro. J Control Release 92:49–67
- Shen H, Hu D, Du J, et al. (2008). Paclitaxel-octreotide conjugates in tumor growth inhibition of A549 human non-small cell lung cancer xenografted into nude mice. Eur J Pharmacol 601:23–9
- Shi C, Cao H, He W, et al. (2015). Novel drug delivery liposomes targeted with a fully human anti-VEGF165 monoclonal antibody show superior antitumor efficacy in vivo. Biomed Pharmacother 73:48–57
- Shiokawa T, Hattori Y, Kawano K, et al. (2005). Effect of polyethylene glycol linker chain length of folate-linked microemulsions loading aclacinomycin A on targeting ability and antitumor effect in vitro and in vivo. Clin Cancer Res 11:2018–25
- Stewart JC. (1980). Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem 104:10–14
- Stoffelen C, Staltari-Ferraro E, Huskens J. (2015). Effects of the molecular weight and the valency of guest-modified poly(ethylene glycol)s on the stability, size and dynamics of supramolecular nanoparticles. J Mater Chem B 3:6945–52
- Sun MJ, Wang Y, Shen J, et al. (2010). Octreotide-modification enhances the delivery and targeting of doxorubicin-loaded liposomes to somatostatin receptors expressing tumor in vitro and in vivo. Nanotechnology 21:475101. doi: 10.1088/0957-4484/21/47/475101
- Susumu K, Uyeda HT, Medintz IL, et al. (2007). Enhancing the stability and biological functionalities of quantum dots via compact multifunctional ligands. J Am Chem Soc 129:13987–96
- Torchilin VP, Levchenko TS, Lukyanov AN, et al. (2001). p-Nitrophenylcarbonyl-PEG-PE-liposomes: fast and simple attachment of specific ligands, including monoclonal antibodies, to distal ends of PEG chains via p-nitrophenylcarbonyl groups. Biochim Biophys Acta 1511:397–411
- Uster PS, Allen TM, Daniel BE, et al. (1996). Insertion of poly(ethylene glycol) derivatized phospholipid into pre-formed liposomes results in prolonged in vivo circulation time. FEBS Lett. 386:243–6
- Wang T, Zhao Z, Wei B, et al. (2010). Spectroscopic investigations on the binding of dibazol to bovine serum albumin. J Mol Struct 970:128–33
- Wang X, Song Y, Su Y, et al. (2016). Are PEGylated liposomes better than conventional liposomes? A special case for vincristine. Drug Deliv 23:1092–100
- Woodle MC. (1998). Controlling liposome blood clearance by surface-grafted polymers. Adv Drug Deliv Rev 32:139–52
- Yamada A, Taniguchi Y, Kawano K, et al. (2008). Design of folate-linked liposomal doxorubicin to its antitumor effect in mice. Clin Cancer Res 14:8161–8
- Yang Q, Guo Y, Li L, Hui S.W. (1997). Effects of lipid headgroup and packing stress on poly(ethylene glycol)-induced phospholipid vesicle aggregation and fusion. Biophys J 73:277–82
- Yuan M, Qiu Y, Zhang L, et al. (2016). Targeted delivery of transferrin and TAT co-modified liposomes encapsulating both paclitaxel and doxorubicin for melanoma. Drug Deliv 23:1171–83
- Zalipsky S. (1995). Functionalized poly(ethylene glycol) for preparation of biologically relevant conjugates. Bioconjug Chem 6:150–65
- Zhang J, Jin W, Wang X, et al. (2010). A novel octreotide modified lipid vesicle improved the anticancer efficacy of doxorubicin in somatostatin receptor 2 positive tumor models. Mol Pharm 7:1159–68