Abstract
Context: Gastric carcinoma (GC) is one of the most common cancers and the second most frequent cause of cancer-related deaths. Chemotherapy is an important therapeutic modality for GC. However, chemoresistance limited its success rate. Combination chemotherapy is often applied to prevent drug-induced resistance in cancers.
Objective: The aim of this study is to evaluate whether the co-delivery of etoposide (ETP) and curcumin (CUR) with one nanoparticle can result in synergistic effects of both drugs.
Methods: ETP- and CUR-loaded nanostructured lipid carriers (ETP-CUR-NLC) were prepared by the solvent injection technique. Their average size, zeta potential and drug loading were evaluated. Human gastric cancer cell lines (SGC7901 cells) were used for the testing of in vitro cytotoxicity studies, and in vivo anti-tumor efficacies of the carriers were evaluated on mice bearing SGC7901 cells xenografts.
Results: ETP-CUR-NLC has a particle size of 114 nm, EPT-loading quantity of 83% and CUR-loading quantity of 82%. ETP-CUR-NLC displayed high cytotoxicity and enhanced antitumor activity in vitro and in vivo. Meanwhile, ETP-CUR-NLC displayed low cytotoxicity in normal tissues in vivo.
Discussion and conclusion: The results demonstrate that ETP-CUR-NLC can achieve impressive anti-tumor activity. By combining CUR, an effective NF-κB inhibitor, with ETP, a powerful anticancer drug, in NLC, we could improve the therapeutic efficacy in cancer treatments. Our results showed that such co-loaded delivery systems could serve as a promising therapeutic approach to improve clinical outcomes against various malignancies.
Introduction
Gastric carcinoma is the fourth most common cancers worldwide and the second leading cause of cancer-related deaths in eastern Asia (Jemal et al., Citation2011; Guggenheim & Shah, Citation2013). Gastric carcinoma has a poor prognosis and many patients present advanced or metastatic disease at the time of diagnosis. Depending on NCCN guidelines for gastric carcinoma, systemic chemotherapy is the mainstay of palliative therapy for advanced or metastatic disease and five classes of cytotoxic agents are currently used in this disease (fluoropyrimidines, platinums, taxanes, topoisomerase inhibitors and anthracyclines (Qu et al., Citation2015).
Etoposide (ETP) is the inhibitor of deoxyribonucleic acid (DNA) topoisomerase II. Its main effect appears to be at the G2 phase of the cell cycle in mammalian cells. ETP has been one of the treatment options for gastric carcinoma but ETP is challenging to administer because of solubility, and undergoes short biological half-life as well as drug resistance (Wang et al., Citation2014; Fatma et al., Citation2015). This necessitates repetitive high dosages and severe side effects. Moreover, deficiencies such as metabolic inactivation, drug resistance, myelosuppression, poor bioavailability and secondary acute myelocytic leukemia, which occur in a several percentage of patients have limited their scopes of applications (Qin et al., Citation2013; Yordanov et al., Citation2013). On the other hand, curcumin (CUR) is a natural remedy, and has been shown to enhance the antitumor efficacy of ETP for prostate cancer (Shukla et al., Citation2014). Therefore, in this study, substantial therapeutic benefits from the combination of drugs (ETP and CUR) were anticipated in the treatment of stomach cancer.
Curcumin can be used extensively to treat a variety of solid tumors, including stomach, breast and colorectal carcinoma (Cui et al., Citation2006; Ahmad et al., Citation2015; Da et al., Citation2015). In gastric cancer cell lines, CUR protects against chemoresistance in human gastric cancer cell lines (SGC7901 cells) by down-regulating NF-κB and subsequent NF-κB-mediated anti-apoptotic genes, such as Bcl-2 and Bcl-xL (Chung et al., Citation2013). It has been also reported that CUR suppresses the expression of epidermal growth factor receptor (EGFR) and was capable of suppressing cell proliferation and migration through inhibition of STAT3 phosphorylation, which contributes to gastric carcinogenesis (Uehara et al., Citation2014; Ahmad et al., Citation2015). In spite of excellent therapeutic potential, low-aqueous solubility and rapid systemic elimination limit its application in medicine. Recently, the development in the field of nanotechnology has made excellent progresses toward administrative lipophilic drug.
Various nanomedicine-based therapeutic novel drug deliveries such as polymeric nanoparticles, solid lipid nanoparticles (SLNs), nanostructured lipid nanoparticles (NLCs) and so on, have been designed to carry lipophilic drugs containing ETP or CUR (Snehalatha et al., Citation2008; Zhang et al., Citation2011; Ahmad et al., Citation2015; Kuo & Lee, Citation2015). NLCs, a new type of lipid nanoparticles, are mixtures of solid lipids with spatially incompatible liquid lipids, which have advantages of improved drug loading capacity and sustained release properties (Müller et al., Citation2002).
In our previous research, ETP-NLCs have been constructed. It has been found that the half maximal inhibitory concentration (IC50) of ETP using NLCs was 6.3 μg/mL for SGC 7901 cells, while 56.5 μg/mL for the pure drug (Jiang et al., Citation2015). The aim of this study was to further investigate the synergistic effect of ETP and CUR against SGC 7901 cells. Therefore, our strategy is to design NLCs loaded with ETP and CUR (ETP-CUR-NLCs) in order to improve therapeutic efficacy and reduce the frequency of drug administration. ETP-CUR-NLCs were characterized for mean particle size, polydispersity index (PDI), zeta potential and entrapment efficiency (EE). SGC7901 cell lines were used for the testing of in vitro cytotoxicity studies, and mice bearing SGC7901 cells xenografts were utilized as the tumor model to evaluate the synergistic antitumor effect. As expected, combination chemotherapy exhibits better therapeutic effect on gastric carcinoma.
Materials and methods
Materials and animals
Etoposide was provided by Qilu Pharmaceutical Co Ltd. (Ji’nan, China). Curcumin was purchased from Tokyo Chemical Industry (TCI) Co., Ltd. Glycerol monostearate (GM), soybean phosphatidylcholine (SPC), oleic acid, (3-[4,5-dimehyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide (MTT) and dimethyl dioctadecyl ammonium bromide (DDAB) were purchased from Sigma-Aldrich (St Louis, MO). Polyethylene glycol (PEG)-distearoylphosphatidylethanolamine (DSPE) was purchased from CordenPharma International (Plankstadt, Germany). Labrafac PG (propylene glycol dicaprylocaprate) was a kind offer from Gattefossé (Gennevilliers, France). All other chemicals were of analytical grade or high performance liquid chromatography (HPLC) grade. SGC7901 cells were obtained from the American type culture collection (ATCC, Manassas, VA). BALB/c nude mice (4–6 weeks old, 18–22 g weight) were purchased from Medical Animal Test Center of Shandong Province (Ji’nan, China).
Preparation of ETP-CUR-NLCs
ETP-CUR-NLCs were constructed by the solvent injection technique (Jiang et al., Citation2015). ETP (5 mg), CUR (1 mg), GM (25 mg), SPC (25 mg) and oleic acid (5 mL) were mixed and heated at 70 °C to form the oil phase. The oil phase was then rapidly injected into the aqueous phase containing DDAB. The resulting mixture was continuously stirred at 800 rpm for 40 min. Thereafter, the dispersion was centrifuged at 10 000 rpm for 10 min and aggregates were re-suspended in double distilled water. Single ETP-loaded NLCs (ETP-NLCs), single CUR-loaded NLCs (CUR-NLCs) and drug free blank NLCs (NLCs) were prepared using the same method.
Characterization of NLCs
The particle size, size distribution and zeta potential of the prepared NLCs were measured by photocorrelation spectrometer (Zetasizer 3000HS, Malvern Instruments Co., Ltd., Malvern, UK) (Ledet et al., Citation2014). The amount of ETP and CUR encapsulated in the ETP-CUR-NLCs was determined by UV-Vis method (Katas et al., Citation2014; Wang et al., Citation2014). Samples were dissolved by adding a specific amount of ethanol and the concentration of the ETP or CUR was determined with a UV-Vis spectrophotometer. The selected wavelength for ETP and CUR measurement was 285 and 430 nm, respectively. The drug loading (DL) and drug entrapment efficiency (EE) of the ETP and CUR were calculated as follows:
DL (%) = (Weight of drug in NLCs)/(Weight of NLCs) × 100
EE (%) = (Weight of drug in NLCs)/(Weight of drug totally added) × 100
In vitro drug release
In vitro release of ETP and CUR from ETP-CUR-NLCs was assessed by the dialysis method (Nekkanti et al., Citation2015). Different kinds of drug-loaded NLCs were placed in the dialysis bag separately. Then, the bag was incubated with 50 mL of release medium at 37 °C (0.1% Tween 80 in PBS, pH 7.4). 1 mL of the medium was collected at predetermined time points and replaced with 50 mL of fresh medium. The concentrations of released ETP and CUR were determined by the UV-Vis method mentioned above.
In vitro cytotoxicity study
The SGC7901 cells were treated with ETP-CUR-NLCs (5 mg ETP, 1 mg CUR), ETP-NLCs (10 mg ETP), CUR-NLCs (5 mg CUR), ETP and CUR mixed solution (ETP + CUR solution, 5 mg ETP, 1 mg CUR), ETP solution (10 mg ETP), CUR solution (5 mg CUR) and NLCs (Alexander et al., Citation2015). ETP + CUR solution, ETP solution and CUR solution were prepared using ethyl alcohol to dissolve the drugs. The cells were seeded in a 96-well plate a density of 5000 cells/well and allowed to adhere for 24 h prior to the assay. The cells were then treated with 200 μL serum-free medium containing various concentrations of NLCs. After 72 h of incubation, 20 μL of MTT solution (5 mg/mL) was added to each well of the plate. After incubation for 4 h, 200 μL/well of DMSO was added to dissolve the contents in the plate. Relative viability was obtained from the absorbance at 590 nm of the treated SGC7901 cells divided by the absorbance at 590 nm of the untreated cells.
In vivo tissues distribution and antitumor activity
The tissues distribution and antitumor effects of NLCs was investigated in gastric tumor-bearing BALB/c nude mice models (Liu et al., Citation2012). BALB/c mice were inoculating (s.c.) subcutaneously in the left armpit with SGC7901 cells suspended in PBS. When tumor volume reached about 100 mm3.
On the one hand, ETP-CUR-NLCs and ETP + CUR solution was given into the mice by tail vein injection, separately. At predetermined time intervals, mice were sacrificed and the tumor, heart, liver, spleen, lung and kidney of mice were collected and stored. To determine the amount of drug in each tissue, tissues were first weighed and homogenized with physiological saline.
On the other hand, ETP-CUR-NLCs, ETP-NLCs, CUR-NLCs, ETP + CUR solution, ETP solution, CUR solution, NLCs and 0.9% saline were injected through the tail vein of the mice of each group, once every 3 d. Twenty-one days later, all the mice were sacrificed by cervical dislocation and the tumor tissue samples were taken out. Tumor volume of each mouse was measured with a digital caliper every 3 days, and was calculated according to the below equation:
V (mm3) = A × B2/2, where A and B represented the longest and shortest diameter of the tumors.
The antitumor efficacy of each group was evaluated by tumor inhibition rate (TIR), which was calculated as follows:
TIR (%) = (Tumor weight of the sample group − Tumor weight of the control group)/Tumor weight of the control group × 100.
Statistical analysis
All the results expressed as means ± standard deviation were representative of three independent experiments. Statistical data analysis was performed using the Student’s t-test. A p value less than 0.05 was considered to be statistically significant.
Results
Preparation and characterization of ETP-CUR-NLCs
summarizes the characteristics of ETP-CUR-NLCs, ETP-NLCs, CUR-NLCs and NLCs. The size of all kinds of NLCs tested was around 110 nm. The PDI is also stable after loading of drugs. The zeta potential of ETP-CUR-NLCs was +33.7 mV, which had no obvious difference with single drug-loaded NLCs or blank NLCs. The DL and EE of ETP for ETP-CUR-NLCs was 9.1 and 83.1%, respectively. The DL and EE of CUR for ETP-CUR-NLCs was 14.8 and 81.5%, respectively.
Table 1. Characterization of NLCs.
In vitro drug release
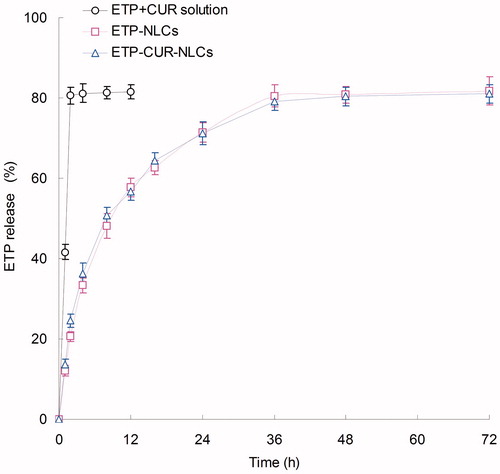
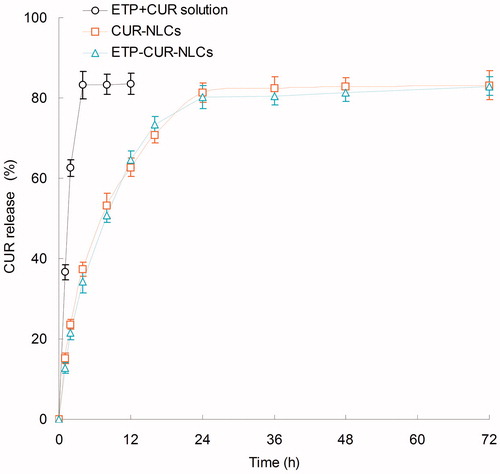
The in vitro release ETP and CUR from different kinds of samples are depicted in and separately. The release of ETP and CUR from ETP + CUR solution was used for the contrast. As shown in the figures, both ETP and CUR released from ETP + CUR solution in a very fast manner. On the contrary, the release of ETP and CUR from the NLCs showed a sustained-release behavior. Nearly complete accumulated releases were achieved after 24 h. The release profiles of ETP from ETP-CUR-NLCs and ETP-NLCs have no obvious difference, indicated the two drugs do not affect the release of each other. The same situation happened in the release of CUR from ETP-CUR-NLCs and ETP-NLCs.
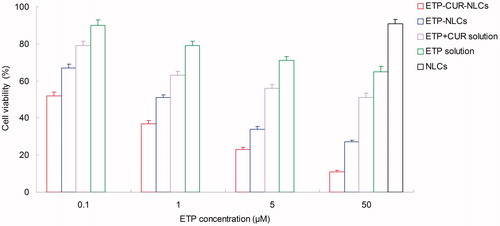
In vitro cytotoxicity study
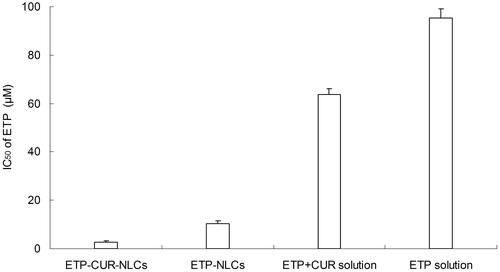
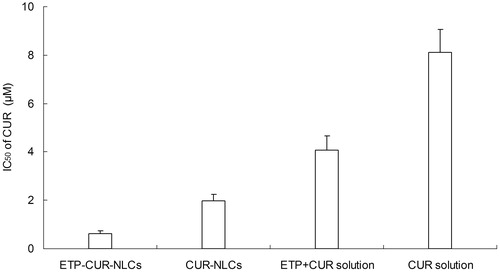
The SGC7901 cells viability of different kinds of samples is displayed in . All of the drug solution and drug-loaded NLCs samples inhibited the growth of the cells. The inhibition effect is increased with the drug concentration. The IC50 values of ETP and CUR on various samples are summarized in and . Cytotoxicity values of drug-loaded NLCs samples were significantly higher than that of drug solution samples (p < 0.05). ETP-CUR-NLCs had the highest cytotoxicity compared with other samples (p < 0.05).
Figure 3. The SGC7901 cells viability of different kinds of samples. SGC7901 cells: Human gastric cancer cell line.
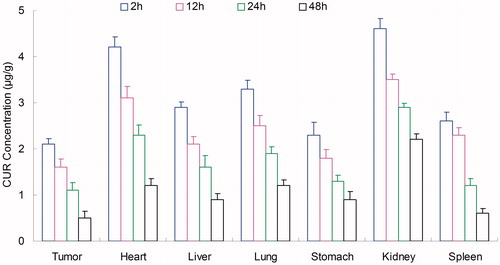
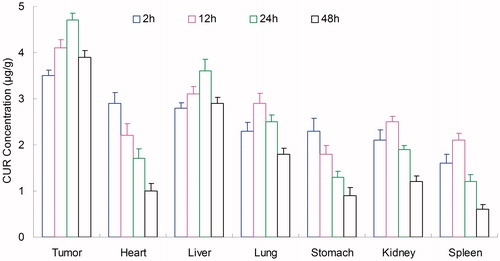
In vivo tissues distribution
Tissues distribution of NLCs was investigated in gastric tumor-bearing BALB/c nude mice models. Tissue distribution results of ETP in ETP + CUR solution and ETP-CUR-NLCs are shown in and . Tissue distribution results of CUR in ETP + CUR solution and ETP-CUR-NLCs are shown in and . illustrates that the ETP + CUR solution mainly distributes in heart and kidney. Distribution of ETP in ETP-CUR-NLCs was higher in the tumor tissue compared with the other tissues (). This could decrease the side effects during the tumor therapy. The higher concentration in the tumor tissue remained relatively stable at all time points until 48 h after injection, indicating the sustained release behavior of the ETP-CUR-NLCs. The same tissue distribution results of CUR were found in ETP-CUR-NLCs and ETP + CUR solution ( and ).
Figure 6. Tissue distribution results of ETP in ETP + CUR solution. ETP: etoposide; CUR: curcumin; ETP + CUR solution: etoposide and curcumin mixed solution.
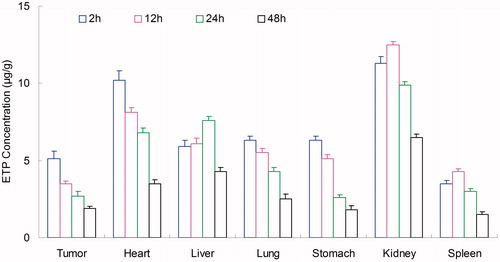
Figure 7. Tissue distribution results of ETP in ETP-CUR-NLCs. ETP: etoposide; CUR: curcumin; ETP-CUR-NLCs: NLCs-loaded with etoposide and curcumin.
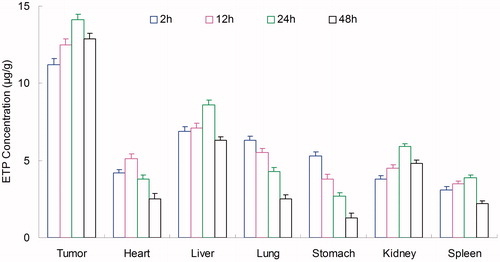
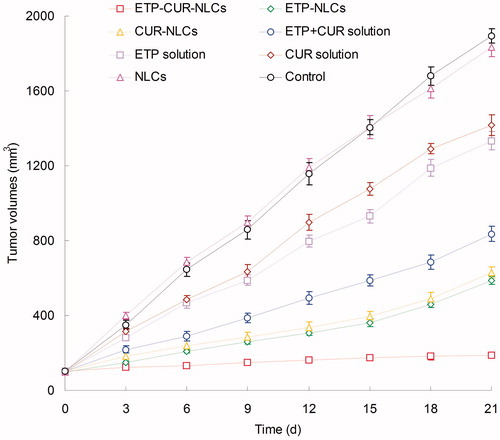
In vivo antitumor activity
illustrates in vivo anti-tumor efficacy of different samples in gastric tumor-bearing mice. The blank NLCs did not have an obvious impact on tumor inhibition, and the same curve was found with the saline control groups (p > 0.05). The increase in tumor volume was substantially inhibited by ETP and CUR-loaded NLCs groups than ETP and CUR solution groups (p < 0.05). Tumor volume of ETP-CUR-NLCs group was obviously the smallest among all groups. The TIR of different groups are illustrated in . The TIR of ETP-CUR-NLCs group (90.02%) was considerably higher than ETP-NLCs group (69.13%) and CUR-NLCs group (66.75%) (p < 0.05). Higher TIR was achieved by ETP-NLCs group than ETP solution group (29.61%) (p < 0.05). The similar phenomenon was found between CUR-NLCs group and CUR solution group (25.17%).
Table 2. Tumor inhibition rates of different samples.
Discussion
Nanostructured lipid nanoparticles, a new type of lipid nanoparticles, have been designed to carry lipophilic drugs (Zhao et al., Citation2010; Seyfoddin & Al-Kassas, Citation2013). In our previous research, ETP-NLCs have been constructed. ETP-NLCs have a narrow size distribution, and high drug entrapment efficiency. ETP-NLCs could significantly enhance in vitro cytotoxicity and in vivo antitumor effect against SGC7901 cells and gastric cancer animal model compared to the free drug (Jiang et al., Citation2015). The aim of this study was to further investigate the synergistic effect of ETP and CUR against SGC 7901 cells. Therefore, ETP-CUR-NLCs were designed in order to improve therapeutic efficacy and reduce the frequency of drug administration.
ETP-CUR-NLCs were prepared by the solvent injection technique (Shah et al., Citation2011). The size of ETP-CUR-NLCs, ETP-NLCs, CUR-NLCs and NLCs was around 110 nm (). This could be concluded that the loading of two drugs have no obvious effect on the size of the NLCs. Particle size is a key factor of the carriers. It has a great impact on the in vitro and in vivo efficiency of the NPs, including prolonged blood circulation time and mediated the tumor targeted effect (Singh et al., Citation2015). The small particle size of ETP-CUR-NLCs is expected to enhance the adhesion with biological cells, increase the cellular uptake of delivery systems, and improved bioavailability of both ETP and CUR. The PDI is also stable after loading of drugs. These could be concluded that the adding of single or double drugs did not affect the size of the NLCs formulations. The zeta potential of ETP-CUR-NLCs was +33.7 mV, which had no obvious difference with single drug-loaded NLCs or blank NLCs. Positive surface charge would let the NLCs easily absorbed onto the passive charged cell surface (Tamilvanan & Kumar, Citation2011). The EE of ETP for ETP-CUR-NLCs was 83.1%. The EE of CUR for ETP-CUR-NLCs was 81.5%. The EE is another fundamental factor influenced the drug therapeutic effect. The high EE of both ETP and CUR achieved by the ETP-CUR-NLCs could proof that the solvent injection technique and the excipients used is appropriate for the loading of drugs into the NLCs.
The in vitro release profiles of ETP and CUR from different NLCs and solution samples are summarized in and . Both ETP and CUR released from the NLCs in a sustained-release manner. The release profiles of ETP from ETP-CUR-NLCs and ETP-NLCs have no obvious difference, indicated that the two drugs do not affect the release of each other. The same situation happened in the release of CUR from ETP-CUR-NLCs and CUR-NLCs. The drug-release mechanism could belong to drug diffusion, lipid matrix swelling, and the carrier erosion or degradation (Fares & Salem, Citation2015). Drugs located near the surface of NLCs were delivered firstly, and then the inner drugs diffused through the lipid matrix (Kawadkar et al., Citation2013). Sustained-release behavior could maintain the drug concentration in the blood circulation and bring about continuous therapeutic effect (Jafarieh et al., Citation2015).
The in vitro cytotoxicity study was carried out on the human gastric cancer cell lines: SGC7901 cells. All of the drug solution and drug-loaded NLCs samples inhibited the growth of the cells in a concentration-dependent pattern (Bao et al., Citation2015). Significantly lower IC50 values of drug-loaded NLCs than that of drug solution samples could be explained that good delivery ability of NLCs formula could facilitate greater cellular uptake (Li et al., Citation2014). ETP-CUR-NLCs had the best tumor cell inhibition efficiency than any other samples. Taking the ETP and CUR dose used for the formulations into consideration, the dose of both drugs used in ETP-CUR-NLCs was obviously lesser than the amount of ETP in ETP-NLCs and the amount of CUR in CUR-NLCs. This could be evidence that the two drugs loaded in the NLCs have synergistic effects and could reduce the amount of drugs used with better antitumor efficiency.
The in vivo tissues distribution of NLCs was investigated in gastric tumor-bearing BALB/c nude mice models. The ETP and CUR in ETP + CUR solution distribute higher in heart and kidney. This could lead to systemic toxicity. Distribution of ETP and CUR in ETP-CUR-NLCs was the highest in the tumor tissue. Solid tumors contributed to an increased leakage, which might provide vascular contents with greater access to extravascular targets (He et al., Citation2010). Higher distribution percent in tumor of ETP-CUR-NLCs might be due to the prolonged blood circulation time. The positively charged ETP-CUR-NLCs exhibited higher distribution percentage in tumor may be because after reaching the leaky tumor microvasculature, NLCs with higher positive charges would be more efficiently depart from the interstitial and to be internalized by the endothelium or tumor cells contiguous to the endothelium (Yan et al., Citation2015). Smaller particle size was favorable for passive targeting to the tumor owing to the “enhanced permeability and retention” (EPR) effects, which resulted in the efficient accumulation in tumor of NPs with diameter of approximately 100-150 nm (Ocal et al., Citation2014).
In vivo anti-tumor efficacy of different samples was investigated in gastric tumor-bearing mice. The inhibition effect of ETP and CUR-loaded NLCs groups were significantly better than ETP and CUR solution groups. The TIR of ETP-CUR-NLCs group was significantly higher than ETP-NLCs group and CUR-NLCs group. Outstanding anti-tumor efficacy results of ETP-CUR-NLCs group may be due to the increased half-life and the targeting effect mediated by the NLCs. Also, with the obvious reduced dosage of ETP and CUR used for the drug-loaded NLCs, ETP-CUR-NLCs showed significantly higher in vivo tumor inhibition effect than the ETP-NLCs and CUR-NLCs. This could prove that the ETP and CUR-loaded in the NLCs have a synergistic effect: the dosage of both drugs could be reduced to a large content (He et al., Citation2015). This could help with the reduction of the unexpected side effect during the cancer therapy procedure, and could also bring about excellent anti-tumor efficiency.
Conclusions
ETP-CUR-NLCs were designed in this study in order to improve the anti-cancer therapeutic efficacy of the drugs. The optimized ETP-CUR-NLCs have small particle size, positive surface charge and high EE. ETP-CUR-NLCs released in a sustained manner, and showed the highest cytotoxicity in vitro. In vivo tissues distribution and antitumor study illustrated that ETP-CUR-NLCs mostly distributed in tumor tissue, and had the highest antitumor activity on gastric cancer animal model with less dosage than single drug-loaded NLCs and drug solution samples. The resulting dual drugs co-loaded lipid nanoparticles could enhance the anticancer activity and could be used as a promising system or the treatment of gastric cancer.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Ahmad MZ, Alkahtani SA, Akhter S, et al (2015). Progress in nanotechnology-based drug carrier in designing of curcumin nanomedicines for cancer therapy: current state-of-the-art. J Drug Target 11:1–21
- Alexander A, Saraf S, Saraf S. (2015). A comparative study of chitosan and poloxamer based thermosensitive hydrogel for the delivery of PEGylated melphalan conjugates. Drug Dev Ind Pharm 41:1954–61
- Bao X, Gao M, Xu H, et al. (2015). A novel oleanolic acid-loaded PLGA-TPGS nanoparticle for liver cancer treatment. Drug Dev Ind Pharm 41:1193–203
- Chung MY, Lim TG, Lee KW. (2013). Molecular mechanisms of chemopreventive phytochemicals against gastroenterological cancer development. World J Gastroenterol 19:984–93
- Cui SX, Qu XJ, Xie YY, et al. (2006). Curcumin inhibits telomerase activity in human cancer cell lines. Int J Mol Med 18:227–31
- Da W, Zhu J, Wang L, et al. (2015). Curcumin suppresses lymphatic vessel density in an in vivo human gastric cancer model. Tumor Biol 36:5215–23
- Fares MM, Salem MS. (2015). Dissolution enhancement of curcumin via curcumin-prebiotic inulin nanoparticles. Drug Dev Ind Pharm 41:1785–92
- Fatma S, Talegaonkar S, Iqbal Z, et al. (2015). Novel flavonoid-based biodegradable nanoparticles for effective oral delivery of etoposide by P-glycoprotein modulation: an in vitro, ex vivo and in vivo investigations. Drug Deliv 21:1–12
- Guggenheim DE, Shah MA. (2013). Gastric cancer epidemiology and risk factors. J Surg Oncol 107:230–6
- He C, Hu Y, Yin L, et al. (2010). Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 31:3657–66
- He Z, Huang J, Xu Y, et al. (2015). Co-delivery of cisplatin and paclitaxel by folic acid conjugated amphiphilic PEG-PLGA copolymer nanoparticles for the treatment of non-small lung cancer. Oncotarget 6:42150–68
- Jafarieh O, Md S, Ali M, et al. (2015). Design, characterization, and evaluation of intranasal delivery of ropinirole-loaded mucoadhesive nanoparticles for brain targeting. Drug Dev Ind Pharm 41:1674–81
- Jemal A, Bray F, Center MM, et al. (2011). Global cancer statistics. CA Cancer J Clin 61:69–90
- Jiang H, Pei L, Liu N, et al. (2015). Etoposide-loaded nanostructured lipid carriers for gastric cancer therapy. Drug Deliv 13:1–4
- Katas H, Abdul Ghafoor Raja M, Ee LC. (2014). Comparative characterization and cytotoxicity study of TAT-peptide as potential vectors for siRNA and Dicer-substrate siRNA. Drug Dev Ind Pharm 40:1443–50
- Kawadkar J, Pathak A, Kishore R, Chauhan MK. (2013). Formulation, characterization and in vitro–in vivo evaluation of flurbiprofen-loaded nanostructured lipid carriers for transdermal delivery. Drug Dev Ind Pharm 39:569–78
- Kuo YC, Lee CH. (2015). Inhibition against growth of glioblastoma multiforme in vitro using etoposide-loaded solid lipid nanoparticles with p-aminophenyl-α-D-manno-pyranoside and folic acid. J Pharm Sci 104:1804–14
- Ledet G, Pamujula S, Walker V, et al. (2014). Development and in vitro evaluation of a nanoemulsion for transcutaneous delivery. Drug Dev Ind Pharm 40:370–9
- Li J, Guo X, Liu Z, et al. (2014). Preparation and evaluation of charged solid lipid nanoparticles of tetrandrine for ocular drug delivery system: pharmacokinetics, cytotoxicity and cellular uptake studies. Drug Dev Ind Pharm 40:980–7
- Liu H, Xu H, Wang Y, et al. (2012). Effect of intratumoral injection on the biodistribution and therapeutic potential of novel chemophor EL-modified single-walled nanotube loading doxorubicin. Drug Dev Ind Pharm 38:1031–8
- Müller RH, Radtke M, Wissing SA. (2002). Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm 242:121–8
- Nekkanti V, Venkatesan N, Wang Z, et al. (2015). Improved oral bioavailability of valsartan using proliposomes: design, characterization and in vivo pharmacokinetics. Drug Dev Ind Pharm 41:2077–88
- Ocal H, Arica-Yegin B, Vural I, et al. (2014). 5-Fluorouracil-loaded PLA/PLGA PEG-PPG-PEG polymeric nanoparticles: formulation, in vitro characterization and cell culture studies. Drug Dev Ind Pharm 40:560–7
- Qin L, Wang M, Zhu R, et al. (2013). The in vitro sustained release profile and antitumor effect of etoposide-layered double hydroxide nanohybrids. Int J Nanomedicine 8:2053–64
- Qu CY, Zhou M, Chen YW, et al. (2015). Engineering of lipid prodrug-based, hyaluronic acid-decorated nanostructured lipid carriers platform for 5-fluorouracil and cisplatin combination gastric cancer therapy. Int J Nanomedicine 10:3911–20
- Seyfoddin A, Al-Kassas R. (2013). Development of solid lipid nanoparticles and nanostructured lipid carriers for improving ocular delivery of acyclovir. Drug Dev Ind Pharm 39:508–19
- Shah M, Chuttani K, Mishra AK, Pathak K. (2011). Oral solid compritol 888 ATO nanosuspension of simvastatin: optimization and biodistribution studies. Drug Dev Ind Pharm 37:526
- Shukla P, Mathur V, Kumar A, et al. (2014). Nanoemulsion based concomitant delivery of curcumin and etoposide: impact on cross talk between prostate cancer cells and osteoblast during metastasis. J Biomed Nanotechnol 10:3381–91
- Singh R, Kesharwani P, Mehra NK, et al. (2015). Development and characterization of folate anchored Saquinavir entrapped PLGA nanoparticles for anti-tumor activity. Drug Dev Ind Pharm 41:1888–901
- Snehalatha M, Venugopal K, Saha RN, et al. (2008). Etoposide loaded PLGA and PCL nanoparticles II: biodistribution and pharmacokinetics after radiolabeling with Tc-99m. Drug Deliv 15:277–87
- Tamilvanan S, Kumar BA. (2011). Influence of acetazolamide loading on the (in vitro) performances of non-phospholipid-based cationic nanosized emulsion in comparison with phospholipid-based anionic and neutral-charged nanosized emulsions. Drug Dev Ind Pharm 37:1003–15
- Uehara Y, Inoue M, Fukuda K, et al. (2014). Inhibition of β-catenin and STAT3 with a curcumin analog suppresses gastric carcinogenesis in vivo. Gastric Cancer 18:774–83
- Wang J, Zhu R, Sun X, et al. (2014). Intracellular uptake of etoposide-loaded solid lipid nanoparticles induces an enhancing inhibitory effect on gastric cancer through mitochondria-mediated apoptosis pathway. Int J Nanomedicine 9:3987–98
- Yan C, Gu J, Hou D, et al. (2015). Improved tumor targetability of Tat-conjugated PAMAM dendrimers as a novel nanosized anti-tumor drug carrier. Drug Dev Ind Pharm 41:617–22
- Yordanov G, Skrobanska R, Evangelatov A. (2013). Colloidal formulations of etoposide based on poly(butyl cyanoacrylate) nanoparticles: preparation, physicochemical properties and cytotoxicity. Colloids Surf B Biointerfaces 101:215–22
- Zhang T, Chen J, Zhang Y, et al. (2011). Characterization and evaluation of nanostructured lipid carrier as a vehicle for oral delivery of etoposide. Eur J Pharm Sci 43:174–9
- Zhao XL, Yang CR, Yang KL, et al. (2010). Preparation and characterization of nanostructured lipid carriers loaded traditional Chinese medicine, zedoary turmeric oil. Drug Dev Ind Pharm 36:773–80