Abstract
Clonazepam (CZ) is an anti-epileptic drug used mainly in status epilepticus (SE). The drug belongs to Class II according to BCS classification with very limited solubility and high permeability and it suffers from extensive first-pass metabolism. The aim of the present study was to develop CZ-loaded polymeric micelles (PM) for direct brain delivery allowing immediate control of SE. PM were prepared via thin film hydration (TFH) technique adopting a central composite face-centered design (CCFD). The seventeen developed formulae were evaluated in terms of entrapment efficiency (EE), particle size (PS), polydispersity index (PDI), zeta potential (ZP), and in vitro release. For evaluating the in vivo behavior of the optimized formula, both biodistrbution using 99mTc-radiolabeled CZ and pharmacodynamics studies were done in addition to ex vivo cytotoxicty. At a drug:Pluronic® P123:Pluronic® L121 ratio of 1:20:20 (PM7), a high EE, ZP, Q8h, and a low PDI was achieved. The biodistribution studies revealed that the optimized formula had significantly higher drug targeting efficiency (DTE = 242.3%), drug targeting index (DTI = 144.25), and nose-to-brain direct transport percentage (DTP = 99.30%) and a significant prolongation of protection from seizures in comparison to the intranasally administered solution with minor histopathological changes. The declared results reveal the ability of the developed PM to be a strong potential candidate for the emergency treatment of SE.
Introduction
Status epilepticus (SE) represents a medical emergency that is associated with high morbidity and mortality (Manno, Citation2003). It is defined as continuous or intermittent seizures lasting more than 5 min, without full recovery of consciousness between seizures (Chen & Wasterlain, Citation2006). It requires immediate intervention (Brophy et al., Citation2012) as the longer the seizures, the greater the risk of cerebral damage (Macri, Citation2010). Treatment involves intravenous administration of a central nervous system (CNS) depressant, namely, of benzodiazepine (BDZ) class (Lockey, Citation2002).
Clonazepam (CZ) is a potent, long-acting nitrobenzodiazepine derivative with anticonvulsant, muscle-relaxant, and anxiolytic properties. It increases the effects of δ-aminobutyric acid (GABA) via modulation of the GABA receptor (Nardi et al., Citation2013). Furthermore, CZ offers advantages over other BDZ due to longer duration of action (Rey et al., Citation1999). Clinical studies also revealed that clinical symptoms resolved more completely with CZ (Lockey, Citation2002).
Oral or intravenous administration of CZ releases the drug directly into the peripheral circulation that results into both limited uptake across the blood-brain barrier (BBB) (Vyas et al., Citation2006) and distribution to non-targeted sites which leads to a number of side effects including palpitation, hair loss and anorexia (Roche, Citation2009).
In addition, oral or intravenous administration of the drug to patients suffering acute SE might be impractical or inconvenient. From one side, intravenous administration requires a qualified personnel or a near hospital facility. From the other side, SE may impair the ability of the patient for swallowing tablets (Anon, Citation2015). Thus, intranasal drug delivery would present a competitive pathway for drug targeting. It protects the drug from first-pass elimination (Illum, Citation2003), circumvents the obstacles of BBB via olfactory region allowing direct delivery to the CNS (Pires et al., Citation2009). Moreover, intranasal delivery is considered to be simple, convenient, and cost-effective (Marx et al., Citation2015).
CZ has been previously formulated as intranasal mucoadhesive microemulsion for brain targeting (Vyas et al., Citation2006), it has been formulated also as solid lipid nanoparticles for parental administration. To our knowledge, CZ has not been formulated as polymeric micelles (PM) nanocarriers for intranasal administration. Thus, herein, mixed PM were developed and optimized as another potential system for brain targeting of CZ.
PM are nanoscopic structure formed by amphiphilic block copolymers composed of hydrophilic and hydrophobic chains that self-assemble in water, above a certain concentration named the critical micelle concentration (CMC) (Chiappetta & Sosnik, Citation2007). They consist of an inner core of assembled hydrophobic segments capable of solublizing lipophilic substances and an outer hydrophilic corona serving as a stabilizing interface between the hydrophobic core and the external aqueous environment (Francis et al., Citation2004).
PM have the advantage of by-passing the P-glycoprotein (P-gp) efflux since they are transported into the cells via receptor-mediated endocytosis in contrast to the typical free drug diffusion (Srivalli & Lakshmi, Citation2012). P-gp are drug efflux protein that hinders distribution of many drugs to the brain, intestine, and multidrug-resistant (MDR) tumors (Amin, Citation2013). However, such systems have the drawbacks of formation of aggregates with a large size, which falls outside the apparent preferred size range for drug delivery systems using nanoscale particles and lack of stability in aqueous dispersion leading to phase separation (Oh et al., Citation2004).
So, the aim of the present study was to formulate and optimize stable PM for rapid brain targeting of CZ. PM are expected to provide rapid nose-to-brain delivery with greater transport and resident of the drug in the brain. This can help to increase drug efficacy, reduce side effects, and decrease the dose and dosing frequency. The performance of the prepared micelles was evaluated in vitro using different criteria, ex vivo for cytotoxic properties and in vivo in mice using biodistribution of 99mTc-clonazepam and appropriate pharmacodynamics models.
Materials and methods
Materials
CZ was a kind gift from Amoun Pharmaceuticals (Elabour city, Egypt), poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (Pluronic® L121, Pluronic® P123), acetonitrile and pentylenetetrazole (PTZ) were procured from Sigma Chemicals Company (St. Louis, MO). Spectra/Pore® dialysis membrane (12 000–14 000 molecular weight cutoff) was purchased from Spectrum Laboratories Inc. (Los Angeles, CA). Technetium-99m was eluted as 99mTcO4− from 99Mo/99mTc generator, Monrol Company, Kocaeli, Turkey. Ethanol, disodium hydrogen phosphate, potassium dihydrogen phosphate, and sodium chloride were from El-Nasr Chemical Co. (Cairo, Egypt).
Experimental design
A three-level three-factor central composite face-centered design (CCFD) was applied. The independent variables were; P123 concentration (X1), Pluronics®:drug ratio (X2), and hydration volume (X3). The levels of each factor were designated as (−1, 0, +1) and their corresponding actual values are shown in . The composition of the 17 formulae of the 33 CCFD is shown in . Analysis of variance (ANOVA) was carried out to estimate the significance of model and terms. Probability p values (p < 0.05) denoted significance.
Table 1. Variables in 33 central composite face-centered design (CCFD) for CZTable Footnotea polymeric micelles.
Table 2. Factors’ levels for the 33 central composite face-centered design (CCFD) used to prepare CZ polymeric micelles.
Preparation of CZ-loaded PM
PM were prepared adopting thin film hydration (TFH) technique (Dua et al., Citation2012). In brief, CZ (10 mg) and mixture of Pluronics® (L121 and P123) – predetermined weights – were accurately weighed and dissolved in acetonitrile (10 ml) in a one liter round-bottomed flask. Acetonitrile was slowly evaporated under vacuum at 50 °C using rotary evaporator (Heidolph VV 2000, Burladingen, Germany) at 90 rpm such that a thin dry film of the components was formed on the inner wall of the flask. The dried thin film was hydrated with the designed amount of distilled water () by rotating the flask in water bath at 30 °C using rotary evaporator at 210 rpm for 1 h under normal pressure. To increase the stability of the formed PM, the obtained dispersion was sonicated for 1 min in a bath sonicator (Crest Ultrasonic Corp., Trenton, NJ).
In vitro evaluation of the formulated PM
Determination of entrapment efficiency
Ethanol was selected as an appropriate solvent for the lysis of the prepared PM (Ryu et al., Citation2000). Total drug content (free + entrapped) of the prepared formulae was determined by dissolving PM (0.5 ml) in ethanol and then measuring the UV absorbance using spectrophotometer (Shimadzu, model UV-1601 PC, Kyoto, Japan) at the predetermined λmax of CZ in ethanol (309 nm) (Patel et al., Citation2012). In order to measure EE%, the PM suspension was filtered through 0.2 μm millipore filter as to remove unentrapped drug (Wiens et al., Citation2004). 0.5 ml of the separated PM were disrupted by sonication with ethanol and the concentration of the entrapped drug was measured spectrophotometrically at the same λmax. The EE% was calculated using the following formula (EquationEquation 1(1) ):
(1)
The measurements were done in triplicates and the mean values ± standard deviation (SD) were calculated.
Determination of particle size (PS), polydispersity index (PDI), and zeta potential (ZP)
The mean PS, PDI, and ZP were determined by Zetasizer Nano ZS (Malvern Instruments Ltd., Worcestershire, UK) at 25 °C. For determining PS, zetasizer system measures the Brownian motion of the particles in the sample using dynamic light scattering (DLS). As for ZP, it is measured using a combination of electrophoresis and laser Doppler velocimetry techniques. These techniques measure how fast a particle moves in a liquid when an electrical field is applied – i.e. its velocity. The formulations were properly diluted with distilled water to have a suitable scattering intensity (Abdelbary & Tadros, Citation2013). The results were recorded in triplicates.
In vitro release
The CZ release from the developed PM was assessed in triplicates using the membrane diffusion technique (Samia et al., Citation2012). Calculated volume of the filtered PM containing 1 mg of the drug according to the predetermined EE, was placed in a dialysis bag (soaked overnight). The bag was then immersed in 50 ml of the release medium in amber colored bottles (due to light sensitivity of the drug) (Shaji & Aditi, n.d.). Because of very limited solubility of the drug, the release medium consisted of ethanol/water mixture in the ratio of 1:1 (Sharma et al., Citation2014). The bottles were then placed in a thermostatically controlled shaking water bath operating at 100 shake per minute and a temperature of 37 ± 0.5 °C (Yang et al., Citation2013). About 3 ml of the release medium were withdrawn at predetermined time intervals (0.25, 0.5, 1, 2, 4, 6, 8 h) and immediately replaced by an equal volume of fresh release medium. Percentage of drug released was calculated and plotted versus time. The release of the drug solution was done simultaneously. The drug release profiles were fitted to zero, first, and Higuchi diffusion models (Higuchi, Citation1963). The model with the highest coefficient of determination (R2) was considered the best fitting. The time required for the release of 50% of the loaded drug (t50%) was calculated and checked statistically.
Selection of the optimized PM formula
Desirability was calculated using Design-Expert® software (Version 7, Stat-Ease Inc., Minneapolis, MN) and considered to optimize the studied responses depending on the provided results. The significant responses were taken into considerations while the non-significant factors were not. The PM formula with the highest desirability value (close to 1) was taken for further investigation.
Differential scanning calorimetry (DSC)
Samples of pure drug, components (Pluronic® P123 and Pluronic® L121) and drug-loaded PM (PM7) were heated in an aluminum pan at a rate of 5 °C/min in an atmosphere of nitrogen to 400 °C and the thermograms were recorded (Salama et al., Citation2012).
Transmission electron microscopy (TEM)
One drop of the optimized formula (PM7) was placed on a copper grid and the excess was removed using a filter paper and left to dry at room temperature. Then, one drop of phosphotungstic acid aqueous solution (2%, w/v, negative staining) was added and the excess was similarly removed and similarly dried. Finally, the grid was examined under a transmission electron microscope (Jeol JEM 2100, Tokyo, Japan).
Effect of storage
The investigated formula (PM7) was assessed following storage at controlled room temperature (25 °C ± 2) over 4 weeks (Han et al., Citation2009). At the end of the storage period, the PM were evaluated with respect to their appearance, EE%, PS, and Q8 h. Statistical analysis of the obtained results was performed by Student’s t-test using SPSS 17.0® software (SPSS Inc., Chicago, IL). Difference at p < 0.05 was considered significant. The release profile of the stored PM was compared to that of the freshly prepared ones according to the model independent mathematical approach of Moore & Flanner (Citation1996). The similarity factor (f2) was calculated according to the following equation (EquationEquation 2(2) ):
(2)
where n is the number of sampling points, Rt and Tt are the mean percent released from reference (fresh) and from test (stored) at time t. An f2 value ≥ 50 indicates that the release profiles are similar, whereas smaller values may imply dissimilar release profiles.
Ex vivo assessment of nasal cytotoxicity
Histopathological analysis of isolated sheep mucosa treated with CZ-loaded PM of choice was done to assess the possible local cytotoxic effects of the developed formula.
Isolation of sheep nasal mucosa
The head of a 1.3-year-old sheep weighing 55 kg was obtained from the local slaughter house (Cairo, Egypt), within 10 min of the sacrifice. The nasal cavity was exposed with a longitudinal excision through the lateral wall of the nose without damaging the septum (Vandekerckhove et al., Citation2009). The mucous membrane was then carefully removed and immediately washed and immersed in ice-cold Ringer’s solution (Du et al., Citation2006).
Application of PM and controls
Three segments from each of the anterior and posterior sections of the nasal mucosa were separated, each segment was then excised into three pieces. The parts were randomly distributed into three groups so that each group contains equal number of anterior and posterior segments. Group 1 was treated with pH 6.4 phosphate-buffered saline (PBS) (negative control) (Jagtap et al., Citation2015), Group 2 received isopropyl alcohol (positive control) (Kumar et al., Citation2009), and Group 3 was exposed to CZ-loaded PM. All the three groups received equal volumes (2 ml) of the treatment. After 2 h, the pieces were washed with distilled water and preserved in 10% formalin in saline solution (Al-Saraj, Citation2010).
Histopathological studies
Sections of 5 μm were stained with hematoxylin and eosin and then examined using light microscope (Chen et al., Citation2014) (National model 138, China).
Biodistribution in mice
Radiolabelling of CZ
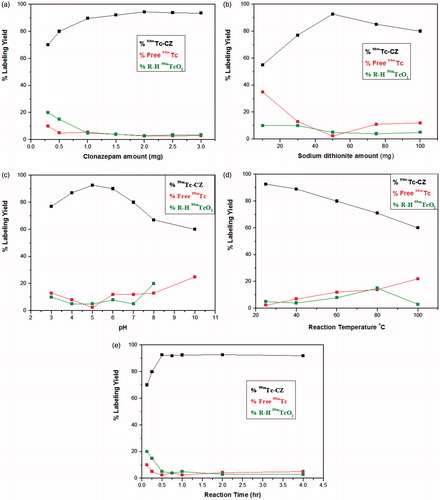
Direct labeling method was used to prepare 99mTc-CZ under reductive conditions in the presence of sodium dithionite (Na2S2O4) as a reducing agent (Geskovski et al., Citation2013). In a 10 ml amber colored penicillin vial, 1.2 ml of CZ solution in absolute ethanol containing 0.3–3 mg of CZ was placed. Then, 1 ml of freshly prepared Na2S2O4 solution in distilled water (containing 10–100 mg of Na2S2O4) was added followed by 100 μl of freshly eluted 99mTc (7.2 MBq) then the pH was adjusted using different volumes of 0.1 M HCl and/or 0.1 M NaOH solutions. The reaction mixture was shaken by electrical vortex and left at ambient temperature for predetermined time intervals before calculating the radiochemical yield.
Different factors that affect the radiolabeling process (sodium dithionite amount, CZ amount, reaction pH, temperature, and time) were studied and optimized to obtain the highest radiochemical yield. Experiments studying each factor were done in triplicate. Differences in the data were evaluated with one-way ANOVA test. The level of significance was set at p < 0.05. Results for p are reported and all the results are given as mean ± standard deviation.
Radiochemical yield assessment
The radiochemical yield and the in vitro stability of 99mTc-CZ complex were assessed by paper chromatography (PC) and thin layer chromatography (TLC).
Acetone was used as a mobile solvent to evaluate the percent of free 99mTcO4− while the reduced hydrolyzed 99mTcO2 was determined using ethanol:water:ammonium hydroxide mixture (2:5:1, v/v/v) (Sakr et al., Citation2013; Essa et al. Citation2015).
Preparation of radiolabelled CZ-loaded PM
The radiolabeled CZ (99mTc-CZ) was used to prepare PM formula of choice (radiolabeled PM7) via TFH technique adopting the aforementioned procedures with slight modification, namely, the radiolabeled drug (99mTc-CZ) was added to the water of hydration. This is due to the interaction of the organic solvent (acetonitrile) with the chemicals used in drug radiolabeling.
Animal study
The protocol of the study (code: PI 1114) was reviewed and approved by Research Ethics Committee-Faculty of Pharmacy, Cairo University (REC-FOPCU) in Egypt.
The studies were carried out using male Swiss albino mice (20–25 g). The animals were housed under constant environmental (room temperature 25 ± 0.5 °C relative humidity; 65% with a 12 h on/off light schedule) and nutritional (fed with standard mice diet with free access to water) conditions throughout the experimental period. On the study day, the mice were divided into 3 groups (18 mice per group). The conscious animals were administered intranasal (I.N) 99mTc-CZ solution (Group A), intranasal (I.N) 99mTc-PM7 formula (Group B), and intravenous (I.V) 99mTc-PM7 formula (Group C) at a CZ dose equivalent to 6 μg/g body weight. For intranasal administration, the mice were held from the back in a slanted position. The preparations were administered at the openings of the nostrils (Abd-Elal et al., Citation2016) using micropipette (200 μl) fixed with low density polyethylene tube having 0.1 mm internal diameter at the delivery site. The procedure was performed gently, allowing the animals to inhale all the preparation (Salama et al., Citation2012). For I.V administration, 99mTc-PM7 was injected through the tail vein of Group C mice. At different time intervals (0.25, 0.5, 1, 2, 4, 8 h), 3 mice were sacrificed. To calculate the percentage uptake, all mice tissues and organs are separated and counted individually for their radioactivity uptake. Since its impossible to separate all muscles, bones and blood of mice, a sample of each of them is separated and a known factor for each of them is used to calculate the whole muscle, bone, and blood radioactivity level. Blood, bone, and muscles were assumed to be 7, 10, and 40% of the total body weight, respectively (Motaleb et al., Citation2012; Rashed et al., Citation2014). Subsequently, the brain was dissected, washed with normal saline, made free from adhering tissue/fluid, and weighed. The weight of the individual tissue/organ was determined. The radioactivity of each sample as well as the background was counted in a well-type NaI (Tl) crystal coupled to SR-7 scaler ratemeter. Percent injected dose per gram (% ID/gram ± SD) in a population of three mice for each time point were reported.
The pharmacokinetics parameters of different CZ preparations were determined for each mice including: maximum CZ radioactivity uptake % injected dose per gram tissue (%ID/g) (blood or brain) (Cmax) and time to reach Cmax (Tmax). The area under the concentration-time curves from zero to 8 h (AUC0–8 h%ID/g) and area under the curve from zero to infinity (AUC0–∞ h%ID/g) were estimated using Kinetica® 2000 software (Innaphase, Philadelphia, PA).
The relative bioavailability of intranasal PM prepared using 99mTc-CZ in comparison to 99mTc-CZ solution was calculated adopting the following formula (Serralheiro et al., Citation2014) (EquationEquation 3(3) ):
(3)
The ability of formula of choice (PM7) for brain targeting following intranasal administration can be calculated in terms of drug targeting efficiency (DTE) (Zhao et al., Citation2007), drug targeting index (DTI) (Khan et al., Citation2009), and nose-to-brain direct transport percentage (DTP) (Zhang et al., Citation2006).
DTE represents time average partitioning ratio of the drug between brain and blood and can be calculated using the following equation (EquationEquation 4(4) ):
(4)
DTI values of CZ formulations were obtained from the following equation (EquationEquation 5(5) ):
(5)
where AUCbrain is the area under brain CZ concentration-time curve from zero to 8 h and AUCblood is the area under blood CZ concentration-time curve from zero to 8 h.
For the direct nose-to-brain transport (DTP) which represents the percentage of drug directly transported to the brain through the olfactory and trigeminal neural pathway, the following equation is used (EquationEquation 6(6) ):
(6)
where Bi.n is the total brain AUC(0–8) following intranasal administration and Bx is a fraction of the brain AUC(0–8) contributed by the systemic circulation through the BBB following the intranasal administration and was calculated according to EquationEquation (7)
(7) :
(7)
where Bi.v is the brain AUC(0–8) following intravenous administration, Pi.v is the blood AUC(0–8) following intravenous administration and Pi.n is the blood AUC(0–8) following intranasal administration.
Pharmacodynamics study
The pharmacodynamics study was conducted according to the protocol described by Florence et al. (Citation2011). Male Swiss albino mice weighing from 25 to 35 g were allocated randomly to four different groups (n = 60). Convulsions were introduced by intraperitoneal injection of PTZ (100 μg/g of body weight) (Jelenkovic et al., Citation2008). Animals were treated with normal saline administered intransally as negative control and with different CZ preparations, namely, CZ solution intranasally (CZSi.n), CZ solution intravenously (CZSi.v), and CZ intranasal PM (PM7) in a dose of 4 μg/g of body weight (Coté et al., Citation2013). CZ treatments were administered 15, 30, and 45 min before i.p injection of PTZ. The time required for the onset of seizures from the time of injection of PTZ was recorded and taken as the evaluation parameter. Statistical analysis of the obtained results was performed by ANOVA followed by post-hoc test using SPSS 17.0® software (Chicago, IL).
Results and discussion
Presence of Pluronics® with different hydrophilic–lipophilic balance (HLB) could help in achieving the optimum thermodynamic and kinetic stabilities for the formed micelles. It was assumed that low HLB Pluronics® would increase the thermodynamic stability of the micelles due to the tight hydrophobic interactions with propylene oxide blocks (Dutra et al., Citation2015). On the other hand, the high HLB Pluronics® would increase the kinetic stability of the micelles due to the steric hindrance that minimize micelle aggregation (Lee et al., Citation2011)
The 17 developed formulae were successfully prepared using TFH technique adopting a CCFD. This design requires much fewer experiments than a full-factorial design. Generation and evaluation of the experimental design was carried out using the Design-Expert® software. The design consisted of 8 factorial points, 6 axial points, and 3 center points, giving a total of 17 formulae. The factorial points help in estimating the linear terms and two factor interactions, the axial points help in estimating the quadratic terms, and the center points were repeated three times to estimate the pure experimental uncertainty at the factor levels (Aboelwafa & Makhlouf, Citation2012).
The results of the measured responses are given under the following headings:
In vitro evaluation
Entrapment efficiency
The entrapment efficiency ranged from 12.7% (PM11) to 85.62% (PM7) (). The statistical analysis revealed that the three investigated factors can significantly (p < 0.0001) affect the ability of the drug to be incorporated in the PM formed .The reduced equation, after omitting the non-significant model terms, in terms of coded variables, was as follows:
Table 3. The measured responses of the central composite face-centered design (CCFD) of CZ polymeric micelles (mean ± SD, n = 3).
For X1 (P123 conc.) it was found that increasing P123 conc. would lead to a decrease in the entrapment ability of the drug due to its low lipophilicity (HLB = 8) compared to L121 (HLB = 1). This can be explained as follows, the increase in L121 conc. was associated with the decrease of P123 conc. Abundance of L121 provides higher lipophilicity due to an HLB value of 1 (Batrakova et al., Citation2003) which provides a favorable medium for the incorporation of the water insoluble molecule of CZ (El-Dahmy et al., Citation2014). Similar results were obtained by Xu et al. (Citation2012) who observed the increase in folate loading after incorporation of L121 in the formulated mixed micelles. As for X2 (Pluronics®:drug ratio), it was observed that elevation of Pluronic® amount resulted in higher drug incorporation. This can be explained on the basis that increasing Pluronics® ratio would result in subsequent increase in the presentation of the triblock copolymer L121 amount having longer hydrophobic segments favoring drug interaction. All this in addition to the possible hydrogen bond formed between the drug molecule and the Pluronics® that increases with the increase of their amount (Kim et al., Citation2010).
Regarding X3 (hydration volume): increasing water volume was found to significantly increase entrapment. Enough water molecules must exist in the Pluronic® – water mixture to bind all EO segments. Increasing the water content higher than the amount of water needed to bind EO segments will swell only the shrunk-bulky EO blocks (the PO blocks remain anhydrous) (Kunieda et al., Citation2001). Swelling of the EO blocks gives an increased interface area per EO block which would alter the interface curvature (Svensson et al., Citation2000), so that the Pluronic® micelle aggregates shapes generally appear as spheres entrapping more drug. Adequate precision was calculated by the Design-Expert® software to demonstrate the signal to noise ratio to ensure that the model could be applied to navigate the design space, whereas a ratio greater than 4 is desirable (de Lima et al., Citation2011). Also, predicted R2 was calculated as a measure of how good the model could predict a response value by comparing the calculated value with the adjusted R2 (Annadurai et al., Citation2008). Adequate precision was 35.398 with reasonable difference between the predicted R2 (0.9384) and the adjusted R2 (0.9817).
Particle size (PS), polydispersity index (PDI), and zeta potential (ZP)
The mean PS of the prepared PM formulae ranged from 83.77 nm (PM3) to 132.7 nm (PM6) (). Polynomial analysis using quadratic model revealed the absence of statistical significant between the studied variables. This is expected in case of PS analysis due to separation of the formed PM using 0.2 μm millipore filter extruding all particles above 200 nm. Also it is worth noticing that the difference between the highest PS and the lowest PS is only 48.93 nm.
Concerning PDI, the values obtained ranged between 0.19 and 0.45 () which could be within the acceptable range (Cho et al., Citation2014). Interestingly, polynomial statistical analysis using quadratic model revealed that two of the investigated factors (X1 and X3) can significantly affect PDI values.
The reduced equation, after omitting the non-significant model terms, in terms of coded variables, was as follows:
Knowing that the Mwt of P123 is 5800 (Sang & Coppens, Citation2011) and that of L121 is 4400 (BASF, Citation2004), increasing P123 conc. (X1) was associated with increase in the average Mwt of Pluronics® mixture resulting in less kinetically restricted encapsulation process of the drug on Pluronic® surface so a less uniform distribution of PS (higher PDI) was obtained (Abdelbary et al., Citation2015). As for hydration volume (X3), increased levels of phase volume ratio and water volume decreased the PDI. This might be explained by the formation of more nucleation sites per unit volume of the antisolvent. Hence, less drug molecules precipitated per nucleation site and a more uniform distribution for the PS was obtained resulting in lower PDI (Aghajani et al., Citation2012).
ZP can be considered as an important indicator of physical stability of nanodispersions (Heurtault et al., Citation2003). A higher electric charge on the surface of the nanoparticles will prevent aggregation because of the strong repellent forces among particles giving more stable dispersions (Honary & Zahir, Citation2013). Generally, ZP values above 20 mV indicate that nanosuspensions are well dispersed with considerable stability (Hornig et al., Citation2009). Results of ZP are compiled in , it ranged from −7.12 mV (PM2) to −28 mV (PM15) indicating that some formulae had better stability (higher than 20 mV) than others. Quadratic model analysis of the measured values showed that none of the studied variables (p > 0.05) could significantly affect the ZP of PM.
In vitro release
Release of CZ from PM was done in ethanol in water (1:1). This is due to the very limited solubility of CZ in water, (saturated solubility in water ≈0.00522 mg/ml) (Patel & Purohit, Citation2009). In vitro cumulative release profiles of the drug from different formulations are shown in . CZ release from drug solution was investigated as control. It reached ≈100% within 3 h, this suggested that the drug could freely diffuse through dialysis membrane (Wei et al., Citation2009). Regarding ANOVA analysis of the amount released after 8 h (Q8h) and time required for release of 50% of the drug (t50%), two factors interaction model was adopted. The results revealed that X1 = P123 conc. had a statistical significant change on the measured variables. The reduced equations, after omitting the non-significant model terms, in terms of coded variables, for Q8h and t50% were: Q8h = 78.71–7.52X1 and t50% = 4.18 + 0.8X1, respectively.
Figure 1. In vitro CZ release profiles from investigated polymeric micelle and the drug solution in ethanol:water (1:1) at 37 ± 0.5 °C, mean ± SD, n = 3.
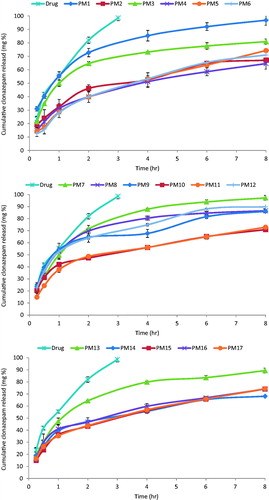
Increasing P123 conc. lead to decrease in release rate of the drug. This could be explained on the basis that P123 has a higher molecular weight in comparison to L121 which means more abundance of O and OH points that enhance attachment to the drug molecule via hydrogen bonds leading to slower release rate (Tang et al., Citation2012). It worth noticing that the PM exhibited biphasic release. This included an initial burst release of the drug located in the shell or at the core–shell interface, followed by a slow release phase corresponding to the diffusion of the drug from the core (Torchilin & Amiji, Citation2010). This indicates that the PM could not only solubilize the poorly soluble drug (CZ), but also sustained its release.
The in vitro drug release profiles of the investigated PM could be best fitted to Higuchi-diffusion model (highest R2, ). This is in line with the results reported earlier by Gaber et al. (Citation2006) who formulated beclomethasone dipropionate as PM intended for pulmonary delivery.
Table 4. Fitting CZ release to zero, first, and Higuchi diffusion models.
Selection of the optimized PM formula
Desirability was estimated to predict the composition of the formula of choice by maximizing EE and Q8h and minimizing PDI and t50%. PS and ZP were not taken into consideration as the results showed statistical insignificant differences (Nour et al., Citation2015). The highest desirability value obtained was 0.921 and it was associated with the independent variables, namely, X1 = 50%, X2 = 40, and X3 = 10 corresponding to formula PM7. Consequently, this formula was selected for further investigation.
Differential scanning calorimetry
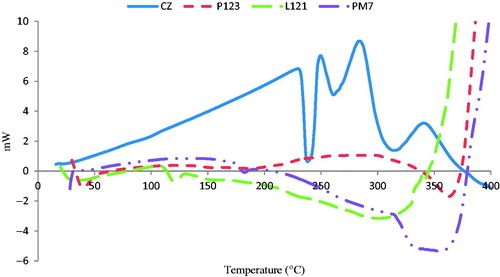
The DSC study was done for CZ, Pluronics® (P123, L121) and for CZ-loaded polymeric micelle candidate formula (PM7).
shows the DSC thermogram of CZ with a sharp characteristic endothermic peak at 238 °C (Roni et al., Citation2011) indicating its crystalline state. Concerning thermograms of P123 and L121, small endothermic peaks were detected at 39.4 °C and 120.35 °C, respectively, indicating their boiling points. Regarding the DSC thermogram of CZ-loaded formula, a very small peak was observed at 182.47 °C indicating a micellization endotherm. This is in accordance with Juggernauth et al. (Citation2011) working on encapsulation of laponite in nanoparticles containing Pluronic® F127. On the other hand, the disappearance of the characteristic endothermic peak of CZ indicates the entrapment of the drug in the developed PM (Leyva‐Gómez et al., Citation2014).
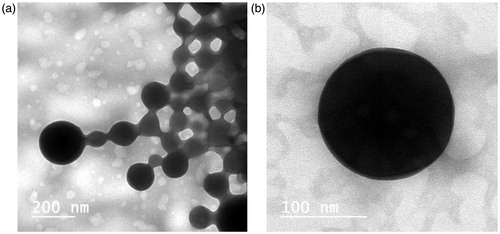
Transmission electron microscopy (TEM)
Photomicrographs of CZ-loaded PM (PM7) are illustrated in . It is clear that the developed micelles were fairly dispersed in aqueous medium () and formed homogeneous small-sized spherical micellar structures with a smooth surface. A closer look on the photomicrograph () would show a perfect spherical shape of the formed PM.
Characteristics of stored PM
There was no observed aggregation or change in the appearance of CZ PM (PM7) after storage at controlled room temperature for 4 weeks. Such findings are in harmony with that obtained by Oh et al. (Citation2004) who found that Pluronics® L121/F127 mixtures (in ratio, 1:1 w/w) formed stable dispersions with small PS. In the present study, the recorded EE, PS, and Q8h for the stored PM7 formula were 82.66% ± 2.18, 131.5 nm ± 5.78, and 98.6% ± 0.15, respectively. The respective values for the freshly prepared PM7 were 85.62% ± 0.63, 124.15 nm ± 5.56 ,and 97.21% ± 2.04. Statistical analysis revealed that there was no significant difference (p > 0.05) in the measured variables of the stored PM when compared to the freshly prepared ones. Calculating similarity factor produced a value of 66.70 indicating that the storage at the specified conditions had no marked effect on the release of the drug (Han et al., Citation2009).
Nasal toxicity
The local toxicity effect of the candidate PM was examined on sheep nasal mucosa in both anterior and posterior regions in comparison to pH 6.4 PBS (negative control) and isopropyl alcohol (positive control). The results are illustrated in and .
Figure 4. Photomicrographs of the anterior segments of sheep nasal mucosa treated with pH 6.4 PBS (negative control, a), isopropyl alcohol (positive control, b), and CZ-loaded polymeric micelles (c) (100×).
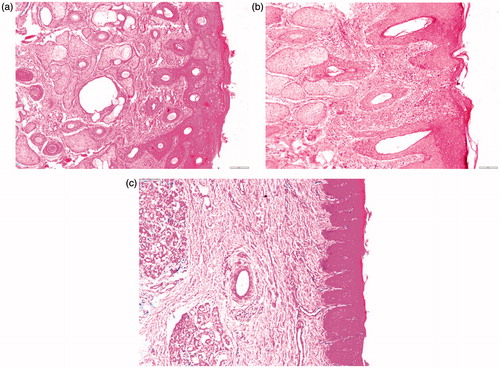
Figure 5. Photomicrographs of the posterior segments of sheep nasal mucosa treated with pH 6.4 PBS (negative control, a), isopropyl alcohol (positive control, b), and CZ-loaded polymeric micelles (c) (100×).
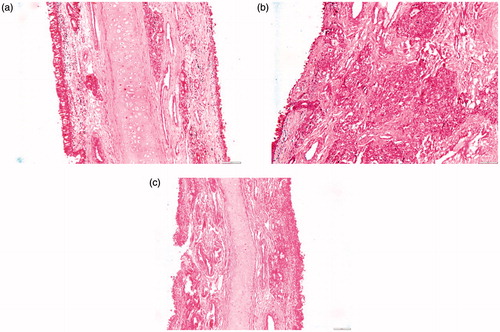
As depicted in , nasal mucosa treated with PBS, revealed no change in the histological structures with normal stratified squamous epithelium and intact underlying connective tissue containing sebaceous glands and hair follicles. Upon exposure to isopropyl alcohol (), sloughing of the epidermal lining with disfiguration of the underlying tissue was observed. Applying formula PM7 to the anterior part of the nasal mucosa showed no change with normal epidermis, dermis, and connective tissue ().
Examining the posterior part, treated with pH 6.4 PBS as a negative control, revealed normal pseudostratified columnar epithelium with submucosa, submucosal glands, and cartilaginous layer (). On exposure to isopropyl alcohol, sloughing of the epithelium was noticed with complete distortion of the submucosal layer (). On the other hand, with PM7 minor thinning of the epithelium was noticed (). This results are in line with Kolsure & Rajkapoor (Citation2012) who formulated zolmitriptan in nanomicellare carrier using Pluronic® F127 and pluronic® F68, histopathological studies revealed the absence of significant effect on the microscopic structure of mucosa as the surface epithelium lining and the granular cellular structure of the nasal mucosa were totally intact.
Radiolabeling of CZ
The highest radiochemical yield of 99mTc-CZ was 94.3 ± 0.25%. Such maximum yield was obtained using 2 mg CZ and 50 mg sodium dithionite. Radiolabeling reaction was done at ambient temperature (27 ± 3 °C) for 30-min reaction time at pH 5 (). 99mTc-CZ complex showed good in vitro stability up to 24 h.
Biodistribution study
The ability and extent of an intranasal formula to deliver the drug to the brain can be mathematically calculated using different parameters, namely, (i) relative bioavailability, (ii) DTE (Zhao et al., Citation2007), (iii) DTI (Khan et al., Citation2009), and (iv) DTP percentage (Zhang et al., Citation2006).
In the current study, radiolabeled preparations were administered to mice as follows: (i) intranasal 99mTc-CZ solution, (ii) intranasal 99mTc-PM7, and (iii) intravenous 99mTc-PM7. The radioactivity was determined in blood and brain at different time intervals up to 8 h.
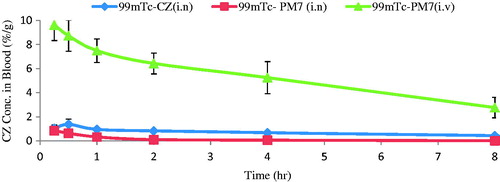
reveals that CZ conc. in brain of mice receiving intranasal 99mTc-PM7 was higher than both intranasal 99mTc-CZ solution and intravenous 99mTc-PM7 (p < 0.05). Concerning blood results (), intravenous 99mTc-PM7 showed the highest blood accumulation of the drug due to direct delivery of the drug to the blood, followed by intranasal 99mTc-CZ solution and then intranasal 99mTc-PM7. These differences were proved to be statistically significant (p < 0.05).
Figure 7. CZ concentration in brain at different time intervals following administration of intranasal 99mTc-CZ solution, intranasal 99mTc-PM7 and intravenous 99mTc-PM7, mean ± SD, n = 3, in male Swiss albino mice.
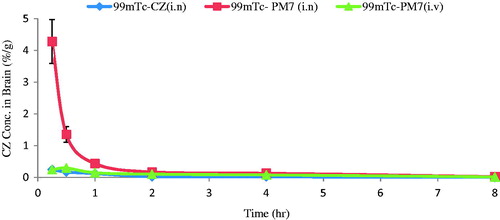
Figure 8. CZ concentration in blood at different time intervals following administration of intranasal 99mTc-CZ solution, intranasal 99mTc-PM7 and intravenous 99mTc-PM7, mean ± SD, n = 3, in male Swiss albino mice.
Brain/blood ratios computed for different radiolabeled preparations () were obtained by dividing brain reading by blood reading for each mouse at each time interval. Statistically higher brain/blood ratios of intranasal 99mTc-PM7 (p < 0.05), in comparison to the intranasally administered solution and to the intravenously administered PM7 formula, indicates the brain targeting ability of the optimized PM formula.
Table 5. Brain/blood distribution of CZ administration as intranasal 99mTc solution, intranasal 99mTc-PM7, and intravenous 99mTc-PM7 in male Swiss albino mice (mean ± SD, n = 3).
The pharmacokinetic behavior of the three administered preparations were mathematically evaluated by the calculation of Cmax, Tmax, and AUC0–∞ for brain and blood (). For the brain, the values were (0.24, 4.28, 0.29) %ID/g, (0.25, 0.25, 0.5) h and (0.32, 2.68, 0.80) h%ID/g for intranasal 99mTc-CZ solution, intranasal 99mTc-PM7 and intravenous 99mTc-PM7, respectively (). The significantly higher Cmax and AUC0–∞ values of the intranasal 99mTc-PM7 confirm direct delivery of the drug to the brain in comparison to the other two administered radiolabeled preparations. This is further proved by the relative bioavailability () which was found to be 812.96% and 11.83% for brain and blood, respectively.
Table 6. Pharmacokinetics parameters for CZ administration as intranasal 99mTc solution, intranasal 99mTc-PM7, and intravenous 99mTc-PM7 in male Swiss albino mice (mean ± SD, n = 3).
Drug administered intranasally can reach brain using mainly two different pathways: (i) either through reaching the systemic circulation then crossing BBB into the brain or (ii) direct nose-to-brain transport from the nasal mucosa through the olfactory region and the trigeminal nerve bypassing the BBB (Illum, Citation2003). Based on the results of the biodistribution study, DTE, DTI, and DTP values were calculated for both intranasal 99mTc-CZ solution and intranasal 99mTc-PM7 (). DTE% represents time average partitioning of drug between brain and blood (Haque et al., Citation2014), while DTI is a measure of the differential targeting between intranasal and intravenous delivery (Taylor et al., Citation2010) and DTP% represents the percent of drug directly transported to the brain by the olfactory and trigeminal neural pathway (Haque et al., Citation2014). Their values were 242.39%, 5.78%, 144.25, 3.46, and 99.3, 70.07 for intranasal 99mTc-PM7 and intranasal 99mTc-CZ solution, respectively.
Table 7. The DTE%, DTI%, and DTP% of intranasal 99mTc-CZ solution and intranasal 99mTc-PM7 polymeric micelles relative to the intravenous 99mTc-PM7 in male Swiss albino mice (mean ± SD, n = 3).
These results are in accordance with Jain et al. (Citation2010) and Kanazawa et al. (Citation2011) who found that intranasal PM have a very high potential for brain targeting of zolmitriptan and coumarin, respectively.
Pharmacodynamic studies
The ability of the preparations to protect mice from PTZ-induced seizures after intravenous and intranasal administrations was evaluated to compare the preparations and their delivery routes (Florence et al., Citation2011). PTZ was administered after predefined intervals of 15-, 30-, and 45-min posttreatment with CZ preparations. The onset of seizures in animals treated with different preparations and routes is shown in . The saline-treated control group produced convulsions with an onset of 58 secs, on average, at the three time intervals. CZ solution (CZS) was administered intravenously 15, 30, and 45 min prior to PTZ challenge. It offered protection against PTZ-induced convulsions by delaying the onset significantly (p < 0.05) for 30 (160.6 ± 7.02 s) and 45 min (139.0 ± 8.18 s) treatment group in comparison with the control group (less than 60 s). However, intravenous CZS failed to induce significant protection after 15 min (p > 0.05).
Table 8. Time (s) for the development of seizures in male Swiss albino mice (mean ± SD, n = 5).
Although intranasal CZ solution offered prolongation of the onset of PTZ-induced seizures at all time intervals in comparison to control groups (), these differences were found to be statistically insignificant. This may be due to the limited ability of the i.n. solution to deliver the drug in adequate conc. to the brain. On the other hand, the offered protection produced by intranasal PM7 is significantly higher (p < 0.05) than all treatment groups and the control at all time intervals. It reached 424.33 ± 31.5, 332.33 ± 41.1, and 314.66 ± 24.58 after 15, 30, and 45 min, respectively. This confirms the ability of the PM to directly deliver the drug to the brain in high concentration depending on the ability of the Pluronics® to overcome the P-gp efflux mechanism, in addition to offering a solubilized form of the drug that allows its immediate uptake and improved efficacy.
Conclusion
Kinetically and thermodynamically stable PM were successfully developed using TFH technique. The ability of the optimized polymeric micelle formula (PM7) with an acceptable PS range and ZP, the lowest PDI and the highest EE for incorporation of the drug was confirmed by TEM and DSC results. PM7 produced minor histopathological changes without affecting the integrity of the sheep nasal mucosa. In addition, the biodistribution and pharmacodynamics studies demonstrated the rapid and effective brain uptake of CZ in mice following intranasal administration of the suggested formula. This may represent an alternative to intravenous administration in the management of acute SE especially when oral administration is not feasible or it is clinically not possible to treat the patient before hospitalization. However, clinical benefits to risk ratio of the developed formulation have to be established for its appropriateness in the clinical practice.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Abd-Elal RM, Shamma RN, Rashed HM, et al. (2016). Trans-nasal Zolmitriptan Novasomes: in-vitro preparation, optimization and in-vivo evaluation of brain targeting efficiency. Drug delivery. doi:10.1080/10717544.2016.1183721
- Abdelbary AA, Al-Mahallawi AM, Abdelrahim ME, et al. (2015). Preparation, optimization, and in vitro simulated inhalation delivery of carvedilol nanoparticles loaded on a coarse carrier intended for pulmonary administration. Int J Nanomedicine 10:6339–53
- Abdelbary GA, Tadros MI. (2013). Brain targeting of olanzapine via intranasal delivery of core-shell difunctional block copolymer mixed nanomicellar carriers: in vitro characterization, ex vivo estimation of nasal toxicity and in vivo biodistribution studies. Int J Pharm 452:300–10
- Aboelwafa AA, Makhlouf AIA. (2012). In vivo evaluation and application of central composite design in the optimization of amisulpride self-emulsifying drug delivery system. Am J Drug Discov Deliv 2:1–16
- Aghajani M, Shahverdi AR, Amani A. (2012). The use of artificial neural networks for optimizing polydispersity index (PDI) in nanoprecipitation process of acetaminophen in microfluidic devices. AAPS PharmSciTech 13:1293–301
- Al-Saraj A. (2010). Use of saturated sodium chloride solution as a tissue fixative. Iraqi J Vet Sci 24:53–8
- Amin L. (2013). P-glycoprotein inhibition for optimal drug delivery. Drug Target Insights 7:27–34
- Annadurai G, Ling LY, Lee JF. (2008). Statistical optimization of medium components and growth conditions by response surface methodology to enhance phenol degradation by Pseudomonas putida. J Hazard Mater 151:171–8
- Anon. (2015). American speech-language-hearing association. 2015 ICD-10-CM Diagnosis Codes,1-38. Available at: http://www.asha.org/uploadedFiles/ICD-10-Codes-SLP.pdf
- BASF. (2004). Pluronic® L121. Technical bulletin, 6, pp. 8–11. Available at: http://worldaccount.basf.com/wa/NAFTA/Catalog/ChemicalsNAFTA/doc4/BASF/PRD/30085763/.pdf?asset_type=pi/pdf&language=EN&urn=urn:documentum:eCommerce_sol_EU:09007bb28001f6f3.pdf
- Batrakova EV, Li S, Alakhov VY, et al. (2003). Optimal structure requirements for Pluronic block copolymers in modifying P-glycoprotein drug efflux transporter activity in bovine brain microvessel endothelial cells. J Pharmacol Exp Therap 304:845–54
- Brophy GM, Bell R, Claassen J, et al. (2012). Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 17:3–23
- Chen H, Chen CC, Acosta C, et al. (2014). A new brain drug delivery strategy: focused ultrasound-enhanced intranasal drug delivery. PLoS One 9:e108880
- Chen JW, Wasterlain CG. (2006). Status epilepticus: pathophysiology and management in adults. Lancet Neurol 5:246–56
- Chiappetta DA, Sosnik A. (2007). Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: improved hydrosolubility, stability and bioavailability of drugs. Eur J Pharm Biopharm 66:303–17
- Cho HJ, Park JW, Yoon IS, et al. (2014). Surface-modified solid lipid nanoparticles for oral delivery of docetaxel: enhanced intestinal absorption and lymphatic uptake. Int J Nanomedicine 9:495–504
- Coté CJ, Lerman J, Anderson B. (2013). A practice of anesthesia for infants and children: expert consult: online and print. Elsevier Health Sciences. 495
- de Lima LS, Araujo MDM, Quináia SP, et al. (2011). Adsorption modeling of Cr, Cd and Cu on activated carbon of different origins by using fractional factorial design. Chem Eng J 166:881–9
- Du G, Gao Y, Nie S, et al. (2006). The permeation of nalmefene hydrochloride across different regions of ovine nasal mucosa. Chem Pharm Bull 54:1722–4
- Dua JS, Rana AC, Bhandari AK. (2012). Liposomes: methods of preparation and applications. Int J Pharm Stud Res III:14–20
- Dutra LMU, Ribeiro MENP, Cavalcante IM, et al. (2015). Binary mixture micellar systems of F127 and P123 for griseofulvin solubilization. Polímeros 25:433–9
- El-Dahmy RM, Elsayed I, Elshafeey AH, et al. (2014). Optimization of long circulating mixed polymeric micelles containing vinpocetine using simple lattice mixture design, in vitro and in vivo characterization. Int J Pharm 477:39–46
- Essa BM, Sakr TM, Khedr MA, et al. (2015). 99mTc-amitrole as a novel selective imaging probe for solid tumor: in silico and preclinical pharmacological study. Eur J Pharm Sci 79:102–9
- Florence K, Manisha L, Kumar BA, et al. (2011). Intranasal clobazam delivery in the treatment of status epilepticus. J Pharm Sci 100:692–703
- Francis MF, Cristea M, Winnik FM. (2004). Polymeric micelles for oral drug delivery: why and how. Pure Appl Chem 76:1321–35
- Gaber NN, Darwis Y, Peh KK, et al. (2006). Characterization of polymeric micelles for pulmonary delivery of beclomethasone dipropionate. J Nanosci Nanotechnol 6:3095–101
- Geskovski N, Kuzmanovska S, Simonoska Crcarevska M, et al. (2013). Comparative biodistribution studies of technetium-99 m radiolabeled amphiphilic nanoparticles using three different reducing agents during the labeling procedure. J Labelled Comp Radiopharm 56:689–95
- Han X, Liu J, Liu M, et al. (2009). 9-NC-loaded folate-conjugated polymer micelles as tumor targeted drug delivery system: preparation and evaluation in vitro. Int J Pharm 372:125–31
- Haque S, Md S, Sahni JK, et al. (2014). Development and evaluation of brain targeted intranasal alginate nanoparticles for treatment of depression. J Psychiatr Res 48:1–12
- Heurtault B, Saulnier P, Pech B, et al. (2003). Physico-chemical stability of colloidal lipid particles. Biomaterials 24:4283–300
- Higuchi T. (1963). Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 52:1145–9
- Honary S, Zahir F. (2013). Effect of zeta potential on the properties of nano-drug delivery systems – a review (Part 2). Trop J Pharm Res 12:265–73
- Hornig S, Bunjes H, Heinze T. (2009). Preparation and characterization of nanoparticles based on dextran-drug conjugates. J Colloids Interface Sci 338:56–62
- Illum L. (2003). Nasal drug delivery-possibilities, problems and solutions. J Control Release 87:187–98
- Jagtap P, Jadhav K, Dand N. (2015). Formulation and ex vivo evaluation of solid lipid nanoparticles (SLNS) based hydrogel for intranasal drug delivery. Int J Med Health Biomed Bioeng Pharm Eng 9:43–53
- Jain R, Nabar S, Dandekar P, et al. (2010). Micellar nanocarriers: potential nose-to-brain delivery of zolmitriptan as novel migraine therapy. Pharm Res 27:655–64
- Jelenkovic AV, Jovanovic MD, Stanimirovic DD, et al. (2008). Beneficial effects of ceftriaxone against pentylenetetrazole-evoked convulsions. Exp Biol Med 233:1389–94
- Juggernauth KA, Gros AE, Meznarich NA, et al. (2011). In situ photogelation kinetics of laponite nanoparticle-based photorheological dispersions. Soft Matter 7:10108–15
- Kanazawa T, Taki H, Tanaka K, et al. (2011). Cell-penetrating peptide-modified block copolymer micelles promote direct brain delivery via intranasal administration. Pharm Res 28:2130–9
- Khan S, Patil K, Yeole P, et al. (2009). Brain targeting studies on buspirone hydrochloride after intranasal administration of mucoadhesive formulation in rats. J Pharm Pharmacol 61:669–75
- Kim S, Shi Y, Kim JY, et al. (2010). Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle-cell interaction. Exp Opin Drug Deliv 7:49–62
- Kolsure PK, Rajkapoor B. (2012). Development of zolmitriptan gel for nasal administration. Asian J Pharm Clin Res 5:1–7
- Kumar A, Sharma P, Chaturvedi A, et al. (2009). Formulation development of sertraline hydrochloride microemulsion for intranasal delivery. Int J ChemTech Res 1:941–7
- Kunieda H, Uddin MH, Horii M, et al. (2001). Effect of hydrophilic- and hydrophobic-chain lengths on the phase behavior of A–B-type silicone surfactants in water. J Phys Chem B 105:5419–26
- Lee ES, Oh YT, Youn YS, et al. (2011). Binary mixing of micelles using Pluronics for a nano-sized drug delivery system. Colloids Surf B Biointerfaces 82:190–5
- Leyva-Gómez G, González-Trujano ME, López-Ruiz E, et al. (2014). Nanoparticle formulation improves the anticonvulsant effect of clonazepam on the pentylenetetrazole-induced seizures: behavior and electroencephalogram. Pharm Nanotechnol 103:2509–19
- Lockey AS. (2002). Emergency department drug therapy for status epilepticus in adults. Emerg Med J 19:96–100
- Macri E. (2010). Management of status epilepticus. Available at: http://www.ohsu.edu/health/_resources/uploads/uploads/SE%20symposium-macri.pdf
- Manno EM. (2003). New management strategies in the treatment of status epilepticus. Mayo Clinic Proc 78:508–18
- Marx D, Williams G, Birkhoff M. (2015). Intranasal drug administration – an attractive delivery route for some drugs. Drug Discov Dev 299–320. Available at: https://pharma.aptar.com/sites/default/files/publications/intranasal_drug_administration_inthec.pdf
- Moore IW, Flanner HH. (1996). Mathematical comparison of curves with an emphasis on in-vitro dissolution profiles. Pharm Technol 20:64–74
- Motaleb MA, El-Kolaly MT, Rashed HM, et al. (2012). Radioiodinated paroxetine, a novel potential radiopharmaceutical for lung perfusion scan. J Radioanal Nucl Chem 292:629–35
- Nardi AE, Machado S, Ferreira Almada L, et al. (2013). Clonazepam for the treatment of panic disorder. Curr Drug Targets 14:353–64
- Nour SA, Abdelmalak NS, Naguib MJ. (2015). Bumadizone calcium dihydrate microspheres compressed tablets for colon targeting: formulation, optimization and in vivo evaluation in rabbits. Drug Deliv 22:286–97
- Oh KT, Bronich TK, Kabanov AV. (2004). Micellar formulations for drug delivery based on mixtures of hydrophobic and hydrophilic Pluronic® block copolymers. J Control Release 94:411–22
- Patel R, Purohit N. (2009). Physico-chemical characterization and in vitro dissolution assessment of clonazepam-cyclodextrins inclusion compounds. AAPS PharmSciTech 10:1301–12
- Patel VB, Dave JB, Patel FM. (2012). Spectrophotometric method for identification and estimation of clonazepam in tablet dosage form. Int J Pharm Res Biosci 1:62–70
- Pires A, Fortuna A, Alves G, et al. (2009). Intranasal drug delivery: how, why and what for? J Pharm Pharm Sci 12:288–311
- Rashed HM, Ibrahim IT, Motaleb MA, et al. (2014). Preparation of radioiodinated ritodrine as a potential agent for lung imaging. J Radioanal Nucl Chem 300:1227–33
- Rey E, Tréluyer JM, Pons G. (1999). Pharmacokinetic optimization of benzodiazepine therapy for acute seizures. Focus on delivery routes. Clin Pharmacokinet 36:409–24
- Roche. (2009). Klonopin tablets (clonazepam): prescription information. pp. 1–19. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/017533s045,020813s005lbl.pdf
- Roni MA, Islam MS, Kibria G, et al. (2011). Effects of poloxamer and HPMC on the dissolution of clonazepam-polyethylene glycol solid dispersions and tablets. Indian J Pharm Educ Res 45:139–44
- Ryu J, Jeong YI, Kim IS, et al. (2000). Clonazepam release from core-shell type nanoparticles of poly(epsilon-caprolactone)/poly(ethylene glycol)/poly(epsilon-caprolactone) triblock copolymers. Int J Pharm 200:231–42
- Sakr TM, Moustapha ME, Motaleb MA. (2013). 99mTc-nebivolol as a novel heart imaging radiopharmaceutical for myocardial infarction assessment. J Radioanal Nucl Chem 295:1511–16
- Salama HA, Mahmoud AA, Kamel AO, et al. (2012). Brain delivery of olanzapine by intranasal administration of transfersomal vesicles. J Liposome Res 22:336–45
- Samia O, Hanan R, Kamal ET. (2012). Carbamazepine mucoadhesive nanoemulgel (MNEG) as brain targeting delivery system via the olfactory mucosa. Drug Deliv 19:58–67
- Sang LC, Coppens MO. (2011). Effects of surface curvature and surface chemistry on the structure and activity of proteins adsorbed in nanopores. Supplement Mater 13:1–8
- Serralheiro A, Alves G, Fortuna A, et al. (2014). Intranasal administration of carbamazepine to mice: a direct delivery pathway for brain targeting. Eur J Pharm Sci 60:32–9
- Shaji J, Aditi P. (n.d.). Intranasal clonazepam mucoadhesive microspheres: factorial designing and primary evaluation. Available at: http://priory.com/pharmacy/clonazepam.htm
- Sharma D, Maheshwari D, Philip G, et al. (2014). Formulation and optimization of polymeric nanoparticles for intranasal delivery of Lorazepam using Box-Behnken design: in vitro and in vivo evaluation. BioMed Res Int 2014:1–14
- Srivalli KMR, Lakshmi PK. (2012). Overview of P-glycoprotein inhibitors: a rational outlook. Brazil J Pharm Sci 48:353–67
- Svensson B, Olsson U, Alexandridis P. (2000). Self-assembly of block copolymers in selective solvents: influence of relative block size on phase behavior. Langmuir 16:6839–46
- Tang J, Bian Z, Hu J, et al. (2012). The effect of a P123 template in mesopores of mesocellular foam on the controlled-release of venlafaxine. Int J Pharm 424:89–97
- Taylor MJ, Tanna S, Sahota T. (2010). In vivo study of a polymeric glucose-sensitive insulin delivery system using a rat model. J Pharm Sci 99:4215–27
- Torchilin V, Amiji MM. (2010). Polymeric micelles as versatile carriers for drugs and nucleic acids delivery. In: Handbook of materials for nanomedicine. Danvers (MA): Pan Stanford Publishing, 190–210
- Vandekerckhove A, Glorieux S, Van den Broeck W, et al. (2009). In vitro culture of equine respiratory mucosa explants. Vet J 181:280–7
- Vyas TK, Babbar AK, Sharma RK, et al. (2006). Intranasal mucoadhesive microemulsions of clonazepam: preliminary studies on brain targeting. J Pharm Sci 95:1–11
- Wei Z, Hao J, Yuan S, et al. (2009). Paclitaxel-loaded pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int J Pharm 376:176–85
- Wiens T, Redelmeier T, Av-Gay Y. (2004). Development of a liposome formulation of ethambutol. Antimicrob Agents Chemother 48:1887–8
- Xu W, Cui Y, Ling P, et al. (2012). Preparation and evaluation of folate-modified cationic pluronic micelles for poorly soluble anticancer drug. Drug Deliv 19:208–19
- Yang ZZ, Zhang YQ, Wang ZZ, et al. (2013). Enhanced brain distribution and pharmacodynamics of rivastigmine by liposomes following intranasal administration. Int J Pharm 452:344–54
- Zhang QZ, Zha LS, Zhang Y, et al. (2006). The brain targeting efficiency following nasally applied MPEG-PLA nanoparticles in rats. J Drug Target 14:281–90
- Zhao Y, Yue P, Tao T. (2007). Drug brain distribution following intranasal administration of Huperzine A in situ gel in rats. Acta Pharmacol Sin 28:273–8