?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
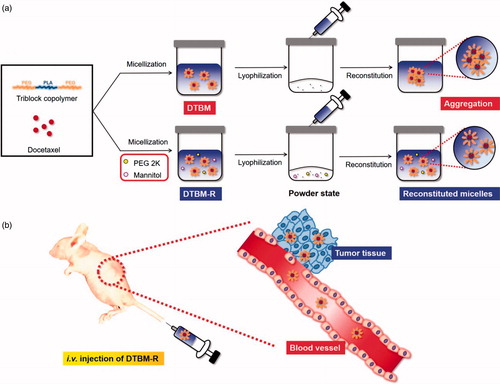
Docetaxel (DTX)-loaded polymeric micelles (DTBM) were formulated using the triblock copolymer, poly(ethylene glycol)–polylactide–poly(ethylene glycol) (PEG–PLA–PEG), to comprehensively study their pharmaceutical application as anticancer nanomedicine. DTBM showed a stable formulation of anticancer nanomedicine that could be reconstituted after lyophilization (DTBM-R) in the presence of PEG 2000 and D-mannitol (Man) as surfactant and protectant, respectively. DTBM-R showed a particle size less than 150 nm and greater than 90% of DTX recovery after reconstitution. The robustly formed micelles might minimize systemic toxicity due to their sustained drug release and also maximize antitumor efficacy through increased accumulation and release of DTX from the micelles. From the pharmaceutical development point of view, DTBM-R showing successful reconstitution could be considered as a potent nanomedicine for tumor treatment.
Introduction
Recently, various nanocarriers have shown potential to overcome the limitations of drugs that exhibit high efficacy but low bioavailability due to poor solubility through tumor-targeted drug delivery (Peer et al., Citation2007; Wicki et al., Citation2015; Choi et al., Citation2016; Shi et al., Citation2017; Choi & Han, Citation2018). There has been great interest in polymeric micelles based on amphiphilic block copolymers as nanocarriers for anticancer therapeutics (Kataoka et al., Citation2001; Gaucher et al., Citation2005). In aqueous environments, amphiphilic block copolymers with a higher concentration than the critical micelle concentration (CMC) form a core–shell structure as a thermodynamically stable state by self-assembly. The hydrophobic core plays a crucial role in the incorporation of hydrophobic anticancer agents (Adams et al., Citation2003). Furthermore, the properties of block copolymers can be optimized by altering their chemical compositions for successful pharmaceutical formulation and drug delivery (Allen et al., Citation1999; Rösler et al., Citation2001; Torchilin, Citation2007).
In previous reports, poly(ethylene glycol)–polylactide–poly(ethylene glycol) (PEG–PLA–PEG) was developed for tumor-targeted drug delivery (Song et al., Citation2016; Hoang et al., Citation2017a,Citationb). As a biocompatible water-soluble polymer, PEG has widely been utilized as a hydrophilic block because of its outstanding water solubility, chain mobility, nontoxicity, and non-immunogenicity (Yamaoka et al., Citation1994). Poly(lactide) (PLA) is a biocompatible and biodegradable polymer with low immunogenicity and favorable mechanical properties for pharmaceutical and biomedical applications (Nampoothiri et al., Citation2010; Rasal et al., Citation2010; Oh, Citation2011). The triblock copolymer was synthesized by Steglich esterification and showed promising properties including high stability, high drug loading efficiency, and successful reconstitution, compared to PEG–PLA diblock copolymer (Song et al., Citation2016). In addition, PEG–PLA–PEG showed potential as an anticancer nanomedicine for metastatic breast cancer by tumor-targeted drug delivery with high therapeutic efficacy (Hoang et al., Citation2017a,Citationb).
From a pharmaceutical point of view, micellar formulations in aqueous solution may result in the degradation of incorporated drugs and the excipients including polymers, during storage. The stability issue might be overcome by using a powder state of micellar formulation that could be reconstituted to a colloidal system by simply adding excipients such as surfactants and protectants. Furthermore, this is a practical approach with respect to in vivo applications, large-scale preparation of the product, and drug stability (Abdelwahed et al., Citation2006; Oh et al., Citation2008, Fonte et al., Citation2016).
In this study, docetaxel (DTX) was loaded into triblock copolymer micelles using PEG–PLA–PEG (DTBM) and the pharmaceutical application of the nanomedicine was comprehensively studied and compared with a commercial product, Nanoxel M, composed of docetaxel loaded polymeric micelle. Preparation of DTBM, physicochemical characterization, optimization of a DTX micellar formulation with successful reconstitution (DTBM-R), in vitro study, and in vivo therapeutic evaluation were conducted to demonstrate the potential of the polymeric nanocarrier using PEG–PLA–PEG from the point of view of pharmaceutical development.
Materials and methods
Materials
Poly(ethylene glycol) 2000 (MW 2 kDa, PEG 2K), dimethylformamide (DMF), PVA, Pluronic F68, PEG 5000 (PEG 5K), PEG 400, tween 80, trehalose, sucrose, lactose, glycine, and D-mannitol (Man) were purchased from Sigma-Aldrich (St. Louis, MO). Tetrahydrofuran (THF), toluene, acetone, and dichloromethane (DCM) were purchased from Honeywell Burdick & Jackson® (Muskegon, MI). Docetaxel (DTX) and the commercial DTX formulation, Nanoxel M were purchased from Samyang Biopharmaceuticals Corp. (Seongnam-si, Gyeonggi-do, Korea). Diethyl ether and hexane were purchased from Samchun Chemical (Hunt Valley, MD). Cyanine 5.5 amine (Cy5.5 amine) was purchased from Lumiprobe (Waltham, MA). KB cells were obtained from Korean Cell Line Bank (Jongno-gu, Seoul, Korea). RPMI 1640 medium, DPBS, penicillin–streptomycin solution, trypsin-EDTA solution, and fetal bovine serum (FBS) were purchased from Welgene (Gyeongsan-si, Gyeongsangbuk-do, Korea). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies, Inc. (Rockville, MD). PEG–PLA–PEG triblock copolymers were synthesized using procedures described previously (Hoang et al., Citation2016, Citation2017b; Song et al., Citation2016). Detailed synthesis and characterization of PEG–PLA–PEG are described in the Supporting Information.
Methods
Preparation of drug-loaded micelles
For the preparation of DTX-loaded micelles, 0.5 mg DTX was mixed with 5 mg of triblock copolymer in 7 ml of a mixture of DMF and distilled water (DMF:DW = 2:5) and dialyzed (MWCO 3.5 kDa, REPLIGEN, Waltham, MA, USA) against distilled water for 24 h. The solutions were centrifuged at 5000 rpm for 5 min to precipitate unloaded drug. Supernatant containing drug-loaded micelles was collected and analyzed.
Measurement of DTX concentration by HPLC
The DTX concentrations of the micelles and other samples were analyzed using a high-performance liquid chromatography system (HPLC, Agilent 1200 series, Agilent Tech., CA) equipped with an auto-injector, high-pressure gradient pump, and UV–Vis detector. A reverse-phase C18 column (ZORBAX Eclipse Plus C18, 4.6 × 150 mm, pore size 5 μm, Agilent Tech., CA) was used for separation. The mobile phase consisting of an isocratic system using acetonitrile:DW (55:45) solvent was delivered at a flow rate of 1 ml/min using a pump. The column effluent was detected at 230 nm, and the concentration of DTX was calculated based on a linear calibration curve of standard DTX. The drug loading content and efficiency were calculated by the following equations:
Particle size measurement
The particle sizes (effective hydrodynamic diameters) of micelles were measured by photon correlation spectroscopy using Zetasizer Nano-ZS (Malvern Instruments, UK) equipped with the Multi Angle Sizing Option (BI-MAS). Measurements were performed in a thermostatic cell at a scattering angle of 173° (backscatter, NIBS Default). Software provided by the manufacturer was used to calculate effective hydrodynamic diameter values. The average Deff. was calculated from three measurements of each sample (n = 3).
Morphology
Diluted DTBM dispersions in DW (0.1 mg/ml) were deposited onto a slide glass and dried in a vacuum. The morphology of DTBM was observed after platinum (Pt) coating using field emission scanning electron microscopy (FE-SEM, Sigma, Carl Zeiss, Germany).
Redispersion of the micellar formulation
To prepare a powder state of docetaxel-loaded triblock micelles (DTBM-R), the effect of various surfactants (PVA, Pluronic F68, PEG 5000 (PEG 5K), PEG 2000 (PEG 2K), PEG 400, and tween 80) and protectants (trehalose, sucrose, lactose, glycine, and D-mannitol (Man)) on redispersion of polymeric micelle without docetaxel (blank micelle) was studied.
Blank micelle (0.1%) was prepared by dialysis method using DW and PBS and collected. Surfactants and protectants were added to blank micelle solution (9 ml) before lyophilization and it was lyophilized in a freeze dryer (FDB-5503, Operon, Gimpo-si, Gyeonggi-do, Korea). The powder state of the formulation was reconstituted by simply adding 9 ml of DW with gentle manual agitation in 10-ml vials (clear 10-ml crimp finish vials, with thicker sealing lip, SUPELCO, Bellefonte, PA) (Oh et al., Citation2008). The rehydration process was observed visually.
Based on the optimization using blank micelle, DTBM-R was prepared with DTBM, PEG 2K (0.5 w/v %), and Man (0.5 w/v %) using the procedure described above. Briefly, DTBM, PEG 2000, and Man were mixed homogenously in 10 ml glass vials before lyophilization. DTBM was reconstituted by gentle agitation after addition of 9 ml of DW. Particle size and DTX recovery of the formulations were compared using DLS and HPLC, respectively.
Drug release profile
To determine the drug release, DTBM-R and the commercial product (Nanoxel M) with 25 mg of DTX were dispersed in PBS at pH 7.4 and transferred to a Spectra/Por dialysis membrane tube with a molecular weight cutoff of 3500. Each membrane tube was immersed in a vial containing 25 ml of 0.9% NaCl solution. The release of DTX from DTBM-R was tested under mechanical shaking (100 rpm) at 37 °C. At predetermined time intervals, 1 ml samples of the outer phase of the dialysis membrane were collected for analysis of drug concentration and replaced with the same amount of fresh medium to maintain a sink condition (Sandhu et al., Citation2015). The released DTX in the continuous outer phase was evaluated by HPLC analysis.
In vitro anticancer effect
KB cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum and grown in a humidified incubator at 37 °C in a 5% CO2 atmosphere. Cells were harvested from growing monolayers and seeded into 96-well plates (5000 cells in 100 μl of RPMI 1640 per well) for 24 h prior to cytotoxicity tests. After the incubation, the media was removed, and cells were washed with DPBS. Cells were treated with the Nanoxel M, DTBM, or DTBM-R at different concentrations by incubation at 37 °C in 5% CO2 for 48 h. The viability of KB cells was determined by CCK assay. Briefly, fresh medium containing CCK solution (10 vol%) was added to each well and the plate was incubated for an additional 2 h. The absorbance of each well was then read on a Flexstation 3 microplate reader (Molecular Devices, Sunnyvale, CA) using a wavelength of 450 nm. The viability of cells treated with samples was compared with non-treated cells in the same medium. IC50 values were calculated with GraphPad Prism 5 software. (GraphPad Software, La Jolla, CA, USA).
Animal care
Animal care and all animal experiments were performed in accordance with the National Institute of Health Guidelines Principles of Laboratory Animal Care and the Animal Protection Law in Republic of Korea and were approved by the Institutional Animal Care and Use Committee (IACUC) of Chung-Ang University, Seoul, Republic of Korea. Tumor xenografts were established by subcutaneous injection of 1 × 107 KB cells suspended in 0.1 ml of DPBS into BALB/c nude mice (Orient Bio Inc., Seoul, Korea). Tumor volume was calculated with the following equation: tumor volume = length × (width)2/2 (Duncan, Citation2003; Oh et al., Citation2013, Citation2014; Kwag et al., Citation2014). Studies of biodistribution and anticancer effects of the micelles were started when the tumor volume reached approximately 10–30 mm3.
Biodistribution of drug-loaded micelles
For near-infrared fluorescence real-time tumor imaging, polymeric micelles containing 10 wt% of Cy5.5–PEG–PLA–PEG and 90 wt% of PEG–PLA–PEG, which has no functional groups, in 0.9 M NaCl were injected into the tail vein of mice bearing KB tumors. The biodistribution of micelles at different time points after injection was monitored using a Fluorescence In Vivo Imaging System (FOBI system, Neo Science, Suwon, Korea) with a red channel for Cy5.5. At 24 h post-injection, the tumor and other main organs were isolated to check for accumulation of micelles. The in vivo and ex vivo fluorescence levels were determined with NEOimage software (Neo Science, Suwon, Korea).
In vivo anticancer efficacy and toxicity
BALB/c nude mice bearing tumors were randomly divided into four groups. PEG–PLA–PEG, Nanoxel M, and DTBM-R were injected intravenously into tumor-bearing mice through tail veins at a dose of 2 mg/kg. Mice in the control group received intravenous injection of saline (0.2 ml) into the tail vein. The relative tumor volume (%) was defined as the volume percentage of a tumor at predetermined time intervals (0–15 days) relative to the initial volume of the tumor. Changes in tumor sizes and body weights of mice were monitored every 3 days for 15 days.
Results and discussion
Optimization of the micellar formulation
Considering ‘particle isolation’ hypothesis (Allison et al., Citation2000) and ‘water replacement hypothesis (Crowe et al., Citation1994; Allison et al., Citation1998; Chen et al., Citation2010), the protection level against lyophilization process would be depend on properties and concentrations of the excipients and nanoparticle (Picco et al., Citation2018). To determine the best combination of surfactant and protectants for DTBM-R, the effect of various excipients on micelle reconstitution were checked using polymeric micelle without DTX (blank micelle) by DLS.
Blank micelles prepared by PBS and DW showed approximately 163 nm and 120 nm, respectively. First, various surfactants (0.5 w/v%) were added and lyophilized to check the effect on micelle reconstitution using 0.1% of blank micelle () (Abdelwahed et al., Citation2006; Oh et al., Citation2008; Fonte et al., Citation2016). Reconstituted micelles using PEG 2K showed the most similar particle size to the original micelles among numerous surfactants (). Similarly, various protectants were also applied to blank micelles with relatively high amount (4 w/v%). Even though other protectants showed smaller particle size, glycine and Man were selected as protectants with high potential to enable successful redispersion. Lyophilized micelles including glycine and Man showed proper appearance with ‘good cake’ without any collapsed state that could prevent successful reconstitution of the formulation () (Fonte et al., Citation2016).
Figure 1. Optimization of the formulation by checking the particle size of reconstituted blank micelles using DLS: effect of various (a) surfactants (0.5%) and (b) protectants (4%) on the reconstitution of blank micelles (0.1%) made by PBS (blank micelle/PBS) and DW (blank micelle/DW). (c) Particle size of the reconstituted blank micelles depending on the amount of PEG 2K. Effect of (d) Man and (e) glycine on the particle size of reconstituted blank micelle. Combination of surfactant (PEG 2K, 0.5%) and protectant (Man or glycine, 0.5%) showed the most similar particle size of reconstituted micelle with the original one without any excipients before lyophilization.
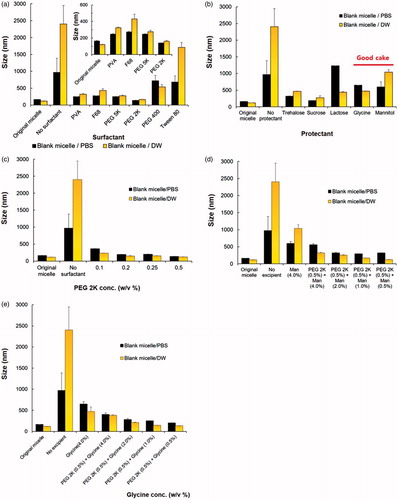
To decrease the content of PEG 2K, particle size of blank micelle after redispersion was compared depending on the concentration of PEG 2K. It was optimized as 0.5 w/v % because reconstituted micelles with a lower concentration of PEG 2K showed a larger particle size (). To determine the protectant, various combination of protectants with PEG 2K (0.5 w/v%) was added to the blank micelles. Since reconstituted micelle prepared using Man (0.5 w/v%) showed the smallest particle size with approximately 125 nm, Man was selected as protectant for the micellar formulation ().
Furthermore, micellar formulation prepared by DW showed smaller particle size than one prepared by PBS. The presence of salt would affect the particle size of the formulation by dehydration of PEG that could result in a modification of inter- and intra-micellar interactions (Carale et al., Citation1994; Jain et al., Citation1999; Mata et al., Citation2005). Considering the tumor targeting ability, DTBM and DTBM-R was prepared by DW to form nanoparticles less than 200 nm (Maeda et al., Citation2000; Fang et al., Citation2011).
Characterization of DTBM
DTX, a common anticancer drug, was chosen as a typical insoluble drug for loading into micelles based on PEG–PLA–PEG triblock copolymers using the dialysis method. DTBMs prepared for 10, 20, and 30 wt% target drug loading amounts are characterized in . As the target loading amount (wt%) of DTX increased, the DTBMs showed slightly decreased particle size, the polydispersity index (PDI), and drug loading efficiency. When the target loading amount was increased to 20 and 30 wt%, the DTX loading content in DTBM decreased compared with a 10 wt% targeted loading amount. Based on these results, the loading capacity of DTBM for DTX is shown to be approximately 7.4 wt%. High DTX loading capacity in the micelles may be due to the hydrophobic PLA block that enabled the physical entrapment of hydrophobic drug in polymeric micelles by hydrophobic interaction between the drug molecule and the hydrophobic core of micelles (Shuai et al., Citation2004; Gao et al., Citation2012; Zhang et al., Citation2014; Hoang et al., Citation2017a; Qi et al., Citation2017). DTBM prepared with 10% DTX loading exhibited particles of 125 nm in size with a spherical structure and narrow size distribution in DW ().
Figure 2. Characterization of DTBM (targeting 10%). (a) Particle size distribution by DLS and (b) morphologies by FE-SEM.
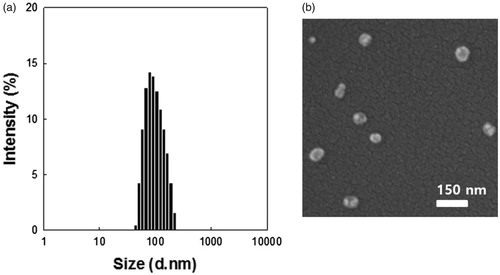
Table 1. Characterization of DTBM (n = 3).
Optimization of DTBM-R
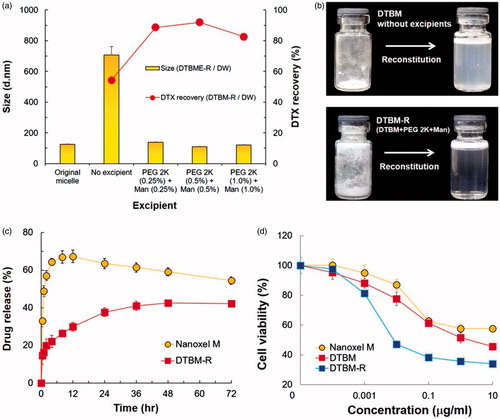
Since PEG 2K and Man were determined as the best excipients for the micellar formulation (), they were further optimized for the formulation of DTBM with successful reconstitution (DTBM-R). The effect of the excipients concentration was compared by particle size of the reconstituted micelles and DTX concentration recovery (). DTBM-R containing PEG 2K (0.5 w/v%) and Man (0.5 w/v%) showed the nano-sized particles of approximately 110 nm and the highest DTX recovery greater than 90%. Successful reconstitution of DTBM-R was also visualized by optical imaging compared with poor reconstitution of DTBM without PEG 2K and Man (). PEG 2K and Man stabilized nanoparticles and prevented their aggregation during lyophilization by acting as a surfactant and a protectant against freezing and drying stresses (Abdelwahed et al., Citation2006; Fonte et al., Citation2016). Because of the presence of PEG on the surface of micelles, the proximity among the micellar particles, and addition of PEG 2K as a surfactant, intra- and inter particular connections of crystallized PEG might be formed during lyophilization, which could prohibit the reconstitution of the micelles (Abdelwahed et al., Citation2006). The addition of Man would maintain the PEG corona in a pseudo-hydrated state through intramolecular hydrogen-bonding that enables the redispersion of micellar formulation (De Jaeghere et al., Citation1999; Zambaux et al., Citation1999).
Figure 3. Optimization and characterization of DTBM-R. (a) Effect of excipients with different concentrations on reconstitution of DTBM-R using PBS and DW that includes PEG 2K (0.5 w/v %) as a surfactant and Man (0.5 w/v %) as a protectant. (b) Comparison of reconstitution: Successful reconstitution of DTBM-R containing PEG and Man and poor reconstitution of lyophilized polymeric micelles without excipients. (c) Drug release profile and (d) cytotoxicity in KB cells treated with the Nanoxel M, DTBM, or DTBM-R for 48 h.
Drug release and cell viability
To investigate DTX release from the formulation, Nanoxel M and DTBM-R were exposed to PBS at pH 7.4 (). Nanoxel M showed a drastic increase in DTX release. It gave a maximum release rate of drug at 12 h followed by slight decrease. At 12 h, the drug released from the commercial product might be entirely dissolved as a supersaturated form in the release medium. Moreover, with the elapsed time, the slight decrease after the maximum at 12 h would be caused by recrystallization or precipitation of the solubilized drug released from micelles (Xie et al., Citation2017; Schver & Lee, Citation2018). In contrast, DTBM-R showed sustained drug release with less than 50% maximum cumulative drug release over 72 h. The sustained release of DTX from DTBM-R would minimize drug loss and reduce systemic toxicity during blood circulation (Qureshi et al., Citation2017; Su et al., Citation2018). This might be attributed to the high stability of DTBM-R caused by PEG–PLA–PEG (2K–6K–2K). As previously reported, the triblock copolymer formed stable polymeric micelles and sustained a particle size less than 200 nm for 7 days because the density and thickness of PEG on the surface of the micelle could provide high steric stabilization (Hoang et al., Citation2017a). Thus, the high stability of the polymeric micelle would prevent burst release of DTX and showed a potential to minimize drug loss and systemic toxicity.
DTBM-R clearly showed higher cytotoxicity than Nanoxel M in the range of concentration of DTX (). Compared to Nanoxel M (IC50 = 8.872 μg/ml), DTBM and DTBM-R showed approximately 28-fold and 129-fold higher efficacy against KB cells (IC50 = 1.924 μg/ml and 0.06874 μg/ml, respectively). Since the triblock copolymer and the other excipients do not appear to induce toxicity (Figure S2), the high toxicity of DTBM and DTBM-R might be attributed to increased translocation due to the nano-sized particles (Peer et al., Citation2007; van Vlerken et al., Citation2007; Hillaireau & Couvreur, Citation2009).
Biodistribution and pharmacodynamic studies
Evaluation of the tumor targeting ability of PEG–PLA–PEG in tumor-bearing nude mice by high-resolution fluorescent imaging using Cy5.5-tagged PEG–PLA–PEG revealed that the micelle gradually accumulated at tumor sites over 24 h (). At 24 h, marked concentration at tumor sites was evident. To identify the biodistribution of micelles, the nude mice were sacrificed after 24 h and excised organs were examined ex vivo. Representative fluorescence images and fluorescence intensity of various organs indicate that most of the micelles were intensively concentrated at tumor sites (). Relative biodistribution of PEG–PLA–PEG micelles was determined by quantitative fluorescence intensity (FI) of tumor and main organs. As expected, accumulation of the micelles at the tumor site was significantly higher than accumulation in other organs; the relative biodistribution of the spleen, liver, and kidney compared to the tumor was 33.3%, 10.1%, and 14.8%, respectively ().
Figure 4. Non-invasive in vivo fluorescent imaging of Cy5.5-tagged micelles after intravenous injection into the tail vein of KB tumor-bearing nude mice. (a) Whole body imaging at predetermined time points after i.v. injection. (b) Ex vivo optical and fluorescent imaging of tumor and organs obtained 24 h post-injection. (c) Relative biodistribution of PEG-PLA-PEG micelles by quantitative fluorescence intensity (FI) of tumors and main organs. The relative biodistribution of the spleen, liver, and kidney compared to the tumor was 33.3%, 10.1%, and 14.8%, respectively. In vivo (d) relative tumor volume and (e) body weight in KB tumor-bearing mice injected with saline (control), PEG–PLA–PEG, Nanoxel M, docetaxel-loaded triblock micelle (DTBM) (equivalent to 2 mg/kg DTX, n = 3). Relative tumor volume was defined as volume ratio of tumor at predetermined time intervals (0–15 days) compared to the initial volume.
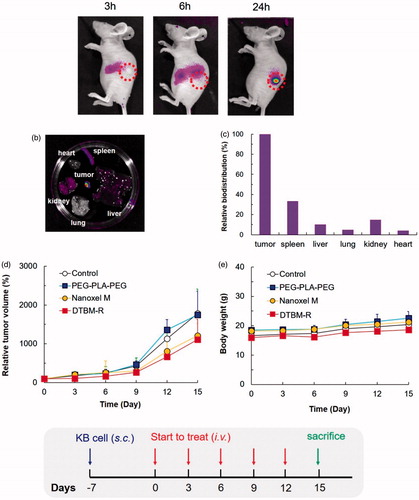
Tumor growth inhibition was studied using a tumor-bearing mouse model with the same cell line (). For the in vivo study, the commercial product and DTBM-R were utilized with doses equivalent to 2 mg/kg DTX. Compared with the commercial product, DTBM-R showed higher tumor growth inhibition due to its tumor targeting ability. Interestingly, DTBM-R and the commercial product showed similar suppression of tumor growth. Furthermore, changes in the body weight of nude mice treated with saline, PEG–PLA–PEG (2K–6K–2K), Nanoxel M, and DTBM-R were negligible. Since the micelles would highly accumulate in the tumor, DTBM-R showed no obvious toxicity throughout the body ().
Based on these results, the pharmaceutical application and tumor growth inhibition by DTBM-R are summarized in . DTBM-R could be reconstituted after lyophilization because the use of PEG 2K and Man as surfactant and protectant respectively stabilized the micelles against freezing and drying stresses and prevented their aggregation during lyophilization. DTBM-R could incorporate DTX into the hydrophobic cores of the micelles formed by hydrophobic PLA blocks. Intravenously injected DTBM-R could circulate within blood vessels without significant extravasation for a longer duration and minimized DTX loss through sustained release due to its highly stable and robust micellar structure (Song et al., Citation2016; Hoang et al., Citation2017a). The circulated DTBM-R could then be distributed and accumulate in tumor sites through the enhanced permeability and retention (EPR) effect (Maeda, Citation2001; Fang et al., Citation2011; Bertrand et al., Citation2014; Danhier, Citation2016; Qureshi et al., Citation2017). At the tumor site, the DTBM-Rs could release DTX and inhibit the tumor with low systemic toxicity.
Conclusion
From the pharmaceutical development point of view, DTBM-R represents a practical formulation of anticancer nanomedicine for storage that could be reconstituted after lyophilization. The robustly formed micelles minimize systemic toxicity due to the sustained drug release. DTBM-R might also maximize antitumor efficacy through increased accumulation and release of DTX. DTBM-R with successful reconstitution could be considered as a potent nanomedicine for tumor treatment.
Supplemental Material
Download MP4 Video (16 MB)Supplemental Material
Download MS Word (746.7 KB)Supplemental Material
Download PDF (349.3 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abdelwahed W, Degobert G, Stainmesse S, Fessi H. (2006). Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev 58:1688–713.
- Adams ML, Lavasanifar A, Kwon GS. (2003). Amphiphilic block copolymers for drug delivery. J Pharm Sci 92:1343–55.
- Allen C, Maysinger D, Eisenberg A. (1999). Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf B Biointerfaces 16:3–27.
- Allison SD, Molina MDC, Anchordoquy TJ. (2000). Stabilization of lipid/DNA complexes during the freezing step of the lyophilization process: the particle isolation hypothesis. Biochim Biophys Acta Biomembr 1468:127–38.
- Allison SD, Randolph TW, Manning MC, et al. (1998). Effects of drying methods and additives on structure and function of actin: mechanisms of dehydration-induced damage and its inhibition. Arch Biochem Biophys 358:171–81.
- Bertrand N, Wu J, Xu X, et al. (2014). Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev 66:2–25.
- Carale TR, Pham QT, Blankschtein D. (1994). Salt effects on intramicellar interactions and micellization of nonionic surfactants in aqueous solutions. Langmuir 10:109–21.
- Chen C, Han D, Cai C, Tang X. (2010). An overview of liposome lyophilization and its future potential. J Control Release 142:299–311.
- Choi JY, Thapa RK, Yong CS, Kim JO. (2016). Nanoparticle-based combination drug delivery systems for synergistic cancer treatment. J Pharm Investig 46:325–39.
- Choi YH, Han H-K. (2018). Nanomedicines: current status and future perspectives in aspect of drug delivery and pharmacokinetics. J Pharm Investig 48:43–60.
- Crowe JH, Leslie SB, Crowe LM. (1994). Is vitrification sufficient to preserve liposomes during freeze-drying? Cryobiology 31:355–66.
- Danhier F. (2016). To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release 244:108–21.
- De Jaeghere F, Allémann E, Leroux J-C, et al. (1999). Formulation and lyoprotection of poly (lactic acid-co-ethylene oxide) nanoparticles: influence on physical stability and in vitro cell uptake. Pharm Res 16:859–66.
- Duncan R. (2003). The dawning era of polymer therapeutics. Nat Rev Drug Discov 2:347–60.
- Fang J, Nakamura H, Maeda H. (2011). The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63:136–51.
- Fonte P, Reis S, Sarmento B. (2016). Facts and evidences on the lyophilization of polymeric nanoparticles for drug delivery. J Control Release 225:75–86.
- Gao X, Huang Y, Makhov AM, et al. (2012). Nanoassembly of surfactants with interfacial drug-interactive motifs as tailor-designed drug carriers. Mol Pharmaceutics 10:187–98.
- Gaucher G, Dufresne MH, Sant VP, et al. (2005). Block copolymer micelles: preparation, characterization and application in drug delivery. J Control Release 109:169–88.
- Hillaireau H, Couvreur P. (2009). Nanocarriers' entry into the cell: relevance to drug delivery. Cell Mol Life Sci 66:2873–96.
- Hoang NH, Lim C, Sim T, et al. (2016). Characterization of a triblock copolymer, poly (ethylene glycol)-polylactide-poly (ethylene glycol), with different structures for anticancer drug delivery applications. Polym Bull 74:1595–1609.
- Hoang NH, Lim C, Sim T, et al. (2017a). Characterization of a triblock copolymer, poly(ethylene glycol)-polylactide-poly(ethylene glycol), with different structures for anticancer drug delivery applications. Polym Bull 74:1595–609.
- Hoang NH, Lim C, Sim T, Oh KT. (2017b). Triblock copolymers for nano-sized drug delivery systems. J Pharm Investig 47:27–35.
- Jain NJ, George A, Bahadur P. (1999). Effect of salt on the micellization of pluronic P65 in aqueous solution. Colloids Surf A Physicochem Eng Asp 157:275–83.
- Kataoka K, Harada A, Nagasaki Y. (2001). Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev 47:113–31.
- Kwag DS, Oh KT, Lee ES. (2014). Facile synthesis of multilayered polysaccharidic vesicles. J Control Release 187:83–90.
- Maeda H. (2001). The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul 41:189–207.
- Maeda H, Wu J, Sawa T, et al. (2000). Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 65:271–84.
- Mata JP, Majhi PR, Guo C, et al. (2005). Concentration, temperature, and salt-induced micellization of a triblock copolymer Pluronic L64 in aqueous media. J Colloid Interface Sci 292:548–56.
- Nampoothiri KM, Nair NR, John RP. (2010). An overview of the recent developments in polylactide (PLA) research. Bioresour Technol 101:8493–501.
- Oh JK. (2011). Polylactide (PLA)-based amphiphilic block copolymers: synthesis, self-assembly, and biomedical applications. Soft Matter 7:5096–108.
- Oh KT, Lee ES, Kim D, Bae YH. (2008). l-Histidine-based pH-sensitive anticancer drug carrier micelle: reconstitution and brief evaluation of its systemic toxicity. Int J Pharm 358:177–83.
- Oh NM, Oh KT, Youn YS, et al. (2013). Poly(L-aspartic acid) nanogels for lysosome-selective antitumor drug delivery. Colloids Surf B Biointerfaces 101:298–306.
- Oh NM, Oh KT, Youn YS, Lee ES. (2014). Artificial nano-pin as a temporal molecular glue for the targeting of acidic tumor cells. Polym Adv Technol 25:842–50.
- Peer D, Karp JM, Hong S, et al. (2007). Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2:751.
- Picco AS, Ferreira LF, Liberato MS, et al. (2018). Freeze-drying of silica nanoparticles: redispersibility toward nanomedicine applications. Nanomedicine 13:179–90.
- Qi D, Gong F, Teng X, et al. (2017). Design and evaluation of mPEG-PLA micelles functionalized with drug-interactive domains as improved drug carriers for docetaxel delivery. J Biomater Sci Polym Ed 28:1538–55.
- Qureshi OS, Kim H-S, Zeb A, et al. (2017). Sustained release docetaxel-incorporated lipid nanoparticles with improved pharmacokinetics for oral and parenteral administration. J Microencapsul 34:250–61.
- Rasal RM, Janorkar AV, Hirt DE. (2010). Poly (lactic acid) modifications. Prog Polymer Sci 35:338–56.
- Rösler A, Vandermeulen GW, Klok H-A. (2001). Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv Drug Deliv Rev 53:95–108.
- Sandhu PS, Beg S, Mehta F, et al. (2015). Novel dietary lipid-based self-nanoemulsifying drug delivery systems of paclitaxel with p-gp inhibitor: implications on cytotoxicity and biopharmaceutical performance. Expert Opin Drug Deliv 12:1809–22.
- Schver GCRM, Lee PI. (2018). Combined effects of supersaturation rates and doses on the kinetic-solubility profiles of amorphous solid dispersions based on water-insoluble Poly(2-hydroxyethyl methacrylate) hydrogels. Mol Pharmaceutics 15:2017–26.
- Shi J, Kantoff PW, Wooster R, Farokhzad OC. (2017). Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer 17:20–37.
- Shuai X, Ai H, Nasongkla N, et al. (2004). Micellar carriers based on block copolymers of poly(ε-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J Control Release 98:415–26.
- Song H-T, Hoang NH, Yun JM, et al. (2016). Development of a new tri-block copolymer with a functional end and its feasibility for treatment of metastatic breast cancer. Colloids Surf B Biointerfaces 144:73–80.
- Su C-Y, Liu J-J, Ho Y-S, et al. (2018). Development and characterization of docetaxel-loaded lecithin-stabilized micellar drug delivery system (LsbMDDs) for improving the therapeutic efficacy and reducing systemic toxicity. Eur J Pharm Biopharm 123:9–19.
- Torchilin VP. (2007). Micellar nanocarriers: pharmaceutical perspectives. Pharm Res 24:1–16.
- Van Vlerken LE, Vyas TK, Amiji MM. (2007). Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm Res 24:1405–14.
- Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. (2015). Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release 200:138–57.
- Xie T, Gao W, Taylor LS. (2017). Impact of Eudragit EPO and hydroxypropyl methylcellulose on drug release rate, supersaturation, precipitation outcome and redissolution rate of indomethacin amorphous solid dispersions. Int J Pharm 531:313–23.
- Yamaoka T, Tabata Y, Ikada Y. (1994). Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J Pharm Sci 83:601–6.
- Zambaux M, Bonneaux F, Gref R, et al. (1999). MPEO-PLA nanoparticles: effect of MPEO content on some of their surface properties. J Biomed Mater Res 44:109–15.
- Zhang P, Lu J, Huang Y, et al. (2014). Design and evaluation of a PEGylated lipopeptide equipped with drug-interactive motifs as an improved drug carrier. AAPS J 16:114–24.