Abstract
To achieve sufficient blood–brain barrier (BBB), penetration is one of the biggest challenges in the development of diagnostic and therapeutic for central nervous system (CNS) disorders. Here, we conducted a systematic review and meta-analysis to assess the preclinical evidence and possible mechanisms of borneol for improving co-administration of CNS drug delivery in animal models. The electronic literature search was conducted in six databases. Fifty-eight studies with 63 comparisons involved 1137 animals were included. Among 47 studies reporting the assessments of CNS drug concentration, 45 studies showed the significant effects of borneol for improving CNS drug delivery (p<.05), whereas 2 studies showed no difference (p>.05). Nineteen comparisons showed borneol up-regulated BBB permeability (p<.05) using brain EB content (n = 8), Rh 123 content (n = 4), brain imaging agent content (n = 2), brain water content (n = 1) and observing ultrastructure of BBB (n = 4), whereas three studies showed no difference or unclear results. Seven studies reported the safety, in which one study showed borneol was reversible changes in the BBB penetration; six studies showed borneol did not increase co-administration of blood drugs concentration of peripheral tissues (p > .05). Effects of borneol are closely associated with inhibition of efflux protein function, releasement of tight junction protein, increasement of vasodilatory neurotransmitters, and inhibition of active transport by ion channels. In conclusion, borneol is a promising candidate for CNS drug delivery, mainly through mediating a multi-targeted BBB permeability.
1. Introduction
A key obstacle for therapeutic drugs administered for central nerve system (CNS) disease is passage across the blood–brain barrier (BBB) (Abbott, Citation2013). The BBB is a specialized non-permeable barrier constituted by endothelial cells, a basal lamina and astrocytic endfeet (Zlokovic, Citation2008). It serves a predominant role in regulating supply of essential nutrients to the brain as well as protecting the CNS from many potentially harmful compounds (Abbott et al., Citation2010). The property of selective impermeable BBB is mainly due to the presence of tight junctions between adjacent endothelial cells and the existence of various BBB transporters, e.g. efflux transporters P-glycoprotein (P-gp). The tight junctions are against the access of about 100% of large-molecule neurotherapeutics and ∼98% of all small-molecule drugs to the brain (Pardridge, Citation2005). The BBB transporters are against the accumulation of a wide range of drugs in brain (Demeule et al., Citation2002). Thus, the BBB maintains the brain homeostasis and also inhibits the entry of potentially useful diagnostic and therapeutic agents, which consequently restricts the therapeutic effects of majority of drugs on many CNS disorders (Abbott et al., Citation2006).
The past 30 years have seen a great deal of research on the CNS drug delivery, and several strategies have been tried to deal with the problem (Banks, Citation2016). For example, highly invasive strategies, i.e. intracerebral or intracerebroventricular administration are useful for local CNS delivery in specific cases e.g. in well-defined tumors, but they are risky, costly, and of limited value for the administration of therapeutic agents that are directed toward less localized diseases such as diffused tumors, Alzheimer’s disease, and multiple sclerosis (Garcia et al., Citation2005). Furthermore, higher concentrations of drug facilitate entry, but efficacy is limited by dose-dependent toxicity of peripheral tissues (Banks, Citation2016). What is more, approaches that disrupt an intact BBB in an attempt to let in a candidate drug also let in circulating substances that are normally excluded by the BBB and can be quite toxic to the CNS (Kroll & Neuwelt, Citation1998). Thus, numerous intravascular drugs delivery strategies which consider BBB as a therapeutic target have been proposed gradually and tested in hope of enhancing BBB penetration instead of disrupting BBB to achieve a widespread transport of the infused drug across the whole brain parenchyma (Tosi et al., Citation2008). Up to now, a number of intravascular strategies have been explored to improve the transport of drug across BBB, such as osmotic and chemical modifications of BBB, enhanced transcellular transport, nanoparticle carriers, and cell-based drug delivery (Hersh et al., Citation2016). This is a promising but difficult area of drug development, as specific features, advantages, and limitations in every strategy (Hersh et al., Citation2016), and few drugs have been successfully applied to the clinic (Zhang et al., Citation2017). This complexity confounds simple strategies for drug delivery to the CNS, but provides a wealth of opportunities and approaches for drug development (Banks, Citation2016).
Borneol, highly lipid-soluble bicyclic terpene chemicals extracted from Cinnamomum camphora (L.) Presl. and Blumea balsamifera (L.) DC. or chemically transformed on the basis of camphor and turpentine oil (State Pharmacopoeia Committee, Citation2010), is widely used as a messenger drug in many traditional Chinese herbal prescriptions such as Angong Niuhuang pill, a well-known formula for treating stroke (Guo et al., Citation2014). According to traditional Chinese medicine (TCM) Emperor-Minister-Assistant-Courier theory, this principle guides the combination of multiple herbal medicines in a specific manner when creating TCM compound prescriptions. Borneol is classified as a ‘Courier herb’ that guides the herbs upward to target organ, especially in the upper part of the body, such as the brain. This studies showed that borneol is not only an effective penetration enhancer through corneal (Yang et al., Citation2009), intestinal mucosa (Zhang et al., Citation2012), and nasal cavity mucosa (Lu et al., Citation2011) but also an effective BBB penetration enhancer for a greater access of drug to the brain (Wang et al., Citation2014). The increased CNS concentrations of carbamazepine and valproate after the co-administration of borneol in epileptic patients with few side effects have been reported in clinical trials (Xu et al., Citation2016; Armulik et al., Citation2010). However, insufficient evidence and unknown mechanism limited the application of borneol in clinic (Zhang et al., Citation2017). Thus, we conducted a preclinical systematic review to provide the preclinical evidence and possible mechanisms of borneol on up-regulation of BBB permeability to enhance CNS drug concentrations.
2. Methods
2.1. Search strategy
The systematically electronic literature search was conducted via PubMed, Chinese National Knowledge Infrastructure, VIP Database, Wanfang database, and Chinese Biomedical Database from their inceptions to December 2017. The search terms were as follows: ‘borneol OR camphol’ AND ‘blood brain barrier’ in Chinese or in English. All searches were limited to animal studies.
2.2. Eligibility criteria
Studies of borneol for CNS drug delivery through enhancing BBB permeability in vivo were included. There was no restriction on animal species or publication status. Eligibility criteria were: (Abbott, Citation2013) borneol for animal, regardless of its mode, dosage and the administration frequency; (Zlokovic, Citation2008) the primary outcome measures were the co-administration of drug concentrations in CNS, and the second outcome measures were the safety of borneol, the various indexes of BBB permeability, and possible mechanisms of borneol for enhancing BBB permeability; (Abbott et al., Citation2010) interventions for control group were isasteric and nonfunctional liquid (normal saline) or no treatment. Exclusion criteria were predefined as follows: (Abbott, Citation2013) case reports, reviews, abstracts, news, comments, editorials, and in vitro studies; (Zlokovic, Citation2008) compared with medicine or another agent with potential similar effect; (Abbott et al., Citation2010) was not tested on the primary and/or second outcome measures; (Pardridge, Citation2005) lack of control group; (Demeule et al., Citation2002) duplicate publication.
2.3. Data extraction
Two authors independently reviewed each included study and extracted following aspects of details: (Abbott, Citation2013) name of first author, year of publication and method of anesthesia and/or model; (Zlokovic, Citation2008) details (species, number, sex, and weight) of animals for each study; (Abbott et al., Citation2010) the use of anesthesia in the experiment and the methods to establish animal models; (Pardridge, Citation2005) the information on the method of administration was obtained from both treatment and control group including drug, dose, mode and frequency; (Demeule et al., Citation2002) the outcome measures and samples for individual comparison were included. A comparison was defined as the qualitative and/or quantitative assessments of co-administration of drug concentrations in CNS and/or the safety of borneol and/or the various indexes of BBB permeability in treatment and corresponding control group after the administration of borneol or vehicle with a given dose, mode, and frequency. In case of lack of vehicle group, the group receiving no adjunct intervention was used as control group for individual comparison. If a drug concentration was used for outcome assessment, both the drug and the method of drug administration were obtained. All available data from quantitative assessments of primary and second outcomes were extracted for every comparison including mean outcome and standard deviation (Abbott et al., Citation2006). The efficacy result was summarized as increased or decreased according to whether a significantly increasing or decreasing outcomes in each study. If there was no statistical difference of effects of borneol between treatment and control groups, the efficacy results were summarized as no difference. In instances of absence of statistical analysis within comparison as well as available original data, the efficacy result of the comparison was listed as “increased?” or “decreased?”
2.4. Quality of evidence
Two authors independently conducted the quality assessment of included studies according to a ten-item modified scale with minor modification: (Abbott, Citation2013) peer-reviewed publication; (Zlokovic, Citation2008) statement of physiological parameters control, such as temperature; (Abbott et al., Citation2010) random allocation; (Pardridge, Citation2005) blinded conduct of the experiments; (Demeule et al., Citation2002) blinded assessment of outcome; (Abbott et al., Citation2006) use of anesthetic without significant intrinsic neuroprotective activity; (Banks, Citation2016) appropriate animal and/or model (brain tumor model, epilepsy, intracranial infection, cognitive dysfunction or Parkinsonism); (Garcia et al., Citation2005) sample size calculation; (Kroll & Neuwelt, Citation1998) compliance with animal welfare regulations; (Tosi et al., Citation2008) statement of potential conflict of interests (Landis et al., Citation2012; Macleod et al., Citation2004).
2.5. Statistical analysis
The statistical analysis was conducted via RevMan version 5.3 software in Copenhagen, Denmark. To estimate the effect of borneol on CNS drug delivery and/or BBB permeability across studies, a summary statistic was calculated for each comparison with 95% confidence intervals by using the random effects method. When the outcome measurements in all included studies in meta-analysis were based on the same scale, weighted mean difference (WMD) was calculated as a summary statistic. On the contrary, when the same outcome measurements were measured in a variety of ways across studies in meta-analysis, standardized mean differences (SMD) was used as a summary statistic. Heterogeneity between study results was investigated based on a standard chi-square test and I2 statistic. A probability value .05 was considered statistically significant.
3. Results
3.1. Study selection
A total of 630 potentially relevant articles were identified, of which 54 were reduplicated and irrelevant articles. By reviewing titles and abstracts, 444 studies were excluded for at least one of following reasons: (Abbott, Citation2013) case reports, reviews, abstracts, news, comments, and editorials; (Zlokovic, Citation2008) not test the effect of borneol on BBB permeability; (Abbott et al., Citation2010) not in vivo studies. After examining the remaining 152 studies through reading the full text, we removed 93 records. Of which, 18 studies were lack of outcome assessments for BBB integrity, 67 studies did not test on co-administration of drug concentrations in CNS and/or the safety of borneol and/or the various indexes of BBB permeability, 2 studies compared with medicine or another agent with potential similar effect, 2 studies were lack of control group, 10 studies were in vitro studies, and 13 studies were duplicate publications. Ultimately, 58 studies (Wang et al., Citation1992; Liang et al., Citation1993; Liu et al., Citation1994; Xu & Wang, Citation1995; Dong et al., Citation2002; Lin et al., Citation2003; Jia et al., Citation2004; Wu et al., Citation2004; Zhang et al., Citation2005; Chen, Citation2005; Zhou et al., Citation2005; Wang, Citation2006; Wang et al., Citation2006; Zheng et al., Citation2007; Xiao et al., Citation2007; Chen et al., Citation2007; Zhang et al., Citation2007; Liu & Gao, Citation2007; Lin et al., Citation2008; Liu et al., Citation2016; Zhou et al., Citation2008; Shi & Zhao, Citation2008; Li et al., Citation2008; Liu et al., Citation2008; Ge et al., Citation2008; Gao et al., Citation2009; Wu et al., Citation2009; Wang et al., Citation2009; Xiao & Ping, Citation2009; Chai et al., Citation2009; Zhu, Citation2009; Wei et al., Citation2010; Zhang, Citation2011; Wu, Citation2011; Zhang et al., Citation2011; Wang et al., Citation2011; Yu et al., Citation2011; Wu et al., Citation2011; Dong et al., Citation2012; Yu et al., Citation2012; Wang et al., Citation2012; Cao, Citation2013; Yu et al., Citation2013; Diao et al., Citation2013; Huang et al., Citation2013; Zhang, Citation2014; Xin et al., Citation2014; Liu, Citation2015; Zhang et al., Citation2015; Guo et al., Citation2015; Yu et al., Citation2015; Zhao et al., Citation2015; Ren, Citation2016; Wei, Citation2016; Wu, Citation2016; Tang et al., Citation2016; Hou et al., Citation2016; Yin et al., Citation2017) were selected for eligibility ().
3.2. Study characteristics
Fifty-eight studies reported effect of borneol CNS drug delivery and/or the BBB permeability involved 1137 animals. Eleven species were used, including Sprague-Dawley (SD) rats (n = 316) (Xu & Wang, Citation1995; Lin et al., Citation2003; Liu et al., Citation2008; Gao et al., Citation2009; Chai et al., Citation2009; Zhu, Citation2009; Zhang, Citation2011; Zhang et al., Citation2011; Dong et al., Citation2012; Yu et al., Citation2012; Yu et al., Citation2013; Diao et al., Citation2013; Zhang et al., Citation2015; Guo et al., Citation2015; Hou et al., Citation2016), Wistar rats (n = 198) (Liang et al., Citation1993; Liu et al., Citation1994; Dong et al., Citation2002; Jia et al., Citation2004; Wang et al., Citation2006; Xiao et al., Citation2007; Gao et al., Citation2009; Zhang et al., Citation2011; Xin et al., Citation2014; Zhao et al., Citation2015; Ren, Citation2016), Kunming mice (n = 298) (Xu & Wang, Citation1995; Dong et al., Citation2002; Jia et al., Citation2004; Li et al., Citation2008; Wu et al., Citation2009; Wu, Citation2011; Yu et al., Citation2011; Wang et al., Citation2012; Huang et al., Citation2013; Wei, Citation2016; Tang et al., Citation2016), ICR mice (n = 58) (Chen, Citation2005; Wang et al., Citation2006; Zhou et al., Citation2008), Balb/c mice (n = 6) (Zhang, Citation2011), NIH rats (n = 20) (Yu et al., Citation2015), FVB rats (n = 12) (Wu, Citation2016), C57BL/6 mice (n = 20) (Yin et al., Citation2017), New Zealand rabbits (n = 44) (Wang et al., Citation1992; Liang et al., Citation1993; Zheng et al., Citation2007; Liu, Citation2015), Japanese White Rabbits (n = 92) (Zhou et al., Citation2005; Zhang et al., Citation2007; Liu & Gao, Citation2007; Shi & Zhao, Citation2008; Li et al., Citation2008; Gao et al., Citation2009), Guinea pigs (n = 20) (Zhang et al., Citation2005) and the remaining animals (n = 58) (Wu et al., Citation2004; Chen et al., Citation2007; Liu et al., Citation2008; Wang et al., Citation2009; Xiao & Ping, Citation2009) that reported as mouse or rabbit but without species details. The weight of rats ranged from 150 to 350 g, the weight of mice ranged from 15 to 30 g and the weight of rabbits ranged from1.8g to 3.0 kg. Chloral hydrate was used in 19 studies (Chen et al., Citation2007; Liu & Gao, Citation2007; Li et al., Citation2008; Gao et al., Citation2009; Zhu, Citation2009; Zhang, Citation2011; Dong et al., Citation2012; Yu et al., Citation2012; Cao, Citation2013; Huang et al., Citation2013; Xin et al., Citation2014; Guo et al., Citation2015; Hou et al., Citation2016), pentobarbital in 5 studies (Wang et al., Citation1992; Zhang et al., Citation2005; Wang, Citation2006; Wang et al., Citation2009; Chai et al., Citation2009), urethane in 3 studies (Zhou et al., Citation2005; Zheng et al., Citation2007; Shi & Zhao, Citation2008), ether in 1 study (Wei, Citation2016), avertin in 1 study (Wu, Citation2016), while no information on anesthetics in the rest 29 studies. As for the method of administration, 21 studies (Wang et al., Citation1992; Liu et al., Citation1994; Dong et al., Citation2002; Lin et al., Citation2003; Wu et al., Citation2004; Zhang et al., Citation2005; Zhou et al., Citation2005; Wang et al., Citation2006; Zhou et al., Citation2008; Ge et al., Citation2008; Zhu, Citation2009; Wu, Citation2011; Yu et al., Citation2011; Dong et al., Citation2012; Cao, Citation2013; Huang et al., Citation2013; Xin et al., Citation2014; Zhang et al., Citation2015) used synthetic borneol, 3 studies (Chen, Citation2005; Yu et al., Citation2013; Yin et al., Citation2017) used L-borneol, 11 studies declared the administration of natural borneol (Chen et al., Citation2007; Liu & Gao, Citation2007; Shi & Zhao, Citation2008; Liu et al., Citation2008; Gao et al., Citation2009; Guo et al., Citation2015; Ren, Citation2016; Tang et al., Citation2016) but without reporting the type of borneol, and the remaining studies used borneol without further information provided. Eighteen studies conducted more than two dose gradients of borneol. Among them, 10 studies (Lin et al., Citation2003; Chen, Citation2005; Ge et al., Citation2008; Wang et al., Citation2011; Yu et al., Citation2013; Zhang et al., Citation2015; Guo et al., Citation2015; Ren, Citation2016; Wei, Citation2016) investigated two dose groups, 7 studies (Zhang et al., Citation2005; Wang et al., Citation2006; Liu et al., Citation2016; Wang et al., Citation2011; Liu, Citation2015; Tang et al., Citation2016; Yin et al., Citation2017) investigated three dose groups, 2 studies (Dong et al., Citation2002; Zhu, Citation2009) investigated four dose groups and 1 study (Yin et al., Citation2017) investigated five dose groups. The mode of borneol application involved oral gavage in 48 studies, intravenous injection in 4 studies (Wang et al., Citation2006; Wu et al., Citation2009; Zhang et al., Citation2015; Hou et al., Citation2016), nasal administration in 4 studies (Zhang et al., Citation2005; Liu et al., Citation2008; Chai et al., Citation2009; Liu, Citation2015) and acupoint injection in 1 study (Lin et al., Citation2003). The frequency of borneol treatment varied from once only (Wang et al., Citation1992; Xu & Wang, Citation1995; Lin et al., Citation2003; Liu et al., Citation2016; Shi & Zhao, Citation2008; Chai et al., Citation2009; Zhang, Citation2011; Wu, Citation2011; Wu et al., Citation2011; Yu et al., Citation2012; Cao, Citation2013; Diao et al., Citation2013; Liu, Citation2015; Guo et al., Citation2015; Wei, Citation2016; Hou et al., Citation2016) to once daily for the duration of 3–14 d (Yu et al., Citation2011; Wang et al., Citation2012; Yu et al., Citation2015; Zhao et al., Citation2015). Borneol compared with vehicle in 33 studies and with no adjunct intervention in other 25 studies (Lin et al., Citation2003; Chen, Citation2005; Chen et al., Citation2007; Lin et al., Citation2008; Liu et al., Citation2008; Wu et al., Citation2009; Chai et al., Citation2009; Wei et al., Citation2010; Zhang, Citation2011; Yu et al., Citation2011; Wang et al., Citation2012; Xin et al., Citation2014; Zhang et al., Citation2015; Yu et al., Citation2015; Ren, Citation2016; Wu, Citation2016; Hou et al., Citation2016; Yin et al., Citation2017). About outcomes for assessing CNS drug delivery and/or BBB permeability, 47 studies used the CNS drug concentration to assess the effects of borneol for CNS drug delivery, including 17 studies (Wang et al., Citation2006; Xiao et al., Citation2007; Zhu, Citation2009; Zhang, Citation2011; Zhang et al., Citation2011; Wang et al., Citation2011; Wu et al., Citation2011; Yu et al., Citation2012; Zhang, Citation2014; Zhang et al., Citation2015; Guo et al., Citation2015; Zhao et al., Citation2015; Wei, Citation2016; Tang et al., Citation2016; Yin et al., Citation2017) reporting the brain concentration, 25 studies (Liu et al., Citation1994; Dong et al., Citation2002; Jia et al., Citation2004; Wu et al., Citation2004; Chen, Citation2005; Wang, Citation2006; Lin et al., Citation2008; Zhou et al., Citation2008; Liu et al., Citation2008; Wu et al., Citation2009; Chai et al., Citation2009; Zhang, Citation2011; Wu et al., Citation2011; Cao, Citation2013; Xin et al., Citation2014; Guo et al., Citation2015; Wei, Citation2016) reporting the brain to serum concentration ratio, 7 studies (Chen et al., Citation2007; Liu & Gao, Citation2007; Gao et al., Citation2009; Wei et al., Citation2010; Diao et al., Citation2013; Liu, Citation2015) reporting the cerebrospinal fluid (CSF) concentration, 4 studies (Zhou et al., Citation2005; Zhang et al., Citation2007; Shi & Zhao, Citation2008; Li et al., Citation2008) reporting the CSF to serum concentration ratio of the drug, and 6 studies (Wu et al., Citation2009; Yu et al., Citation2012; Wang et al., Citation2012; Cao, Citation2013Diao et al., 2013; Xin et al., Citation2014) reporting the blood drug concentration. In addition, nine studies (Liang et al., Citation1993; Xu & Wang, Citation1995; Lin et al., Citation2003; Zhang et al., Citation2005; Zhu, Citation2009; Yu et al., Citation2011; Wu et al., Citation2011; Huang et al., Citation2013; Yin et al., Citation2017) performed the quantitative assessments of brain for EB, and four studies (Yu et al., Citation2011; Wang et al., Citation2012; Yu et al., Citation2013; Wu, Citation2016) for rhodamine 123 (Rh 123), four studies (Zhang et al., Citation2007; Ge et al., Citation2008; Yu et al., Citation2011; Yu et al., Citation2013) reported the ultrastructure of BBB, two studies used imaging such as CT (Wang et al., Citation1992) and immunofluorescence image (Zhang, Citation2011), and one study (Wang et al., Citation2011) for water content (). About possible mechanisms of borneol for enhancing BBB permeability, 7 studies (Xiao et al., Citation2007; Chen et al., Citation2007; Zhang et al., Citation2011; Wang et al., Citation2012; Cao, Citation2013; Diao et al., Citation2013; Yin et al., Citation2017) refer to 5-hydroxytryptamine and histamine, 10 studies (Xiao et al., Citation2007; Chen et al., Citation2007; Zhu, Citation2009; Yu et al., Citation2011; Wang et al., Citation2012; Diao et al., Citation2013; Yu et al., Citation2015; Ren, Citation2016; Tang et al., Citation2016; Yin et al., Citation2017) refer to P-gp, 6 studies (Chen, Citation2005; Xiao et al., Citation2007; Chen et al., Citation2007; Zhou et al., Citation2008; Yu et al., Citation2011; Diao et al., Citation2013) refer to NOS, 3 studies (Wang et al., Citation2009; Chai et al., Citation2009; Yu et al., Citation2011) refer to tight junction, 1 study (Wu, Citation2016) refer to a chloride-permeable channel CIC-3, and 1 study (Yu et al., Citation2013) refer to multidrug resistance 1a (Mdr1a), multidrug resistance 1 b (Mdr1b) and multidrug resistance protein 1 (Mrp1).
Table 1. Summary the efficacy of borneol for improving central nervous system drug delivery.
3.3. Quality of included study
The quality scores of studies included varied from 1 to 5 out of 10 points with the average of 2.8. Among them, 1 study scored 1 point; 22 studies scored 2 points; 24 studies scored 3 points; 8 studies scored 4 points; 3 studies scored 5 points (). Forty-seven studies were peer-reviewed publication and 11 studies were Master’s thesis or PhD thesis. Six studies described the control of temperature. Forty-seven studies declared the random allocation. Forty-five studies described the use of anesthetic without significant intrinsic neuroprotective activity. Sixteen studies stated the compliance with animal welfare regulations. Three studies described the application of animal or model with relevant comorbidities. None of the studies included reported the masked conduct of experiments, the blinded assessments of outcome, a sample size calculation or a statement of potential conflict of interests.
Table 2. Quality assessment of included studies.
3.4. Effectiveness
3.4.1. Co-administration of drug concentrations in CNS
Forty-seven studies reporting the assessments of co-administration of drug concentrations in CNS, of which 45 studies showed the significant effects of borneol for improving CNS drug delivery and 2 studies showed no difference (Chen, Citation2005; Liu, Citation2015). Among the 45 studies, several main categories of drugs were reported, including antineoplastic drugs, antibiotics, antiviral drugs, drugs for epileptic, Parkinsonism and cognition. Some Chinese herbal medicines also were mentioned. Eight types of the drugs were reported more than once. There studies investigated the effect of borneol on tetramethylpyrazine concentration-curve in brain tissue (Wang et al., Citation2006; Li et al., Citation2008; Xiao & Ping, Citation2009) and in CSF (Liu, Citation2015); three studies (Dong et al., Citation2002; Jia et al., Citation2004; Yin et al., Citation2017) on the brain concentration of cisplatin; two studies on the brain concentration (Gao et al., Citation2009) and on the CSF concentration of methotrexate (Guo et al., Citation2015); two studies (Zhang, Citation2014; Zhang et al., Citation2015) on the brain concentration of Kaempferol; two studies (Zhou et al., Citation2008; Zhang et al., Citation2011) on the brain concentration and one study (Zhou et al., Citation2005) on the CSF to serum concentration ratio of carbamazepine over time; three studies (Chen et al., Citation2007; Zhang et al., Citation2007; Liu & Gao, Citation2007) on CSF concentration-curve of valproate; two studies (Dong et al., Citation2012; Yu et al., Citation2012) on the main pharmacokinetic parameters of geniposide in brain tissue; two studies on the brain concentration (Liu et al., Citation2008) and the CSF to serum concentration ratio (Zheng et al., Citation2007) of Salvia miltiorrhiza over time ().
Table 3. The classification of drugs transferred into the brain.
3.4.2. BBB permeability and meta-analysis
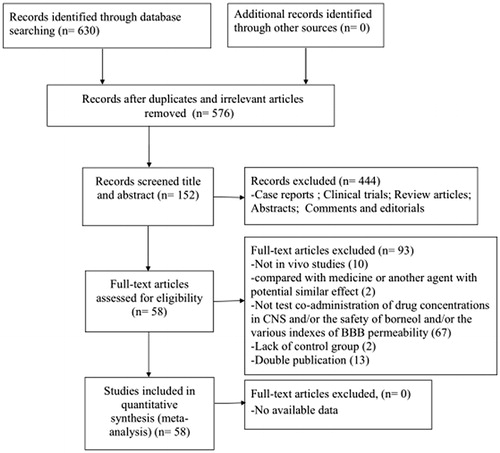
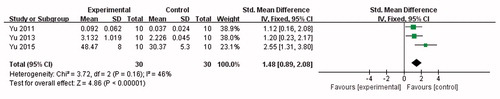
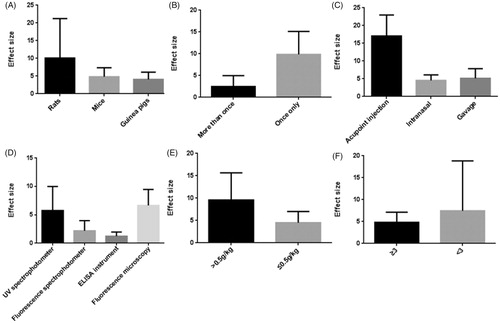
Nine studies (Liang et al., Citation1993; Xu & Wang, Citation1995; Lin et al., Citation2003; Zhang et al., Citation2005; Zhu, Citation2009; Yu et al., Citation2011; Wu et al., Citation2011; Huang et al., Citation2013; Yin et al., Citation2017) used EB content as outcome measures to test the BBB permeability and involved following 11 comparisons: 8 comparisons (Xu & Wang, Citation1995; Lin et al., Citation2003; Zhang et al., Citation2005; Zhu, Citation2009; Yu et al., Citation2011; Wu et al., Citation2011; Huang et al., Citation2013; Yin et al., Citation2017) with increased effects (p<.05), 1 comparison (Xu & Wang, Citation1995) with no difference (p>.05), and 2 comparisons (Liang et al., Citation1993) listed as “increased?” without data. Meta-analysis of 8 (26,28,31,53,59,60,67,80) comparisons with available data showed significant effects of borneol for increasing brain EB content compared with control (n = 141, SMD 5.85, 95% CI: 3.56 ∼ 8.14, p<.00001). There was high heterogeneity among these 8 comparisons (χ2 = 87.54, p<.00001, I2 = 92%). Thus, subgroup analysis was followed according to stratification on animal species, the frequency of administration, the mode of application, the dose of administration and the instrument used for quantification of brain EB content. In the subgroup analyses of these factors, the effect size of rat species was larger than other two animal mice and guinea pigs species (SMD = 11.59 vs. SMD = 4.27 vs. SMD = 4.79, ). The effect size of single administration animals was greater than successive administration animals (SMD = 9.11 vs. SMD = 2.72, ). The mode of application showed great discrepancy in the overall effect of outcome measure, which the administration by acupoint injection with only scale of 7.2% weight accounted for greater effect size than by intranasal administration and gavage (SMD = 17.55 vs. SMD = 4.79 vs. SMD = 4.77, ). The effect size was greater in animals using fluorescence microscopy than in animals using other quantified method, including UV spectrophotometer, fluorescence spectrophotometer, ELISA instrument (). The group that the therapeutic dose of borneol larger than 0.5 g/kg showed greater effect size than the group with 0.5 g/kg or less dose (SMD = 9.37 vs. SMD = 3.93, ). The lower quality studies exhibit larger effect size than the higher ones (SMD = 9.38 vs. SMD = 4.68, ). Four studies (Yu et al., Citation2011; Yu et al., Citation2013; Yu et al., Citation2015; Wu, Citation2016) used Rh 123 content as outcome measures to test the BBB permeability, after removing 1 study (Wu, Citation2016) for concentration-curve of Rh 123, meta-analysis of three studies (Yu et al., Citation2011, Citation2013, Citation2015) indicated that borneol can improve Rh123 concentration in CNS significantly compared with control (n = 30, SMD 1.48, 95% CI: 0.89 ∼ 2.08, p<.00001). There was low heterogeneity among the three included studies (χ2 = 3.72, p = .16, I2 = 46%) (). Compared with controls, two studies (Wang et al., Citation1992; Zhang, Citation2011) showed significant effects of borneol for increasing brain imaging agent entering the brain (p<.05) but failed to obtain primary data for poor analysis, one study (Wang et al., Citation2011) for increasing brain water content (p<.05), four studies (Zhang et al., Citation2007; Ge et al., Citation2008; Yu et al., Citation2011, Citation2013) for increasing the opening effects of the ultrastructure of BBB (p<.05).
Figure 2. Subgroup analysis according to brain Evans blue content. (A) animal species; (B) the frequency of borneol administration; (C) the mode of application; (D) the instrument used for quantification; (E) the therapeutic dose of borneol; (F) the quality of studies. The vertical axis represents effect size point estimates for borneol and 95% confidence intervals.
3.4.3. The safety of co-administration of borneol
Six studies (Wu et al., Citation2009; Yu et al., Citation2012; Cao, Citation2013; Diao et al., Citation2013; Xin et al., Citation2014) indicated that the increased effects of borneol on brain or CSF drug concentration were accompanied by the absence of an increase in the blood drug concentration. One study (Ge et al., Citation2008) reported that the opening of BBB by borneol has been found to be reversible and physiological in accordance with the ultrastructure assessments of BBB, which could last up to 8 h after its intragastric administration in rats.
3.4.4. Possible mechanisms
The possible mechanisms of borneol in an increase of BBB permeability are summarized as follows: (Abbott, Citation2013) inhibition of drug efflux through combining with P-gp competitively and inhibiting its activity (Xiao et al., Citation2007; Chen et al., Citation2007; Zhu, Citation2009; Yu et al., Citation2011; Wang et al., Citation2012; Diao et al., Citation2013; Yu et al., Citation2015; Ren, Citation2016; Tang et al., Citation2016; Yin et al., Citation2017) and decreasing the expressions of both Mdr1a, Mdr1b, and Mrp1 in hippocampus and hypothalamus (Yu et al., Citation2013); (Zlokovic, Citation2008) increasing the amount of 5-hydroxytryptamine and histamine (Xiao et al., Citation2007; Chen et al., Citation2007; Zhang et al., Citation2011; Wang et al., Citation2012; Cao, Citation2013; Diao et al., Citation2013; Yin et al., Citation2017) in the hypothalamus; (Abbott et al., Citation2010) improvement of the circulation by enhancing the expression of NO via up-regulating the expression of NOS (Chen, Citation2005; Xiao et al., Citation2007; Chen et al., Citation2007; Zhou et al., Citation2008; Yu et al., Citation2011; Diao et al., Citation2013); (Pardridge, Citation2005) releasing tight junction between capillary endothelial cells (Wang et al., Citation2009; Chai et al., Citation2009; Yu et al., Citation2011); (Demeule et al., Citation2002) inhibiting the permeability of a chloride-permeable channel CIC-3 (77) (Figure S1).
4. Discussion
4.1. Summary of evidence
This is the first preclinical systematic review to determine the effects of borneol on CNS drug delivery in animal models. Fifty-eight with 1137 animals were selected. The quality of studies included was generally medium. The evidence available from this study showed that the co-administration of borneol is a promising candidate for CNS drug delivery. The effects of borneol are closely associated with the inhibition of efflux protein function, releasement of tight junction protein, increasement of vasodilatory neurotransmitters, and inhibition of active transport by ion channels.
4.2. Limitations
Our study only included two animal species, rodent, and rabbit, which may potentially impose restrictions on the promotion of the findings. The significant heterogeneity across studies indicates that conclusions should have been treated more cautious. The methodological quality of studies included was generally moderate, which is an inherent drawback in the primary study. It was indicated that a lack of blinding outcome assessments attributed to a 27% overestimation of the mean reported effect size (Holman et al., Citation2015). No study reported the data on a sample size calculation, which may inflate the reported effect size. Therefore, the results in this study should be interpreted with caution.
4.3. Implications
In this study, the findings showed the enhanced penetration of a variety of drugs acting on the CNS and increased BBB permeability of EB and Rh 123 after the co-administration of borneol. Thus, we proposed accordingly the co-administration of borneol as a potential approach for effective brain drug delivery with several advantages. First, the administration of borneol is noninvasive and allows for repeated applications by gavage, intravenous injection, and nasal administration. Second, the increased effects of borneol on brain or CSF drug concentration were accompanied by the absence of an increase in the blood drug concentration (Wu et al., Citation2009; Yu et al., Citation2012; Cao, Citation2013; Diao et al., Citation2013; Xin et al., Citation2014), which indicated that the co-administration of borneol did not increase the risk of peripheral adverse effects. Third, the opening of BBB by borneol has been found to be reversible and physiological in accordance with the ultrastructure assessments of BBB, which could last up to 8 h after its intragastric administration in rats (Ge et al., Citation2008) and did not cause an up-regulation of inducible nitric oxide synthase (Baoshe & Qi de, Citation2002), the over-expression that always occurred in the presence of pathological processes, e.g. Hypoxia (Robinson et al., Citation2011). Thus, the co-administration of borneol may be a safe and promising strategy for effective BBB penetration enhancer for CNS drug.
The evidence of mechanisms available from this study showed that borneol enhanced BBB permeability largely through inhibiting efflux protein function, releasing tight junction protein, increasing vasodilatory neurotransmitters, inhibiting active transport by ion channels. Moreover, some studies (Zhang et al., Citation2012; Li et al., Citation2012) reported that borneol can increase the levels of excitatory amino acid greater than the levels of inhibitory amino acids increased in the whole brain, leading to a transient elevation in the excitation ratio, which was conjectured as a reason of the transient and reversible effects of borneol on enhancing BBB permeability. Thus, borneol for opening BBB permeability transiently and reversibly depended on multi-targeted mechanisms.
5. Conclusions
Our findings indicate that borneol is a multi-targeted BBB permeability mediator, suggesting that the co-administration of borneol is a promising candidate for CNS drug delivery. The effects of borneol are closely associated with the inhibition of efflux protein function, the releasement of the tight junction protein, increasement of vasodilatory neurotransmitters, and inhibition of active transport by ion channels.
Supplemental_File.pdf
Download PDF (56 KB)Disclosure statement
The authors declare that there is no conflict of interests regarding the publication of this article.
Additional information
Funding
References
- Abbott NJ, Patabendige AA, Dolman DE, et al. (2010). Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25.
- Abbott NJ, Rönnbäck L, Hansson E. (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41–53.
- Abbott NJ. (2013). Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 36:437–49.
- Armulik A, Genov G, Mäe M, et al. (2010). Pericytes regulate the blood-brain barrier. Nature 468:557–61.
- Banks WA. (2016). From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov 15:275–92.
- Baoshe Z, Qi de L. (2002). The physiological and pathological opening of the blood-brain barrier by the borneol. Trad Chin Drug Res Clin Pharmacol 13:287–8.
- Cao Y. (2013). Experimental Study on the effect of borneol for CPT-11 penetrating across BBB in rats [MD dissertation]. Zhejiang, China: Zhejiang Chinese Medical University.
- Chai G, Pan Y, Li F. (2009). Effect of borneol/mentholum eutectic mixture on nasal-brain delivery of neurotoxin loaded nanoparticles. Zhongguo Zhong Yao Za Zhi 34:698–701.
- Chen Q. (2005). Study on pharmacokinetics of paeonol influenced by borneol and the distribution of paeonol in mice [MD dissertation]. Zhejiang, Hangzhou: Zhejiang University.
- Chen RL, Zhao ZG, Zhang XH, et al. (2007). Effect of Borneol on sodium valproate passing blood-brain barrier. Chin J Rehabil Theory Pract 13:151–3.
- Demeule M, Régina A, Jodoin J, et al. (2002). Drug transport to the brain: key roles for the efflux pump P-glycoprotein in the blood-brain barrier. Vascul Pharmacol 38:339–48.
- Diao Y, Liu XN, Wang OY, et al. (2013). The effect of borneol on penetration of MnTBAP through blood brain barrier in rat. Prog Anat Sci 19:410–2.
- Dong X, Ruan M, Yu B, et al. (2012). Effects of borneol at different doses on concentration of geniposide in rat brains. China Tradit Herb Drugs 43:1366–70.
- Dong XZ, Tang XA, Gao QH, et al. (2002). Study on the Auxo-action of Borneol assisting the penetration of DDB across BBB. Chin Pharm J 37:275–7.
- Gao C, Gao M, Shi WZ, et al. (2009). Experimental study on the effect of borneol for methotrexate penetrating across blood brain barrier. Chin J Clin Pharmacol 25:134–7.
- Garcia E, Andrieux K, Gil S, et al. (2005). Colloidal carriers and Blood-Brain Barrier (Bbb) translocation: a way to deliver drugs to the brain. Int J Pharm 298:274–92.
- Ge C, Han M, Bai R, et al. (2008). Effect of borneol on the ultrastructure of promoting blood brain barriar open. Chin J Integr Med Cardio-/Cerebrovasc Dis 6:1183–4.
- Guo JQ, Zhang R, Duan MM, et al. (2015). Effects of natural borneol on methotrexate penetrating across blood-tumor barrier. Trad Chin Drug Res Clin Pharmacol 1:73–7.
- Guo Y, Yan S, Xu L, et al. (2014). Use of angong niuhuang in treating central nervous system diseases and related research. Evid Based Complement Alternat Med 2014:346918.
- Hersh DS, Wadajkar AS, Roberts NB, et al. (2016). Evolving drug delivery strategies to overcome the blood brain barrier. Curr Pharm Des 22:1177–93.
- Holman L, Head ML, Lanfear R, et al. (2015). Evidence of experimental bias in the life sciences: why we need blind data recording. PLoS Biol 13:e1002190.
- Hou TH, Li XB, Peng CS. (2016). Effects of borneol on the penetration of asiaticoside through the blood brain barrier in rats. Chin Pharm 27:3939–41.
- Huang P, Xia HL, Jia F, et al. (2013). Effect of Benzoinum and Borneo Camphor in different proportion on the mice of cerebral ischemia, anoxia and blood-brain barrier. Pharmacol Clin Chin Mater Clin Med 29:75–8.
- Jia YM, Ding Z, Zeng LY, et al. (2004). Borneol on facilitating permeation for cisplatin through blood brain barrier. Sichuan Med J 25:156–7.
- Kroll RA, Neuwelt EA. (1998). Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery 42:1083–99.
- Landis SC, Amara SG, Asadullah K, et al. (2012). A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490:187–91.
- Li H, Huang J, Shi W, et al. (2008). The pharmacokinetics of temozolomide in rabbits after intragastric administration of Borneol. Proceedings of the Eighth Chinese Conference on Pharmacoepidemiology; Beijing Tiantan Hospital Affiliated to Capital Medical University; Beijing.
- Li WR, Chen RW, Yang L, et al. (2012). Pharmacokinetics of natural borneol after oral administration in mice brain and its effect on excitation ratio. Eur J Drug Metab Pharm 7:39–44.
- Liang MR, Ye SM, Zhang YQ, et al. (1993). The effect of borneol on Evan’s blue staining of rabbit and rat brain tissue. Guangzhou Zhong Yi Xue Yuan Xue Bao 10:211–3.
- Lin XM, Chen HD, Yan JW, et al. (2003). Effect of acupoint injection of camphol-fluid on the permeability of blood-brain barrier in rats. Zhen Ci Yan Jiu 28:99–101.
- Lin ZZ, Yao MC, Lan MX, et al. (2008). Effects of borneol on distribution of sodium ferulate in plasma and brain regions of mice. Chin Tradit Herb Drugs 39:551–6.
- Liu BL. (2015). Study of borneol on nasal absorption of ligustrazine and on nasal mucosal mucin expression level influence [MD dissertation]. Guangdong, China: Guangzhou University of Chinese Medicine;.
- Liu J, Li X, Hu SS, et al. (2016). Studies on the effects of Baras Camphor on the tissue distribution of Salvia miltiorrhiza Bge. In complex Danshen prescription in rabbits. Anal Methods 8:3171–1615.
- Liu N, Gao XC. (2007). The study of blood-brain barrier opening by Borneol. Acta Acad Med Weifang 29:398–400.
- Liu Q, Liang M, Chen Z, et al. (1994). The influence of borneol on the passing of gentamycin through blood-brain barrier. J Guangzhou Univ Tradit Chin Med 11:37–40.
- Liu YD, Sun HJ, Li R, et al. (2008). Influence of borneol on nasal absorption of Ligustrazinee. Zhongguo Zhong Yao Za Zhi 33:259–61.
- Lu Y, Du SY, Chen XL, et al. (2011). Enhancing effect of natural borneol on the absorption of geniposide in rat via intranasal administration. J Zhejiang Univ Sci B 12:143–8.
- Macleod MR, O’Collins T, Howells DW, et al. (2004). Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 35:1203–8.
- Pardridge WM. (2005). The blood-brain barrier: bottleneck in brain drug development. NeuroRx 2:3–14.
- Ren ZY, 2016. Research on the impact of borneol on blood brain barrier and pglycoprotein [MD dissertation]. Shandong, China: Shandong University of Traditional Chinese Medicine.
- Robinson MA, Baumgardner JE, Otto CM. (2011). Oxygen-dependent regulation of nitric oxide production by inducible nitric oxide synthase. Free Radic Biol Med 51:1952–65.
- Shi WZ, Zhao ZG. (2008). Pharmacokinetics study of nimustine (ACNU) through blood brain barrier after intragastric administration of borneol. Chin J Hosp Pharm 28:1933–6.
- State Pharmacopoeia Committee. (2010). The pharmacopoeia of people’s republic of China. Beijing: Chemical Industry Press. 56–136 p.
- Tang DD, Zhang J, Wang JR, et al. (2016). Borneol promotes oral absorption and penetration into brain of puerarin and catalpol. Chin J Chin Mater Med 41:2720–6.
- Tosi G, Costantino L, Ruozi B, et al. (2008). Polymeric nanoparticles for the drug delivery to the central nervous system. Expert Opin Drug Deliv 5:155–74.
- Wang G, Zeng N, Wang J, et al. (2012). Study on the effect of borneol influence on BBB opening for promoting the cerebral absorption of quercetin. Pharmacol Clin Chin Mater Clin Med 28:65–8.
- Wang LP, Feng JF, Hu KL, et al. (2014). Progress in regulation effect of aromatic refreshing traditional Chinese medicine on BBB permeability and its mechanism. Zhongguo Zhong Yao Za Zhi 39:949–54.
- Wang NS, Xie D, Liang MR, et al. (1992). Effect of borneol on rabbit blood brain barrier function by CT dynamic scanning observation. Tradit Chin Drug Res Pharmacol 8:28–31.
- Wang P, Wang C, Lou Y. (2011). Borneol and rhizome of chuanxiong on facilitating permeation for compound Shuyu Jiannao decoction through blood brain barrier. J Hubei Univ Chin Med 13:21–3.
- Wang SX, Miao WL, Fang MF, et al. (2009). Effect of borneol on the tissue distribution of notoginseng R1, ginsenoside Rg1 and Rein rabbits. J Fourth Mil Med Univ 30:2750–2.
- Wang W. (2006). Study on the auxo-action of borneol accelerating transportation of drug through the BBB [MD dissertation]. Zhejiang, China: Zhejiang University.
- Wang Y, Zhang ZY, Xu F, et al. (2006). Effects of borneol on concentration of tetramethylpyrazine in blood and distribution in brain of rat. Zhongguo Yaoye 15:30–1.
- Wei SJ. (2016). Effect of borneol and quercetin on the penetration of erlotinib across the blood-brain barrier in mice [MD dissertation]. Fujian, China: Fujian Medical University.
- Wei Y, Liu P, He X, et al. (2010). Microdialysis and HPLC method for determination of concentration of pantoprazole in rat striatum after combined administration with borneol. Zhongguo Zhong Yao Za Zhi 35:2605–8.
- Wu HB, Wang SN, Shi L, et al. (2009). Effects of borneol on the distribution of azidothymidine palmitate liposomes in mice. Chin Pharmacol J 44:590–3.
- Wu JB. (2016). The role of chloride channels in the enhanced transport of adiamycin and rhodamine across the blood brain barrier induced by borneol [MD dissertation]. Guangdong, China: Jinan University.
- Wu QD. (2011). The studies on the synthesis and pharmacokinetics of borneol derivates [PhD dissertation]. Guangdong, China: Guangzhou University of Chinese Medicine.
- Wu SR, Cheng G, He YX, et al. (2004). Studies on the effects of borneol on the distribution of rifampicin in mice. Chin Pharmacol J 39:289–91.
- Wu X, Ouyang L, Xiang D, et al. (2011). Enhancing effect of borneolum syntheticum and acori talarinowii rhizoma on penetrating blood-brain barrier of hydroxysafflor yellow A. China Tradit Herb Drugs 42:734–7.
- Xiao YQ, Zhang LY, Tang HT, et al. (2007). Study on the role of borneol in improving permeability of arsenic through blood brain barrier. Chin J Neurosurg Dis Res 6:244–6.
- Xiao YY, Ping QN. (2009). Effects of Borneol-β-cyclodextrin inclusion complex on the plasma and brain concentrations of tetramethylpyrazine phosphate in mice. J Chin Pharm Univ 40:412–5.
- Xin HL, He XR, Li W, et al. (2014). The effect of borneol on the concentration of meropenem in rat brain and blood. J Asian Nat Prod Res 16:648–57.
- Xu JY, Zhu LT, Yu YZ, et al. (2016). The effect of Borneol on serum and cerebrospinal fluid sodium valproate concentration in children with intractable epilepsy. Chin J Integr Trad West Med 36:1138–40.
- Xu W, Wang ZR. (1995). Effect of menthol and borneol on the distribution of sulfadiazine sodium and Evan’s blue in the rat and mouse brain. Pharmacol Clin Chin Mater Clin Med 6:31–3.
- Yang H, Xun Y, Li Z, et al. (2009). Influence of borneol on in vitro corneal permeability and on in vivo and in vitro corneal toxicity. J Int Med Res 37:791–802.
- Yin Y, Cao L, Ge HF, et al. (2017). L-Borneol induces transient opening of the blood-brain barrier and enhances the therapeutic effect of cisplatin. Cell Mol Dev Neurosci 28:506–13.
- Yu B, Lv GH, Sun Y, et al. (2011). Effect of electroacupuncture combined with intragastric administration of borneol on the permeability of blood-brain barrier in the mouse. Zhen Ci Yan Jiu 36:335–40.
- Yu B, Ruan M, Dong X, et al. (2012). The influence of borneol treatment interval on the concentration of geniposide in rat brains. Chin Pharmacol Bull 28:862–6.
- Yu B, Ruan M, Dong X, et al. (2013). The mechanism of the opening of the blood-brain barrier by borneol: a pharmacodynamics and pharmacokinetics combination study. J Ethnopharmacol 150:1096–1108.
- Yu H, Zhou GL, Mao BB. (2015). Effect of borneol on the function of P-glycoprotein in blood brain barrier. J Anhui Sci Technol Univ 29:34–8.
- Zhang HY, Wu WK, Lu Q, et al. (2012). Effect of borneol on promoting absorption of oral drugs. Chin J Exp Trad Med Formulae 18:294–7.
- Zhang JQ, Wei YH, Duan HG, et al. (2011). Effects of borneol on the pharmacokinetics and brain tissue distribution of carbamazepine. Chin J Hosp Pharm 31:747–50.
- Zhang L. 2011. Aprotinin-conjugated nanoparticles drug delivery system unite with promete penentration-action of borneol for brain targeting [PhD dissertation]. Shanghai, China: Fudan University.
- Zhang N, Liu P, He XR. (2012). Effect of borneol, moschus, storax, and acorus tatarinowii on expression levels of four amino acid neurotransmitters in the rat corpus striatum. Neural Regen Res 7:440–4.
- Zhang Q, Wu D, Wu J, et al. (2015). Improved blood-brain barrier distribution: effect of borneol on the brain pharmacokinetics of kaempferol in rats by in vivo microdialysis sampling. J Ethnopharmacol 162:270–7.
- Zhang Q. (2014). The enhancing effect of borneol on the blood-brain barrier penetration of neuroprotective kaempferol based on blood and cerebral microdialysis [MD dissertation]. Anhui, China: Anhui Medical University.
- Zhang QL, Fu BM, Zhang ZJ. (2017). Borneol, a novel agent that improves central nervous system drug delivery by enhancing blood-brain barrier permeability. Drug Deliv 24:1037–44.
- Zhang RT, Wang H, Chen L, et al. (2005). Study on the effect of borneol nasal drops on the vasopermeability of nasal mucosa and cerebral vessel of guinea pigs. J Chin Pharm 16:1291–3.
- Zhang XH, Liu FQ, Zhao ZG. (2007). The opening effect on the blood-cerebrospinal fluid barrier induced by borneol (Bn, Bingpian) and its improvement of antibiotic treatment in rabbits with experimental bacterial meningitis. Chin J Neuroimmunol Neurol 14:93–6.
- Zhao TJ, Zhang PL, Zhou Y, et al. (2015). Influences of borneol and muscone on blood-brain barrier permeability of nerve growth factor. Chin J New Clin Med 8:728–31.
- Zheng XH, Zhao X, Fang M, et al. (2007). Pharmacokinetic effects of shi herb-borneol on jun herb-Salvia miltiorrhiza. J Xi’an Jiaotong Univ Med Sci 28:170–3.
- Zhou HY, Chen XY, Huang CK, et al. (2008). Effect of borneol on the distribution of carbamazepine in mice. Wenzhou Yi Xue Yuan Xue Bao 38:300–5.
- Zhou HY, Wang P, Lin D, et al. (2005). Studies on the effects of borneol on the pharmacokinetics of carbamazepine in rabbits. Chin Pharmacol Bull 21:1263–6.
- Zhu GD. (2009). Study on the auxo-action of borneol accelerating the penetration of BBB and P-glycoprotein-mediated mechanism [MD dissertation]. Guangdong, China: Guangzhou Medical University.
- Zlokovic BV. (2008). The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178–201.