Abstract
Biologic drugs (e.g. anti-tumor necrosis factors) are effective treatments for multiple chronic inflammatory diseases including rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis. Administration of biologic drugs is usually via subcutaneous self-injection, which provides many patient benefits compared to infusions including increased flexibility, reduced costs, and reduced caregiver burden. However, it is also associated with challenges such as needle phobia, patient treatment misconceptions and incorrect drug administration, and can be impacted by dexterity problems. Evidence suggests these problems, along with other drug administration challenges (e.g. patient forgetfulness, busy lifestyles, and polypharmacy), can reduce patient adherence to treatment. To combat these challenges, patient feedback has been used to develop a range of self-injection devices, including pre-filled syringes, pre-filled pens, and electronic injection devices. Providing different devices for drug administration gives patients the opportunity to choose a device that addresses the challenges they face as an individual. Research suggests involving patients in medical device development, providing patients with a choice of devices and enrolling individuals in patient support programs can empower patients to take control of their treatment journey. By providing a portfolio of self-injection devices, designed based on patient needs, patient experience will improve, potentially improving adherence and hence, long-term treatment outcomes.
1. Introduction
Anti-tumor necrosis factor (anti-TNF) biologic drugs have emerged as effective and safe at treating a range of chronic inflammatory diseases, including axial spondyloarthritis (axSpA), Crohn’s disease (CD), psoriasis (PSO), psoriatic arthritis (PsA) and rheumatoid arthritis (RA) (Sivamani et al., Citation2013; Maruotti & Cantatore, Citation2014; Wang et al., Citation2014; Mease, Citation2015; Trivedi & Hanauer, Citation2015). The use of biological disease-modifying antirheumatic drugs (bDMARDs), often alongside conventional disease-modifying antirheumatic drugs (cDMARDs), such as methotrexate, has resulted in better long-term disease control and reduced functional impairment.
Anti-TNF biologic drugs are protein molecules and so are easily broken down by the digestive system; therefore administration is usually via injection (Schiff et al., Citation2017). To reduce the impact of treatment administration on patients’ lives, self-injection devices, able to inject anti-TNFs subcutaneously, have been developed. Once trained, patients can self-administer their prescribed biologic agent without additional help (Lyseng-Williamson, Citation2017). By removing the need of a healthcare professional (HCP) or caregiver, patient self-injection is associated with a wide range of benefits compared to infusion therapy, including increased flexibility in the time and place of injection administration, reduced cost to both the patient and healthcare system, reduced travel time, and reduced caregiver burden (Cross et al., Citation2006; Keininger & Coteur, Citation2011). Overall, this leads to increased patient control, self-efficacy, and autonomy, reducing the psychological burden of disease and improving quality of life (Salmon & Hall, Citation2003). Self-injection is also associated with a number of challenges. These include needle phobia, fear and anxiety, concerns about pain, stinging, and other injection site reactions, patient lack of confidence, incorrect administration, medication non-adherence, and the struggle to use a self-injection device while suffering from arthritic pain and swelling of the hands (Schwartzman & Morgan, Citation2004; Keininger & Coteur, Citation2011; Schiff et al., Citation2017). Self-injection device design can help overcome some of the challenges associated with self-injection, improving ease of use, and ultimately aiding disease management and improving long-term outcomes (Maniadakis et al., Citation2018).
The aims of this narrative review article are to describe current self-injection devices and the device development process including how patients can influence development, based on a pragmatic review of the literature. In addition, this article explores how using a portfolio of different self-injection devices may help address the needs of patient populations in different disease areas.
2. Device options
Three main types of device have been developed for the administration of biologic agents in chronic inflammatory diseases: pre-filled syringes (PFS), pre-filled pens (PFP), and electronic injection devices (e-Devices). These different devices have been developed in response to the challenges and preferences of patients using self-injection devices with the aim of improving patient experience during self-injection (Sheikhzadeh et al., Citation2012; Lange et al., Citation2014; Schulze-Koops et al., Citation2015; Schiff et al., Citation2016; Lyseng-Williamson, Citation2017).
A PFS is an injection system consisting of a needle and syringe (Sheikhzadeh et al., Citation2012); the syringe is pre-filled with the appropriate drug dose reducing the time taken to inject, decreasing the chance of microbiological contamination and reducing the likelihood of dosing errors () (Makwana et al., Citation2011). The physical features of the syringe (e.g. finger flange, plunger rod, and needle cap) can be designed to help with self-injection, for example, the flanges can be increased in size to help rheumatic patients handle the device (Sheikhzadeh et al., Citation2012).
Table 1. Available PFS for anti-TNF administration and their associated features.
The PFP is a self-injection pen designed to automate the injection process () (Kivitz et al., Citation2006; Kivitz & Segurado, Citation2007; Lange et al., Citation2014; Domańska et al., 2017). Automated activation (e.g. by push button), audible clicks, a hidden needle and a viewing window for observing injection progress all aim to improve ergonomics, make the injection process easier, and increase patient confidence (Freundlich et al., Citation2014; Lange et al., Citation2015; Domańska et al., Citation2017b).
Table 2. Available PFP for anti-TNF administration and their associated features.
Most recently e-Devices have been developed based on the PFP design but offering more enhanced technical features (). They are reusable and electronic with advanced technical functions to support disease management such as on-screen instructions, injection log, skin sensor, and injection speed control. Furthermore, the e-Device can include features to maximize injection safety; for example, an automatic injection stop when skin contact is lost, the same automatic needle insertion and retraction as seen in PFPs, and a medication information chip reader to ensure medication authenticity and use-by-date (Domańska et al., Citation2018). As e-Devices are reusable with disposable medicine cartridges, practice ‘cartridges’ that do not contain medicine have been developed to allow patients to gain confidence using the e-Device (Domańska et al., Citation2018).
Table 3. Available e-Devices for anti-TNF administration and their associated features.
3. Development of devices
Development of self-injection devices is increasingly focused on patient needs, with patient feedback being incorporated at many stages of device design () (Schiff et al., Citation2016; Schulze-Koops et al., Citation2015; Domańska et al., Citation2018). During the early stages of device design, focus groups are often used to collect information about the unmet needs of the intended users (e.g. patients, caregivers, HCPs) of the fully developed medical device. Feedback from these groups is then used to develop early prototype devices (Keininger & Coteur, Citation2011; Schiff et al., Citation2016; Schiff et al., Citation2017).
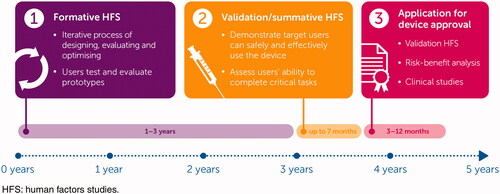
Following the design and development of the prototype device, formative human factors studies (HFS) are used to assess and iteratively improve the prototype design, the packaging and the readability of the instructions for use (Lange et al., Citation2014; Lange et al., Citation2015; Schiff et al., Citation2016; Domańska et al., Citation2018). Participants can include healthy volunteers, patients, caregivers, and HCPs who are asked to simulate using the device (e.g. inject an artificial skin pad) and answer questions on device usability. User feedback is then incorporated into the next iteration of the device design before conducting another formative HFS to assess the updated version. Formative HFS aim to guide the development process and ensure the device is effectively addressing all identified patient needs (Domańska et al., Citation2018). This process helps to guarantee device safety and that patients use the device as intended. This includes identifying any usability problems or use-errors and ensuring any modifications to the prototype design are effective (FDA, Citation2016b).
3.1. Key regulatory steps required for approval
For medical devices to be granted approval in the US or EU, the Food and Drug Administration (FDA) and European Medicines Agency (EMA) require evidence that the device is safe and effective for use by the intended users, for the intended uses, and for the intended use environments. The FDA and EMA both require this evidence to be in the form of a validation HFS (Panescu, Citation2009; French-Mowat & Burnett, Citation2012; FDA Citation2016a; MHRA Citation2017).
Validation HFS are conducted on the finalized device, representative of the launch product, with the aim to validate the safety and effectiveness of both the device and instructions for use. Participants are not trained but are provided with the instructions for use for guidance and are monitored while using the device to perform a simulated self-injection. Users are assessed against a number of pre-defined tasks that have been identified as critical for the patient to use the device safely and effectively; these are termed critical tasks (FDA, Citation2016b). Participants are individuals expected to use the device in a real-world setting. Results of validation HFS are used as part of the submission to the FDA/EMA for device approval. In some cases, clinical studies are also required alongside a validation HFS, for example, bioequivalence studies comparing devices and clinical studies to demonstrate safe and effective use (FDA, Citation2016b).
3.2. Patient empowerment and the implementation of new device technology
Sub-optimal adherence is a challenge associated with all DMARDS including biologic therapies (Fidder et al., Citation2013; Lopez-Gonzalez et al., Citation2015; Maniadakis et al., Citation2018), and has implications for both clinical and patient-reported outcomes, and treatment costs. For example, non-adherence has consistently been associated with increased morbidity and mortality, and reduced treatment benefit and symptomatic improvement (Bluett et al., Citation2015; Pasma et al., Citation2015; Maniadakis et al., Citation2018). Device development is increasingly focusing on patient preference and satisfaction as evidence suggests improved patient experience with drug administration can have a positive effect on adherence to therapy (Schwartzman & Morgan, Citation2004; Sheikhzadeh et al., Citation2012).
Patient preference studies, which compare new devices with already-approved self-injection devices, can provide insights into the impact of modifying device features on patient preference and give an indication of whether these features will help improve treatment experience. In preference studies, patients compare and rate different injection devices based on their individual preferences and satisfaction resulting in a scientific and clinical assessment of patient opinions. Preference studies can provide evidence that a specific device is preferred over competitors, and provide an opportunity for patients to give feedback on why they prefer certain devices over others (Lyseng-Williamson, Citation2017). Patient preference studies can also be feature-specific, asking patients to rank individual features of a medical device to determine the features patients consider most important or useful (Domańska et al., Citation2017a). Additionally, a preference study may be an HFS if comparing a device against a competitor device, or a clinical study if preference between different devices for the same drug is investigated.
Discrete choice experiments (DCE) are another method of investigating user preference of different devices and design features. DCE studies are a quantitative technique to investigate the role each attribute plays in determining patients’ preference for a device or feature (Harrison et al., Citation2015). Users answer multiple questions stating their preference between two or more hypothetical devices. Each hypothetical device is defined by several varying attributes and responses are used to determine patient preference for each attribute and each attribute’s relative importance (Mangham et al., Citation2009). Through DCE and preference studies, patients are given the opportunity to feedback what devices and features they prefer and prioritize (Fraenkel et al., Citation2004; Kivitz et al., Citation2006; Finckh et al., Citation2016; Collier et al., Citation2017). This ability to influence the development of devices empowers patients, giving them a way to guide how treatment is administered.
A more recently developed method to elicit patient feedback is the Parker Model (J⊘rgensen et al., Citation2018). The Parker Model is a composite, three-step, qualitative research model designed to evaluate the development and implementation of new medical devices. The model combines concept mapping of patient views about the e-Device with iterative participatory design sessions to develop e-Device prototypes that capture the feedback of all patients. Finally, patient and disease management stakeholders are involved in group and individual stakeholder evaluations of the e-Device (J⊘rgensen et al., Citation2018). Each step of the model aims to support the flow of information between participants and highlight key themes in users’ responses to new medical devices. This process of evaluation can help identify insights that can feed back into new iterations of medical devices and can help ensure new device technologies are introduced to new users in the most effective way.
4. Factors impacting patient adherence that can be addressed using optimized device design
Multiple factors, both general and specific to chronic inflammatory diseases, influence treatment adherence and successful self-injection. Generally, reasons for treatment non-adherence can be grouped into two categories: conscious and unconscious non-adherence.
Conscious non-adherers actively avoid administering medication for reasons such as concerns about treatment efficacy or side effects, or beliefs about medication or healthcare generally (Michetti et al., Citation2017). Evidence suggests reducing medication concerns and increasing family and HCP support may help reduce this form of non-adherence (van den Bemt & van Lankveld, Citation2007; Gadallah et al., Citation2015; Morgan et al., Citation2015). It has been suggested that a wider recognition of the role of conscious psychological factors, including medication beliefs, could help improve treatment adherence and improve clinical and economic outcomes (Morgan et al., Citation2015).
Unconscious non-adherence is a form of non-intentional non-adherence often driven by patients’ inability to take their medication. This could include forgetfulness, misunderstanding of the drug regimen, a lack of patient motivation, needle phobia, and problems with hand dexterity and drug administration when using non-ergonomically adapted syringes. Many of the factors driving unconscious non-adherence, such as those above, can be addressed by improving the design of a self-injection device.
Evidence suggests forgetfulness (erratic non-adherence) may account for ∼50% of non-adherence, despite patients expressing a strong willingness to try new medicines and adhere to effective treatment (Feldman, Citation2013). Polypharmacy may exacerbate this, with evidence suggesting that taking more than three types of medication daily or increasing the dosing frequency is likely to increase rates of non-adherence (Saini et al., Citation2009; Bugni et al., Citation2012). Limited health literacy and low educational level have also been associated with non-adherence. It has been suggested patient education programs to increase patient understanding of the disease area and potential treatment benefits may improve levels of this type of unconscious non-adherence (Horne et al., Citation2013; Pasma et al., Citation2013; Joplin et al., Citation2015). Applying patient education to self-injection has had some success at improving rates of adherence. For example, an education session about switching from a PFS to a PFP was found to increase adherence following the switch to the PFP in a group of patients prescribed etanercept (Borrás-Blasco et al., Citation2013). However, there are few studies in this area and so more research focusing on the impact of specific educational strategies is needed (Galo et al., Citation2016).
Reduced psychological well-being can also be a reason for unconscious non-adherence. Unmanaged persistent anxiety and depression are associated with poorer health outcomes over time, including raised tender joint count, Disease Activity Score (DAS28) and Health Assessment Questionnaire (HAQ) score in RA (Hider et al., Citation2009; Matcham et al., Citation2016; Maniadakis et al., Citation2018). Low mood coupled with misconceptions and a poor understanding of their chronic conditions may lead to dissatisfaction with clinical consultation and inappropriate avoidance of treatment, non-adherence and poor disease management (Fortune et al., Citation2002; Renzi et al., Citation2002). For example, depressive symptoms predict anti-TNF non-adherence in patients with inflammatory bowel disease (Calloway et al., Citation2017). Low levels of self-efficacy, an individual’s belief in their own ability to succeed in accomplishing a task, have also been linked to non-adherence, suggesting increasing patient self-injection confidence and empowerment may help increase adherence levels (Nafradi et al., Citation2017).
Specific factors related to the disease area (e.g. rheumatology-specific factors) and treatment may also contribute to low adherence (Vangeli et al., Citation2015). Fear and anxiety about self-injection, in the form of needle phobia, is a recognized barrier to patient adherence (Cox & Mohr, Citation2003), and previous bad experiences with self-injection have been found to affect the patient’s future experience of self-injection (Schiff et al., Citation2017). Positive therapeutic clinical relationships and skilled HCPs can help to reduce fear, improve patient wellbeing, individualize person-centered care and foster a feeling of support. For example, the development of rituals surrounding the preparation and act of self-injection can help patients’ control of the process and reduce anxiety (Schiff et al., Citation2017).
5. A portfolio of devices can address a range of patient needs
As discussed above, low patient adherence is caused by a range of factors which are strongly individually determined. The necessity-concern framework proposes that a patient’s adherence decisions are a result of the balance between their perceived need for medication (necessity) and their concerns regarding its use (Horne et al., Citation1999; Phillips et al., Citation2014). If an individual’s view of the necessity of their treatment increases, while their concerns about its use decrease, adherence to their treatment regimen will improve (Horne et al., Citation2013). Effective device design provides one way to reduce patient treatment concerns.
All self-injection devices can be developed to reduce patient concerns about drug administration and help maximize the range of individuals it is suitable for. By involving patients in the design of a device, for example through formative HFS, device manufacturers can ensure they are developing devices that address patient needs (Domańska et al., Citation2018). Syringe flanges or PFP grips can be designed ergonomically to help increase the isometric force patients can apply during an injection. As a result, this helps overcome some of the challenges resulting from patient dexterity problems (Sheikhzadeh et al., Citation2012). Ergonomic syringe flanges were preferred by patients, compared to a standard syringe (Sheikhzadeh et al., Citation2012).
Having access to a portfolio of different injection devices designed specifically for each treatment option allows physicians, through discussions with patients, to adapt treatment administration to different patients’ needs. The availability of choice in self-injection devices is an important piece in the ‘eco-system’ of a comprehensive treatment landscape for chronic disease management. Evidence suggests using a preferred device may increase patient tolerance of self-injection and possibly improve adherence (Gau & Takasawa, Citation2017). Generally, devices have been found to have a positive impact on the self-esteem of elderly patients if they feel they are mastering the device (Thomson et al., Citation2013). However, the same research found that a device could have a negative impact on elderly patients’ self-esteem if they felt they had no other choice but to use it (Thomson et al., Citation2013). This evidence supports the use of a portfolio of devices, as a choice between devices may help prevent patients from feeling they have to use a specific device.
Injection-naïve patients often suffer increased self-injection anxiety (Cox & Mohr, Citation2003; Karter et al., Citation2010). The PFPs and e-Devices have been designed with hidden needles, possibly reducing needle phobia (Domańska et al., Citation2017b), and patient preference studies have found that patients generally find PFPs easier to use than PFSs (Kivitz et al., Citation2006). However, some patients have reported that a PFS allows them more control over self-injection: a comparison study between a PFS and a PFP found patients who preferred the syringe often preferred it because the injection was easier to control and less painful (Kivitz et al., Citation2006). RA is associated with dexterity issues; easy to use self-injection devices, such as PFPs, can improve ease of use and reduce pain, potentially facilitating adherence to treatment (Kivitz & Segurado, Citation2007). In particular, patient comments suggest the larger grip of a PFP (‘it’s sturdy and the material on the handle is anti-slip which made me feel more confident’) and audible indication clicks (‘two clicks [were] reassuring’) increase patient confidence (Domańska et al., Citation2017b).
e-Devices have enhanced technological features that can help with disease management (Collier et al., Citation2017; Domańska et al., Citation2018). These features allow patients to combine the ease of automated self-injection with increased self-injection control. Previous studies have demonstrated that medication reminders and treatment logs can improve treatment adherence (Patel et al., Citation2013; Coorey et al., Citation2018); e-Devices have the potential to incorporate these functions allowing patients to check if it is time to administer an injection, potentially helping to prevent unconscious non-adherence. As a result, this type of self-injection device may appeal to busy patients or patients with cognitive problems who may struggle to remember to carry out injections. The injection log can also be used to foster discussions with HCPs on reasons for non-adherence as injection history can be accessed and missed injections and injection patterns highlighted easily at clinic appointments (Domańska et al., Citation2018). Previous studies have demonstrated that support from HCPs can improve patient adherence (Mansoor et al., Citation2013), and so an injection log that connects to a patient’s electronic health records could identify patients at need of more direct support. The ability to practice injections and the step-by-step on-screen instructions can help patients increase their confidence in their ability to self-inject correctly (Domańska et al., Citation2018). Additionally, e-Devices permit personalization; for example, the technology allows patients to choose between different injection speeds (Domańska et al., Citation2018). However, an e-Device may not be appropriate for patients who are not at ease with technology.
In addition to a range of self-injection devices, patient support services are available that aim to support patients during self-injection. Support services provide education on the injection process, disease and treatment, and give patients an opportunity to share their experiences. This encourages patients to ask about, and therefore resolve, problems with successful self-injection. For example, a patient support program (PSP) for adalimumab patients includes patient education and injection training, delivery and disposal of devices, financial assistance, patient reminders and direct contact with specialist nurses (Bessette et al., Citation2018). Several studies examining the impact of the adalimumab PSP found adherence increased in those using the service, compared to patients who were not (Hill et al., Citation2001; Rubin et al., Citation2017; Bessette et al., Citation2018; Marshall et al., Citation2018). Additionally, providing patients with information about antirheumatic drugs in groups resulted in better outcomes and increased adherence, compared to providing information individually (Homer et al., Citation2009). A review of studies reporting outcomes of PSPs found such services positively impact patient adherence, and patients enrolled in the program have increased Patient Activation Measure (PAM)-13 scores (Ganguli et al., Citation2016; Van den Bosch et al., Citation2017). PAM is a measure of how ‘activated’ a patient is and is a reliable indicator of the level of patient knowledge and confidence regarding their own health and care (Hibbard et al., Citation2004). Although beneficial, additional PSPs can add complexity to patients’ treatment journeys. The development of e-Devices with functions such as connectivity and injection logging, can provide many PSP benefits without further burdening patients. In previous studies, patients described often feeling over‑burdened by self-injection, for example struggling to remember how to inject (Schiff et al., Citation2017). Therefore, any initiatives that seamlessly combine injection devices with support services will likely lead to improved patient experience.
6. The future of drug-delivery devices
Injection is a common method of drug administration, and injection devices are continually being developed. For example, researchers are currently developing a needle-free influenza vaccination – there may be the potential for adapting these innovations for use in biologic administration (McAllister et al., Citation2014). Oral administration is generally preferred by patients and there is some evidence that it may increase adherence (Quante et al., Citation2012). Oral administration of large protein molecules represents unique challenges, however, methods for biologic administration, particularly for gastrointestinal disease indications such as Crohn’s disease, are being developed. For example, oral administration of anti-TNF to a mouse model of inflammatory bowel disease was found to improve symptoms (Bhol et al., Citation2013). Additionally, recent research into the development of an oral biologic delivery system allowing injection of insulin into the stomach lining demonstrated comparable plasma drug levels to subcutaneous injection in animals (Abramson et al., Citation2019). If proven effective in clinical trials, a similar delivery system could be developed for the administration of other large protein molecules, such as anti-TNFs.
With the advent of e-Devices in rheumatology, ‘smart’ self-injection devices can not only make the act of self-injecting easier but can also help patients manage their disease. For example, a future self-injection e-Device, developed with the ability to synchronize with a mobile phone application, could help patients and HCPs track their treatment and symptoms, provide education and increase patient confidence and motivation to adhere to their treatment regimen. This could be used to individualize treatment, help patients self-manage their disease and increase independence. Phone and computer applications available to support self-injection and drug delivery in other disease areas, for example, multiple sclerosis and diabetes, have been found to increase patient ability to track their own health and provide data for improved patient-HCP communication (Mougiakakou et al., Citation2010; Greiner et al., Citation2015).
In recent years, there has been an accelerated drive to utilize artificial intelligence (AI) and machine learning algorithms to support patient care (Jiang et al., Citation2017). Several studies have investigated the potential uses of machine learning in rheumatology. In one study, machine learning was successfully used to predict disease flares by monitoring levels of patient activity using activity trackers (Gossec et al., Citation2018). In another study, machine learning was used to develop a clinical prediction model for RA mortality (Lezcano-Valverde et al., Citation2017). Similar innovations could be developed by coupling machine learning to connected self-injection devices; for example, it may be possible to monitor changes in patient-reported outcome measures using machine learning, this could then be used to predict disease activity and make treatment recommendations.
7. Conclusions
Low adherence to treatment regimens is a well-documented phenomenon in biologic drug research (Elliott, Citation2008; Bluett et al., Citation2015; Lopez-Gonzalez et al., Citation2015). Reasons behind non-adherence are complex but evidence suggests they often originate from a lack of patient confidence or engagement (Nafradi et al., Citation2017; Maniadakis et al., Citation2018). Supporting patients through PSPs and devices that address patient needs can increase confidence and motivation, and improve adherence (Nafradi et al., Citation2017; Rubin et al., Citation2017). To ensure that self-injection devices are best designed to help overcome treatment administration challenges, device design and development should be centered around the patient. Published results from HFS and preference studies demonstrates the heterogeneity that exists in patient priorities and highlights how a single self-injection device cannot support the needs of all patient groups (Fraenkel et al., Citation2004; Kivitz et al., Citation2006; Domańska et al., Citation2017a; Domańska et al., Citation2018; Maniadakis et al., Citation2018). By providing a choice of self-injection devices HCPs will be able to support a larger proportion of patients and empower them to take control of their personal treatment journey. As discussed above, this has the potential to improve patient experience and treatment adherence, improve clinical outcomes, and reduce the economic and societal burden of disease.
Acknowledgments
The authors acknowledge Susanne Wiegratz, UCB Pharma, Monheim, Germany for publication coordination and Emma Phillips, PhD, and Simon Foulcer, PhD, from Costello Medical, UK, for medical writing and editorial assistance in preparing this manuscript for publication, based on the authors’ input and direction.
Disclosure statement
BVB: Received grant/research support from: UCB, Pfizer, AbbVie; Speakers bureau: Pfizer, AbbVie, UCB, Biogen, Sandoz. Delivered consultancy work for UCB, Novartis and Pfizer.LG: Delivered consultancy work for UCB, Sanofi and Jackel Solutions.BD: Employee of UCB Pharma.RB: Employee of UCB Pharma.IM: Employee of UCB Pharma.LEK: Received grant/research support from: UCB, Biogen, Janssen Pharmaceuticals, and Novartis; Speakers bureau: Pfizer, AbbVie, Amgen, UCB, BMS, Biogen, MSD, Novartis, Eli Lilly and Company, and Janssen Pharmaceuticals.
Additional information
Funding
References
- Abramson A, Caffarel-Salvador E, Khang M, et al. (2019). An ingestible self-orienting system for oral delivery of macromolecules. Science 363:611–5.
- Bessette L, Lebovic G, Millson B, et al. (2018). Impact of the adalimumab patient support program on clinical outcomes in ankylosing spondylitis: results from the COMPANION study. Rheumatol Ther 5:75–85.
- Bhol KC, Tracey DE, Lemos BR, et al. (2013). AVX-470: a novel oral anti-TNF antibody with therapeutic potential in inflammatory bowel disease. Inflamm Bowel Dis 19:2273–81.
- Bluett J, Morgan C, Thurston L, et al. (2015). Impact of inadequate adherence on response to subcutaneously administered anti-tumour necrosis factor drugs: results from the Biologics in Rheumatoid Arthritis Genetics and Genomics Study Syndicate cohort. Rheumatology (Oxford) 54:494–9.
- Borrás-Blasco J, Gracia-Perez A, Castera MD, et al. (2013). Educational session as a tool to increase patient satisfaction of switching etanercept from the prefilled syringe to the autoinjection pen. Expert Opin Biol Ther 13:1103–8.
- Bugni VM, Ozaki LS, Okamoto KY, et al. (2012). Factors associated with adherence to treatment in children and adolescents with chronic rheumatic diseases. J Pediatr (Rio J) 88:483–8.
- Calloway A, Dalal R, Beaulieu DB, et al. (2017). Depressive symptoms predict anti-tumor necrosis factor therapy noncompliance in patients with inflammatory bowel disease. Dig Dis Sci 62:3563–7.
- Collier DH, Bitman B, Coles A, et al. (2017). A novel electromechanical autoinjector, AutoTouch, for self-injection of etanercept: real-world use and benefits. Postgrad Med 129:118–25.
- Coorey GM, Neubeck L, Mulley J, et al. (2018). Effectiveness, acceptability and usefulness of mobile applications for cardiovascular disease self-management: Systematic review with meta-synthesis of quantitative and qualitative data. Eur J Prev Cardiol 25:505–21.
- Cox D, Mohr DC. (2003). Managing difficulties with adherence to injectable medications due to blood, injection, and injury phobia and self-injection anxiety. American Journal of Drug Delivery 1:215–21.
- Cross MJ, March LM, Lapsley HM, et al. (2006). Patient self-efficacy and health locus of control: relationships with health status and arthritis-related expenditure. Rheumatology (Oxford) 45:92–6.
- Domańska B, Mountian I, Vinconneau G. (2017a). Patient-preferred design features of TNF inhibitor self-injection devices: insights from a rheumatoid arthritis auto-injector preference study. Value in Health 20:A591–2.
- Domańska B, Stumpp O, Poon S, et al. (2018). Using patient feedback to optimize the design of a certolizumab pegol electromechanical self-injection device: insights from human factors studies. Adv Ther 35:100–15.
- Domańska B, VanLunen B, Peterson L, et al. (2017b). Comparative usability study for a certolizumab pegol autoinjection device in patients with rheumatoid arthritis. Expert Opin Drug Deliv 14:15–22.
- Elliott RA. (2008). Poor adherence to medication in adults with rheumatoid arthritis. Disease Management & Health Outcomes 16:13–29.
- EMA, (2018). Summary of product characteristics: benepali. https://www.ema.europa.eu/documents/product-information/benepali-epar-product-information_en.pdf
- FDA, (2016a). Applying human factors and usability engineering to medical devices: guidance for industry and Food and Drug Administration staff. https://www.fda.gov/downloads/MedicalDevices/…/UCM259760.pdf.
- FDA, (2016b). Human factors studies and related clinical study considerations in combination product design and development: draft guidance for industry and FDA staff. https://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM484345.pdf.
- Feldman SR. (2013). Disease burden and treatment adherence in psoriasis patients. Cutis 92:258–63.
- Fidder HH, Singendonk MM, van der Have M, et al. (2013). Low rates of adherence for tumor necrosis factor-alpha inhibitors in Crohn's disease and rheumatoid arthritis: results of a systematic review. World J Gastroenterol 19:4344–50.
- Finckh A, Escher M, Liang MH, et al. (2016). Preventive Treatments for Rheumatoid Arthritis: Issues Regarding Patient Preferences. Curr Rheumatol Rep 18:51.
- Fortune DG, Richards HL, Kirby B, et al. (2002). A cognitive-behavioural symptom management programme as an adjunct in psoriasis therapy. Br J Dermatol 146:458–65.
- Fraenkel L, Bogardus ST, Concato J, et al. (2004). Patient preferences for treatment of rheumatoid arthritis. Ann Rheum Dis 63:1372–8.
- French-Mowat E, Burnett J. (2012). How are medical devices regulated in the European Union? Journal of the Royal Society of Medicine 105:S22–S28.
- Freundlich B, Kivitz A, Jaffe JS. (2014). Nearly pain-free self-administration of subcutaneous methotrexate with an autoinjector: results of a phase 2 clinical trial in patients with rheumatoid arthritis who have functional limitations. J Clin Rheumatol 20:256–60.
- Gadallah MA, Boulos DN, Gebrel A, et al. (2015). Assessment of rheumatoid arthritis patients' adherence to treatment. Am J Med Sci 349:151–6.
- Galo JS, Mehat P, Rai SK, et al. (2016). What are the effects of medication adherence interventions in rheumatic diseases: a systematic review. Ann Rheum Dis 75:667–73.
- Ganguli A, Clewell J, Shillington AC. (2016). The impact of patient support programs on adherence, clinical, humanistic, and economic patient outcomes: a targeted systematic review. Patient Prefer Adherence 10:711–25.
- Gau M, Takasawa K. (2017). Initial patient choice of a growth hormone device improves child and adolescent adherence to and therapeutic effects of growth hormone replacement therapy. J Pediatr Endocrinol Metab 30:989–93.
- Gossec L, Guyard F, Leroy D, et al. (2018). Detection of flares by decrease in physical activity, collected using wearable activity trackers, in rheumatoid arthritis or axial spondyloarthritis: an application of Machine-Learning analyses in rheumatology. Arthritis Care Res (Hoboken). doi:https://doi.org/10.1002/acr.23768.
- Greiner P, Sawka A, Imison E. (2015). Patient and physician perspectives on MSdialog, an electronic PRO diary in multiple sclerosis. Patient 8:541–50.
- Harrison M, Marra C, Shojania K, et al. (2015). Societal preferences for rheumatoid arthritis treatments: evidence from a discrete choice experiment. Rheumatology (Oxford) 54:1816–25.
- Hibbard JH, Stockard J, Mahoney ER, et al. (2004). Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res 39:1005–26.
- Hider SL, Tanveer W, Brownfield A, et al. (2009). Depression in RA patients treated with anti-TNF is common and under-recognized in the rheumatology clinic. Rheumatology (Oxford) 48:1152–4.
- Hill J, Bird H, Johnson S. (2001). Effect of patient education on adherence to drug treatment for rheumatoid arthritis: a randomised controlled trial. Ann Rheum Dis 60:869–75.
- Homer D, Nightingale P, Jobanputra P. (2009). Providing patients with information about disease-modifying anti-rheumatic drugs: individually or in groups? A pilot randomized controlled trial comparing adherence and satisfaction. Musculoskeletal Care 7:78–92.
- Horne R, Chapman SC, Parham R, et al. (2013). Understanding patients' adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLoS One 8:e80633.
- Horne R, Weinman J, Hankins M. (1999). The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychology & Health 14:1–24.
- Jiang F, Jiang Y, Zhi H, et al. (2017). Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol 2:230–43.
- Joplin S, van der Zwan R, Joshua F, et al. (2015). Medication adherence in patients with rheumatoid arthritis: the effect of patient education, health literacy, and musculoskeletal ultrasound. Biomed Res Int 2015:150658.
- Jørgensen TS, Skougaard M, Taylor PC, et al. (2018). The Parker Model: applying a qualitative three-step approach to optimally utilize input from stakeholders when introducing new device technologies in the management of chronic rheumatic diseases. Patient 11:515–26.
- Karter AJ, Subramanian U, Saha C, et al. (2010). Barriers to insulin initiation: the translating research into action for diabetes insulin starts project. Diabetes Care 33:733–5.
- Keininger D, Coteur G. (2011). Assessment of self-injection experience in patients with rheumatoid arthritis: psychometric validation of the Self-Injection Assessment Questionnaire (SIAQ). Health Qual Life Outcomes 9:2.
- Kivitz A, Cohen S, Dowd JE, et al. (2006). Clinical assessment of pain, tolerability, and preference of an autoinjection pen versus a prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin Ther 28:1619–29.
- Kivitz A, Segurado OG. (2007). HUMIRA pen: a novel autoinjection device for subcutaneous injection of the fully human monoclonal antibody adalimumab. Expert Rev Med Devices 4:109–16.
- Lange J, Richard P, Bradley N. (2014). Usability of devices for self-injection: results of a formative study on a new disposable pen injector. Med Devices (Auckl) 7:195–203.
- Lange J, Richard P, Bradley N. (2015). Usability of a new disposable autoinjector platform device: results of a formative study conducted with a broad user population. Med Devices (Auckl) 8:255–64.
- Lezcano-Valverde JM, Salazar F, León L, et al. (2017). Development and validation of a multivariate predictive model for rheumatoid arthritis mortality using a machine learning approach. Scientific Reports 7:10189.
- Lopez-Gonzalez R, Leon L, Loza E, et al. (2015). Adherence to biologic therapies and associated factors in rheumatoid arthritis, spondyloarthritis and psoriatic arthritis: a systematic literature review. Clin Exp Rheumatol 33:559–69.
- Lyseng-Williamson KA. (2017). Certolizumab pegol administration devices: a profile of their use and usability. Drugs Ther Perspect 33:515–22.
- Makwana S, Basu B, Makasana Y, et al. (2011). Prefilled syringes: an innovation in parenteral packaging. Int J Pharm Investig 1:200–6.
- Mangham LJ, Hanson K, McPake B. (2009). How to do (or not to do) … Designing a discrete choice experiment for application in a low-income country. Health Policy and Planning 24:151–8.
- Maniadakis N, Toth E, Schiff M, et al. (2018). A targeted literature review examining biologic therapy compliance and persistence in chronic inflammatory diseases to identify the associated unmet needs, driving factors, and consequences. Adv Ther 35:1333–55.
- Mansoor SM, Krass I, Aslani P. (2013). Multiprofessional interventions to improve patient adherence to cardiovascular medications. J Cardiovasc Pharmacol Ther 18:19–30.
- Marshall JK, Bessette L, Thorne C, et al. (2018). Impact of the adalimumab patient support program's care coach calls on persistence and adherence in Canada: an observational retrospective cohort study. Clinical Therapeutics 40:415–29.e6.
- Maruotti N, Cantatore FP. (2014). Impact of biological therapy on spondyloarthritis. Eur J Clin Pharmacol 70:1021–7.
- Matcham F, Norton S, Scott DL, et al. (2016). Symptoms of depression and anxiety predict treatment response and long-term physical health outcomes in rheumatoid arthritis: secondary analysis of a randomized controlled trial. Rheumatology (Oxford) 55:268–78.
- McAllister L, Anderson J, Werth K, et al. (2014). Needle-free jet injection for administration of influenza vaccine: a randomised non-inferiority trial. Lancet 384:674–81.
- Mease PJ. (2015). Biologic therapy for psoriatic arthritis. Rheum Dis Clin North Am 41:723–38.
- MHRA, (2017). Human factors and usability engineering – guidance for medical devices including drug-device combination products. London, UK: MHRA.
- Michetti P, Weinman J, Mrowietz U, et al. (2017). Impact of Treatment-Related Beliefs on Medication Adherence in Immune-Mediated Inflammatory Diseases: Results of the Global ALIGN Study. Adv Ther 34:91–108.
- Morgan C, McBeth J, Cordingley L, et al. (2015). The influence of behavioural and psychological factors on medication adherence over time in rheumatoid arthritis patients: a study in the biologics era. Rheumatology (Oxford) 54:1780–91.
- Mougiakakou SG, Bartsocas CS, Bozas E, et al. (2010). SMARTDIAB: a communication and information technology approach for the intelligent monitoring, management and follow-up of type 1 diabetes patients. IEEE Trans Inform Technol Biomed 14:622–33.
- Muller-Ladner U, Flipo RM, Vincendon P, et al. (2012). Comparison of patient satisfaction with two different etanercept delivery systems. A randomised controlled study in patients with rheumatoid arthritis. Z Rheumatol 71:890–9.
- Nafradi L, Nakamoto K, Schulz PJ. (2017). Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PLoS One 12:e0186458.
- Panescu D. (2009). Medical device development. Conf Proc IEEE Eng Med Biol Soc 2009:5591–4.
- Pasma A, Schenk CV, Timman R, et al. (2015). Non-adherence to disease-modifying antirheumatic drugs is associated with higher disease activity in early arthritis patients in the first year of the disease. Arthritis Res Ther 17:281.
- Pasma A, van't Spijker A, Hazes JM, et al. (2013). Factors associated with adherence to pharmaceutical treatment for rheumatoid arthritis patients: a systematic review. Semin Arthritis Rheum 43:18–28.
- Patel S, Jacobus-Kantor L, Marshall L, et al. (2013). Mobilizing your medications: an automated medication reminder application for mobile phones and hypertension medication adherence in a high-risk urban population. J Diabetes Sci Technol 7:630–9.
- Phillips LA, Diefenbach MA, Kronish IM, et al. (2014). The necessity-concerns framework: a multidimensional theory benefits from multidimensional analysis. Ann Behav Med 48:7–16.
- Quante M, Thate-Waschke I, Schofer M. (2012). What are the reasons for patient preference? A comparison between oral and subcutaneous administration. Z Orthop Unfall 150:397–403.
- Renzi C, Picardi A, Abeni D, et al. (2002). Association of dissatisfaction with care and psychiatric morbidity with poor treatment compliance. Arch Dermatol 138:337–42.
- Rubin DT, Mittal M, Davis M, et al. (2017). Impact of a patient support program on patient adherence to adalimumab and direct medical costs in Crohn's disease, ulcerative colitis, rheumatoid athritis, psoriasis, psoriatic arthritis, and ankylosing spondylitis. J Manag Care Spec Pharm 23:859–67.
- Saini SD, Schoenfeld P, Kaulback K, et al. (2009). Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care 15:e22–33.
- Salmon P, Hall GM. (2003). Patient empowerment and control: a psychological discourse in the service of medicine. Soc Sci Med 57:1969–80.
- Schiff M, Koo J, Jin E, et al. (2016). Usability and acceptability of the abatacept pre-filled autoinjector for the subcutaneous treatment of rheumatoid arthritis. Adv Ther 33:199–213.
- Schiff M, Saunderson S, Mountian I, et al. (2017). Chronic disease and self-injection: ethnographic investigations into the patient experience during treatment. Rheumatol Ther 4:445–63.
- Schulze-Koops H, Giacomelli R, Samborski W, et al. (2015). Factors influencing the patient evaluation of injection experience with the SmartJect autoinjector in rheumatoid arthritis. Clin Exp Rheumatol 33:201–8.
- Schwartzman S, Morgan GJ. Jr. (2004). Does route of administration affect the outcome of TNF antagonist therapy? Arthritis Res Ther 6:S19–S23.
- Sheikhzadeh A, Yoon J, Formosa D, et al. (2012). The effect of a new syringe design on the ability of rheumatoid arthritis patients to inject a biological medication. Appl Ergon 43:368–75.
- Sivamani RK, Goodarzi H, Garcia MS, et al. (2013). Biologic therapies in the treatment of psoriasis: a comprehensive evidence-based basic science and clinical review and a practical guide to tuberculosis monitoring. Clinic Rev Allergy Immunol 44:121–40.
- Thomson R, Martin JL, Sharples S. (2013). The psychosocial impact of home use medical devices on the lives of older people: a qualitative study. BMC Health Serv Res 13:467.
- Trivedi I, Hanauer SB. (2015). Balancing the risks and benefits of biologic therapy in inflammatory bowel diseases. Expert Opin Drug Saf 14:1915–34.
- van den Bemt BJ, van Lankveld WG. (2007). How can we improve adherence to therapy by patients with rheumatoid arthritis? Nat Clin Pract Rheumatol 3:681.
- Van den Bosch F, Wassenberg S, Östör A, et al. (2017). Impact of patient support program utilization on patient activation measure scores among patients with rheumatoid arthritis [abstract]. Arthritis Rheumatol 69. https://acrabstracts.org/abstract/impact-of-patient-support-program-utilization-on-patient-activation-measure-scores-among-patients-with-rheumatoid-arthritis/
- Vangeli E, Bakhshi S, Baker A, et al. (2015). A systematic review of factors associated with non-adherence to treatment for immune-mediated inflammatory diseases. Adv Ther 32:983–1028.
- Vermeire S, D'Heygere F, Nakad A, et al. (2018). Preference for a prefilled syringe or an auto-injection device for delivering golimumab in patients with moderate-to-severe ulcerative colitis: a randomized crossover study. Patient Prefer Adherence 12:1193–202.
- Wang D, Li Y, Liu Y, et al. (2014). The use of biologic therapies in the treatment of rheumatoid arthritis. Curr Pharm Biotechnol 15:542–8.