Abstract
Active components of natural products, which include paclitaxel, curcumin, gambogic acid, resveratrol, triptolide and celastrol, have promising anti-inflammatory, antitumor, anti-oxidant, and other pharmacological activities. However, their clinical application is limited due to low solubility, instability, low bioavailability, rapid metabolism, short half-life, and strong off-target toxicity. To overcome these drawbacks, cell membrane-based biomimetic nanosystems have emerged that avoid clearance by the immune system, enhance targeting, and prolong drug circulation, while also improving drug solubility and bioavailability, enhancing drug efficacy, and reducing side effects. This review summarizes recent advances in the preparation and coating of cell membrane-coated biomimetic nanosystems and in their applications to disease for targeted natural products delivery. Current challenges, limitations, and prospects in this field are also discussed, providing a research basis for the development of multifunctional biomimetic nanosystems for natural products.
1. Introduction
Natural products play a vital role in the prevention and treatment of diseases, such as cancer, Alzheimer’s disease, Parkinson’s disease, and other pathological conditions. Natural products have been used for a long time since ancient times, which is directly attributable to the therapeutic values and lower adverse side effects compared to modern medicine. Its efficacy relies on the use of multiple active components, including paclitaxel (PTX), curcumin (Cur), gambogic acid, resveratrol, triptolide and celastrol (Liu et al., Citation2020b; Zheng et al., Citation2022), which show promising anti-inflammatory, antitumor, anti-oxidant, and other pharmacological activities ( and ) (Kocaadam and Sanlier, Citation2017; Hatami et al., Citation2020; Huang et al., Citation2020a; Viegas et al., Citation2020; Kaur et al., Citation2022; Li et al., Citation2022a). However, the development and application of these active components is limited due to poor solubility, low bioavailability, short half-life, and instability in biological environments (Puglia et al., Citation2017).
Figure 1. The active components of natural products mentioned in this review. Original image created by author(s).
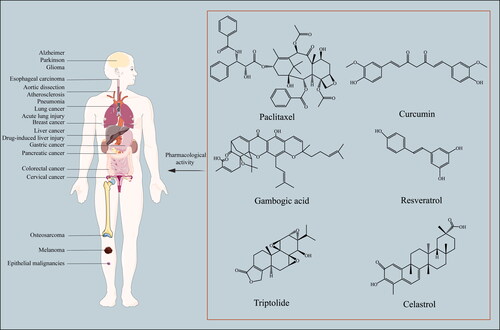
Table 1. Origin and biological activities of active components discussed in this review.
In recent decades, nanodelivery systems such as liposomes, solid lipid nanoparticles, nanoemulsions, micelles and polymer nanoparticles have been widely used to overcome these challenges(Dadwal et al., Citation2018; Patra et al., Citation2018; Swierczewska et al., Citation2018; Liu et al., Citation2023b). Compared to modern medicine, nanocarriers offer better solubility, pharmacokinetics, biodistribution, bioavailability and stability as well as lower toxicity. In addition, they protect drugs from degradation and inactivation and prolong their time in circulation through sustained release (Din et al., Citation2017; Crintea et al., Citation2022). However, they still suffer from fast elimination, low target specificity, and poor tumor permeability. Modifying nanoparticles with polyethylene glycol (PEG) shells can prolong their circulation and reduce their toxicity, but also impede their cellular internalization (Gabizon and Martin, Citation1997; Harris et al., Citation2001; Karakoti et al., Citation2011; Sanchez Armengol et al., Citation2022). In addition, nanodelivery systems modified with ligands such as antibodies, peptides and folic acid may be easily recognized by the mononuclear macrophage system, which removes them from the circulation and inhibits targeted drug delivery (Bajracharya et al., Citation2022; Wang et al., Citation2020a).
Biomimetic nanocarriers have recently emerged as novel nanomaterials with greater ability to target cells and to evade immune responses. These nanoparticles are cloaked in the ‘camouflage’ of membranes derived from red blood cells (RBCs), white blood cells (WBCs), platelets or cancer cells, the resulting camouflaged nanoparticles attain special functions including immune escape, longer circulation times, excellent biosafety and homologous targeting ability (Jin et al., Citation2018; Lu et al., Citation2023). These membranes can even be modified with functional groups to strengthen drug efficacy, and they can be administered in combination with other treatments, such as magnetic hyperthermia, photodynamic therapy (PDT), and acoustic dynamic therapy.
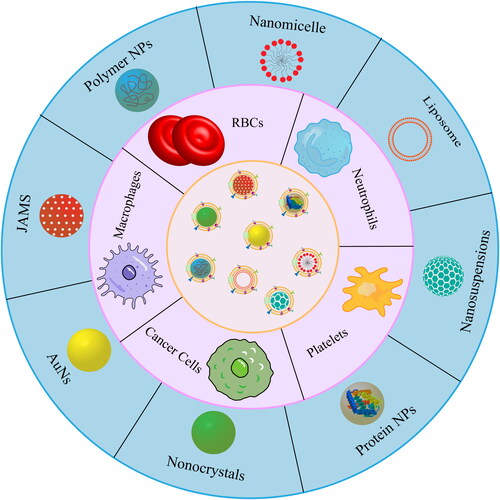
In this review, we summarize the developments in biomimetic nanodelivery systems for natural products over the past five years, focusing on nanopreparations coated with different cell membranes (). This study may serve as a reference for the future application of cell membrane biomimetic technology in the delivery of active but insoluble natural products.
2. Experimental basis of cell membrane-based biomimetic nanoparticles
2.1. Preparation of cell-derived membrane
The first step in the preparation of biomimetic nanopreparations is the extraction and purification of the cell membrane, both of which need to be prudently treated to preserve the structure of the cell membrane and the activity of surface proteins (Liu et al., Citation2019d).
Above all, the cell membrane was extracted by hypotonic cracking, repeated freeze-thawing and mechanical crushing. The principle of hypotonic lysis is that cells will swell and rupture under low osmotic pressure to release the contents, which is widely used in the extraction of erythrocyte membrane (Feng et al., Citation2022; Xu et al., Citation2023a, Citation2023b). Repeated freeze-thawing involves freezing cells at low temperatures and thawing them repeatedly at room temperature to remove the contents (Ji et al., Citation2023; Li et al., Citation2023b). However, freezing and thawing can potentially cause damage such as reduced protein activity. Cells are broken by mechanical crushing due to the intense ultrasonic shock wave and shear stress (Fan et al., Citation2021; Wu et al., Citation2023). This method is efficient but generates large amounts of heat, resulting in the inactivation of cell membrane proteins. Hence, the corresponding cooling measures should be considered. At present, the combined use of these methods can yield satisfactory results.(Chu et al., Citation2023; Liu et al., Citation2023c; Ma et al., Citation2023; Xie et al., Citation2023). For example, neutrophils were first treated by hypotonic lysis and later treated using a homogenizer. Glioma cells and stromal cells were first treated by hypotonic lysis followed by repeated freeze-thawing treatment. Consequently, the cell membrane proteins were preserved and the nanopreparations were successfully functionalized.
Differential centrifugation is generally used to purify cell membranes (Du et al., Citation2023; Li et al., Citation2023a). Some researchers also use sucrose density gradient centrifugation or ultrafiltration to purify cell membranes (Lai et al., Citation2015; Gao et al., Citation2020b; Xie et al., Citation2023). The cell membrane vesicles were resuspended in PBS and saved at −80 °C until use.
2.2. Methods of membrane-preparation fusion
There are three main methods of membrane-preparation fusion, including physical extrusion, sonication, and microfluidic electroporation. Extrusion is the process of repeatedly mechanically pushing a nanopreparation through a 400 nm, 200 nm, and 100 nm polycarbonate porous membrane to fuze it to the cell membrane (Huang et al., Citation2023b; Ying et al., Citation2023). The method is very robust in terms of repeatability and is one of the most used methods for preparing membrane camouflaging nanopreparations. The primary drawback of using this method for time-consuming and labor-intensive, thus cannot achieve batch preparation. Inspired by the merit of simple and efficient operation, researchers increasingly use sonication to generate cavitation bubble for the spontaneous fusion of nanopreparations and cell membrane-derived vesicles (Liu et al., Citation2019c). This technique has the added benefit of losing less material than physical extrusion. However, the size of biomimetic nanopreparations prepared by ultrasonic method is not uniform, and the damage of heat generation on the activity of membrane proteins during ultrasonic process is irreversible. Lastly, a relatively new approach to enable membrane coating is to employ microfluidic electroporation (Rao et al., Citation2017; Liu et al., Citation2019a). This fabrication technique uses electric pulses to create temporary hydrophilic pores in the cell membrane so that the nanomaterials can successfully enter the cell membrane. The bionic nanomaterials obtained have uniform particle size and are efficient and can be mass-produced. However, problems such as high cost and lack of specifications and standards for core technologies need to be addressed.
2.3. Characterization of cell membrane-based biomimetic nanoparticles
To ensure the logic and effectiveness of the membrane coating process design, it is necessary to in vitro verify the structure, morphology, physical and chemical properties, and integrity of the surface proteins of the cell membrane after the successful preparation of biomimetic nanopreparations. The core-shell structure of membrane biomimetic nanopreparations can be directly observed using a transmission electron microscope or scanning electron microscope. The size and potential of membrane-coated nanopreparations can be determined using dynamic light scattering and zeta potential, respectively. The size of the hydrated particles and the electronegativity of the nanoparticles increased when membrane-encapsulated and coincided with the zeta potential of the membrane surface. The integrity of membrane surface proteins was confirmed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis on intact cells, isolated cell membranes, and protein bands coated with nanoparticles. And a Western blot was used to examine the distinctive proteins on the cell membrane. Fluorescent dyes have recently been used to confirm that membrane-mimicking nanopreparations have been successfully prepared (Liu et al., Citation2019b). Fluorescent dyes were employed to identify the cell membrane and the nanoparticle in turn, and then the membrane-coated nanoparticles were observed and analyzed using laser scanning confocal microscopy.
3. Cell membrane-based biomimetic nanoparticles for natural products
3.1. RBC membranes
RBCs, the most abundant cells in the human body, circulate in the blood for up to 120 days and are considered ideal carriers for various bioactive compounds such as enzymes, drugs, proteins, and macromolecules. Mature RBCs lack a nucleus and various organelles, which makes their extraction and purification particularly easy (Pierige et al., Citation2008; Lanao et al., Citation2020). The long-term circulation of RBCs is mediated by several membrane proteins, including integrin-associated protein (CD47), C8-binding protein, homologous restriction protein, and decay acceleration factor; these proteins play a key role in the evasion of immune defenses, including the complement system. CD47 also serves as a ‘do-not-eat-me signal’ that interacts with the glycoprotein signal-regulatory protein-α (SIRP-α) on macrophages to prevent RBCs from being cleared by the immune system (Barclay, Citation2009; Barclay and Van den Berg, Citation2014; Villa et al., Citation2016). Since cell membranes inherit the properties of their parent cells, polymer-based nanodelivery systems coated with RBC membranes (RBCMs) have been widely explored in recent years () (Hu et al., Citation2011), and this work has demonstrated their potential for preventing nanoparticle clearance by the human immune system and improving targeting efficiency for cancer treatment.
Table 2. Examples of drug-loaded nanoparticles coated with red blood cell membranes for disease treatment.
3.1.1. Polymer-modified RBCM-coated nanoparticles
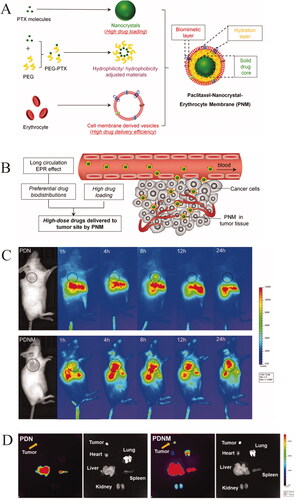
The hydrophobic nature of nanocarriers usually hinders intravenous drug administration, shortening drug half-life in the body and preventing sufficient accumulation at the lesion. Surface modification has emerged as an effective strategy to extend the lifetime of hydrophobic nanocarriers in circulation and prevent immune clearance. For example, hydrophilic PEGylated nanoparticles can evade immune clearance and prevent drug capture by the reticuloendothelial system (RES), prolonging drug circulation in the body (Li and Huang, Citation2010). However, this strategy is effective only after the first injection, and repeated injection may generate anti-PEG antibodies, which rapidly eliminate nanoparticles from the circulatory system (Wu et al., Citation2017a). In contrast, coating of polymer-modified nanodelivery systems with RBCMs stabilizes drugs and increases their bioavailability, thereby strengthening their therapeutic efficacy (Guo et al., Citation2021; Zhou et al., Citation2021; Safarpour et al., Citation2022; Zheng et al. Citation2023). In a recent study, PTX-loaded polymer nanocrystals coated with RBCMs escaped RES uptake and immunorecognition () (Zhai et al., Citation2020). Modification of nanocarriers with poly(lactic-co-glycolic acid) (PLGA), polylactic acid, poly(ε-caprolactone)–poly(ethylene glycol) (PCL-PEG) or polycaprolactone increased the anticancer activity of drugs through the enhanced permeability and retention effect (Xie et al., Citation2019; Wang et al., Citation2021b; Zhang et al., Citation2022).
Figure 3. Illustration of red cell membrane-biomimetic nanoparticles for escaping RES uptake and immunorecognition. (A) The components of PNM. (B) In vivo distributions of the PNM system. (C) In vivo and (D) ex vivo NIR fluorescence imaging of DiR-labeled PDN and PDNM (Zhai et al., Citation2020). © 2020. The Author(s). Reproduced with permission.
3.1.2. Targeted modification of RBCM-coated nanoparticles
Active targeting can lead to better drug bioavailability and fewer off-target side effects in vivo than passive targeting mediated by the enhanced permeability and retention effect. However, RBCMs lack specific proteins and molecules that could support active targeting. Therefore, the RBCM surface has been modified with the tumor-penetrating bispecific recombinant protein anti-EGFR-iRGD (Chen et al., Citation2018a; Zhang et al., Citation2018b), which can bind to integrins overexpressed in tumor blood vessels and cells as well as to the epidermal growth factor receptor overexpressed by most malignant tumors (Sha et al., Citation2015a, Citation2015b; Bian et al., Citation2016). In those studies, the membrane modification was achieved through physical lipid insertion instead of chemical conjugation in order to preserve the immune-evading biological components of RBCMs (Chen et al., Citation2018a; Zhang et al., Citation2018b).
In another study, functionalized RBCM-coated nanoparticles loaded with Cur were modified with DSPE-PEG3400-T807 (T807), allowing the particles to cross the blood-brain barrier (BBB) and target tau aggregates within neurons(Gao et al., Citation2020a). Cur is a natural anti-oxidant that can relieve the symptoms of Alzheimer’s disease (Chen et al., Citation2018b), and T807 binds strongly to hyperphosphorylated tau, inhibiting multiple pathways in Alzheimer’s pathogenesis (Do Carmo et al., Citation2021; Katharina Buerger et al., Citation2003). The surface of RBCMs has also been co-modified with triphenylphosphine cation (TPP) and T807 or rabies virus glycoprotein 29 (RVG29), resulting in nanodelivery systems that can cross the BBB and target mitochondria in neurons (Gao et al., Citation2020c; Han et al., Citation2020b; Liu et al., Citation2022b).
Nanoparticles have been modified by self-assembling hydrophobic stearic acid and hydrophilic carboxymethylcellulose, leading to selective drug release specifically in the acidic tumor microenvironment. In this approach, folic acid and PEG were conjugated to the RBCM surface via lipid chains in order to prolong drug circulation and improve targeting (Song et al., Citation2022).
3.1.3. Comprehensive therapy using RBCM-coated nanoparticles
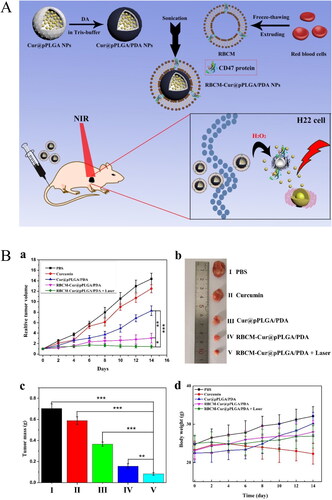
PDT is a rapidly developing cancer treatment because it offers noninvasive therapy at a specific location in the body at a particular time (Felsher, Citation2003). In recent years, various photothermal materials have been used to develop bionic nanodelivery systems encapsulating natural products, including gold nanoparticles (AuNPs) (Zhu et al., Citation2018), 5,10,15,20-tetraphenylchlorin (Pei et al., Citation2018), indocyanine green (Wang et al., Citation2020c; Zhu et al., Citation2023), near-infrared dye IR780 (Zhang et al., Citation2021c), and pyropheophorbide-α (Liu et al., Citation2022c). For example, dopamine was allowed to self-polymerize in a weakly alkaline and aerobic environment such that it formed a polydopamine (PDA) layer on the surface of Cur-loaded nanoparticles, which were then coated with RBCMs. Cur and PDA acted as both chemotherapeutic and photothermal agents, and the combination of this nanosystem with near-infrared laser irradiation effectively promoted H22 cancer cell apoptosis and significantly reduced H22 tumor volume in mice () (Wang et al., Citation2020b).
Figure 4. Illustration of red cell membrane-biomimetic nanoparticles for combining chemotherapeutic and hyperthermia to enhancing antitumor effect. (A) The preparation of RBCM-cur@pPLGA/PDA nanoparticles and in vivo chemo-photothermal therapy. (B) In vivo antitumor efficacy of the various formulations in the H22 tumor model. (a) Tumor growth curves during the drug administration period. (b) Typical photographs of tumors isolated at the end of the administration. (c) Mean weights of the tumors isolated at the end of the administration. (d) Body weight changes in drug administration period (Wang et al., Citation2020b). © 2020. The Author(s). All rights reserved. Reproduced with permission.
3.2. WBC membranes
WBCs are an important part of the body’s defense system against inflammatory diseases, cancer, viral infections, and other disorders. Macrophages derived from monocyte differentiation, lymphocytes, neutrophils, and natural killer cells are all WBCs commonly used in bionic nano-drug delivery systems, which can be recruited to the tumor microenvironment during the development of malignant tumors (Han et al., Citation2018; Xinyue et al., Citation2018; Wang et al., Citation2021a). Therefore, WBC membranes have been used extensively to prepare bionic nanodelivery systems that can extend drug circulation in vivo and enhance active drug targeting, most recently of compounds with anti-inflammatory, anti-tumor or other effects ().
Table 3. Examples of drug-loaded nanoparticles coated with white blood cell membranes for disease treatment.
3.2.1. Macrophage membranes
The WBCs most commonly used for bionic nanodelivery systems are macrophages derived from monocyte differentiation, lymphocytes, neutrophils, and natural killer cells (Parodi et al., Citation2013; Dash et al., Citation2020; Wang et al., Citation2022). Macrophage membranes contain several functional molecules such as CD45, CD11a and polysaccharides that help macrophage membrane-modified nanocarriers evade RES elimination, thereby prolonging drug circulation (Li et al., Citation2018). Other surface markers on the M1 macrophage membrane, such as CD80 and CD86, exert immune effects by activating T lymphocytes and helping the drug carrier target certain cells, while α4 integrin interacts with the vascular cell adhesion molecule-1 (VCAM-1) in cancer cells and binds to metastatic cancer cells, providing a new direction for tumor immunotherapy (Cao et al., Citation2016; Najafi et al., Citation2019). Nanoparticles encapsulated with M1 macrophage membranes were able to take advantage of M1 macrophages’ tendency to gravitate toward sites of inflammation and target tumors to increase the effectiveness of the drug therapy, compared to previous studies in which researchers used non-polarised or M2 macrophage membrane (Hu et al., Citation2020; Li et al., Citation2021a).
1. Targeted modification using macrophage membrane-coated nanoparticles
In a recent study, macrophage membrane-coated Cur-loaded nanocarriers prepared through an ultrasonication method interacted with target cells through surface proteins and vector ligands, avoiding elimination and ensuring selective delivery to disease sites (Shen et al., Citation2021). Another study reported the preparation of a Cur-based bionic drug delivery platform coated with macrophage membrane for the treatment of pneumonia. Platycodon grandiflorum polysaccharide direct nanocarriers to the lungs and target the site of pneumonia with macrophage membranes for dual targeting. The developed nanosystem significantly reduced cytokine storm syndrome and effectively inhibited acute lung injury in a mouse model (Li et al., Citation2022b). Coating macrophage membranes onto nanocarriers modified with albumin and the fusion peptide TAT-NBD enhanced drug delivery selectively to lesions (Cao et al., Citation2020; Liu et al., Citation2021). Together, the albumin and cell membrane layers significantly enhanced the cytotoxicity and apoptosis of paclitaxel, thereby improving the therapeutic efficacy in a melanoma xenograft mouse model. The fusion peptide TAT-NBD enhances the cell membrane penetration of loaded curcumin liposomes to promote their accumulation and penetration, and effectively exerts the anti-inflammatory effects of the drug.
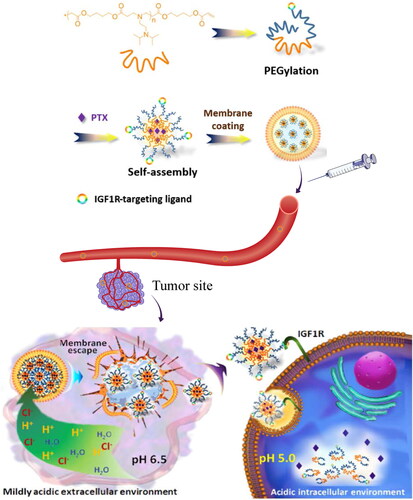
To further improve drug targeting and therapeutic efficacy, a macrophage membrane-coated nanoparticle (cskc-PPiP/PTX@Ma) was developed for tumor-targeted chemotherapy delivery. Amphiphilic bola-pattern polymers with selected side chains were first synthesized via dual-end PEGylation of poly(β-amino ester) for PTX loading. The polymer was then functionalized with a cationic 2-aminoethyldiisopropyl (PPiP) group to enable pH-responsive drug release in the tumor microenvironment. A synthetic D-form oligopeptide with the cskc sequence was also used as the targeting ligand on the nanoparticle surface to enhance uptake by cancer cells (). Cskc-PPiP/PTX@Ma showed high penetration efficiency at pH 6.5 in vitro and strong antitumor activity in mice bearing orthotopic tumors (Zhang et al., Citation2018a).
Figure 5. Illustration of macrophage membrane-biomimetic nanoparticles for escaping and drug-release mechanism (Zhang et al., Citation2018a). © 2018. The Author(s). All rights reserved. Reproduced with permission.
2. Comprehensive therapy using macrophage membrane-coated nanoparticles
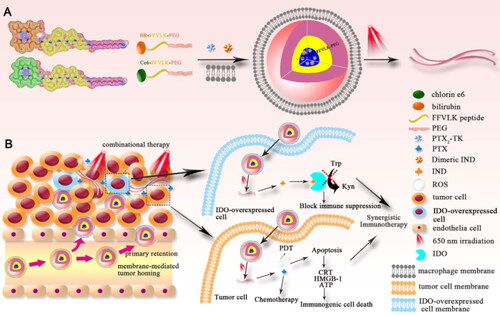
To further improve drug efficacy, several studies have investigated biomimetic nanomedicines that can change their shape or size in response to various stimuli (Liu et al., Citation2020a). For example, one study used macrophage membranes to camouflage laser-responsive, shape-changeable nanoparticles loaded with the photosensitizer chlorin e6, PTX, and indoximod (Liu et al., Citation2020a). The macrophage membrane promoted drug circulation and tumor targeting, while chlorin e6 converted laser light into reactive oxygen species (ROS) to trigger the transformation of spherical micelles into nanofibers for strong retention and enhanced cellular internalization in tumors, leading to long drug circulation and sustained drug release. ROS also directly killed tumor cells by PDT and stimulated the generation of PTX from the PTX dimer, which significantly inhibited tumor growth and induced immunogenic cell death (). Indoximod, for its part, activated antitumor immune responses. These observations indicate that this nanoplatform can achieve multimodal therapy to inhibit the growth of orthotopic breast tumors and lung metastasis.
Figure 6. Illustration of the construction and the drug-action mechanism of macrophage membrane-biomimetic nanoparticles (Liu et al., Citation2020a). © 2020. The Author(s). All rights reserved. Reproduced with permission.
These results also illustrate how nanodelivery systems coated with macrophage membrane can effectively evade RES clearance and accumulate at inflammatory and tumor sites. However, the high plasticity of macrophages means that they may be in different activation states or phenotypes when their membranes are isolated, which should be carefully controlled if the membranes are to be used for coating drug delivery systems.
3.2.2. Neutrophil membranes
Neutrophils are the most abundant type of WBCs in humans and the body’s first line of defense against invading pathogens (Chu et al., Citation2018). Neutrophils are rapidly activated in response to inflammatory stimuli and pass through the vascular endothelium to leave the circulation and migrate to sites of inflammation or tumors (Chu et al., Citation2018; Liu et al., Citation2022a; Tang et al., Citation2023). After activation by inflammatory responses, neutrophils bind to circulating tumor cells, preventing them from giving rise to distant metastatic tumors (Mantovani et al., Citation2008; Nguyen et al., Citation2009). Hence, neutrophils have been extensively studied as carriers for anti-inflammatory or antitumor drugs (Kang et al., Citation2017; Oroojalian et al., Citation2021). For instance, celastrol-loaded PEG methyl ether-block-PLGA nanoparticles were coated with neutrophil membrane to target pancreatic adenocarcinoma (Cao et al., Citation2019). The neutrophil membrane endowed nanoparticles with high affinity for tumor cells, reflecting the ability of neutrophils to accumulate at inflamed sites. In addition, the obtained nanoplatform penetrated the blood-pancreas barrier in mice bearing Panc02 tumors, achieving pancreas-targeted drug delivery and reducing systemic drug toxicity (Cao et al., Citation2019). In another study, the superior antitumor activity of the neutrophil membrane-cloaked NP construct was verified in an ectopic xenograft tumor (Chowdhury et al., Citation2022).
These results suggest that neutrophil membrane coating can significantly improve anticancer efficacy. However, neutrophils have a half-life of only 7 h and cannot be cultured in vitro, which greatly limits their application as drug delivery carriers.
3.3. Platelet membranes
Platelets, also known as thrombocytes, are the smallest non-nucleated blood myeloid cells derived from mature megakaryocytes of the bone marrow (Patel et al., Citation2005; Holinstat, Citation2017). Platelets are normally oval or disk-shaped, but their shape can vary substantially as a result of movement and deformation (Berger, Citation1970). Platelets form clots at the sites of blood vessel injury or blood leakage, and they collaborate with endothelial cells to release pro-inflammatory cytokines. In addition, they promote tumor metastasis by helping tumor cells escape from the immune system (Lefrançais et al., Citation2017; Li et al., Citation2018; Wang et al., Citation2020d; Kunde and Wairkar, Citation2021).
The platelet surface contains several immunoregulatory proteins, such as CD47 and CD55, as well as transmembrane proteins such as P-selectin. CD47 interacts with signals expressed by macrophages to favor immune escape, while P-selectin binds specifically to CD44 receptors upregulated on the surface of cancer cells to target tumor sites (Hu et al., Citation2015a, Citation2015b). Given that platelet membranes can be easily extracted and purified, they have been widely used as a coating to enable nanocarriers to adhere selectively to damaged vessels and pathogens ().
Table 4. Examples of drug-loaded nanoparticles coated with platelet membrane for disease treatment.
3.3.1. Conventional platelet membrane-coated nanoparticles
Adjuvant chemotherapy is commonly used after surgery to prevent cancer recurrence and metastasis. However, the poor targeting capability and dose toxicity of adjuvant chemotherapeutics greatly limit their efficacy (Mei et al., Citation2020). One study explored the construction of a biomimetic platelet membrane-coated nanocrystal system, which combines tumor targeting by platelets with the high drug loading of nanocrystals. The resulting PTX-loaded biomimetic nanocrystals delivered large amounts of PTX selectively to tumor sites and improved mouse survival by targeting the coagulation cascade of nanoparticles (Mei et al., Citation2020). In another study, a biomimetic Cur-loaded Ca2+ nanogenerator was developed based on an ion interference strategy for tumor-specific therapy (Zhang et al., Citation2021a). The platelet membrane coating improved tumor-targeting ability, and Ca2+ and Cur were simultaneously released in the acidic tumor microenvironment. Cur promoted the release of Ca2+ from endoplasmic reticulum and inhibited Ca2+ efflux to increase Ca2+ overload and activate mitochondrial apoptotic signaling, suggesting the potential for synergistic cancer treatment based on Ca2+ overload therapy and chemotherapy.
3.3.2. Polymer-modified nanoparticles coated with platelet membranes
A study used chitosan-modified liposomes loaded with Cur, based on the idea of pH-responsive drug release by swelling and protonation. Through platelet membrane concealment, drugs showed better bioavailability and higher retention in the bloodstream. The integration of chitosan allowed Cur, upon accumulation in tumors via the EPR effect, to react to the intracellular pH of cancer cells and promote the effective release of cytotoxic medicines, which displayed great anticancer efficacy (Wan et al., Citation2023). Cur and resveratrol, two active ingredients in natural products, were encapsulated in PLGA nanoparticles and surface-coated with platelet membrane. Due to the intrinsic affinity of the platelet membrane for the site of inflammation, more medicines can be directed at the injured lung vasculature and successfully inhibit the pulmonary vascular injury model (Jin et al., Citation2022).
3.3.3. Comprehensive therapy using platelet membrane-coated nanoparticles
Atherosclerosis is a cardiovascular disease involving thickening of arterial walls, narrowing of the lumen, chronic inflammatory autoimmune responses, and abnormal lipid metabolism (Kattoor et al., Citation2017; Wu et al., Citation2017b; Wolf and Ley, Citation2019; Saigusa et al., Citation2020). In one treatment approach, the anti-proliferative drug PTX was combined with PDT to inhibit inflammatory macrophages in order to mitigate atherosclerosis (Huang et al., Citation2020b). Specifically, a drug-coated ‘balloon’ was developed using Janus aminated mesoporous silica (JAMS) nanomotors that offered high drug loading and sustained drug release. The nanomotor was co-loaded with PTX and anti-VCAM-1 antibody, then coated with platelet membrane to reduce drug leakage. Near-infrared irradiation promoted the penetration of drug-loaded JAMS into atherosclerotic plaques, where the nanoparticles were retained in part through interaction between the anti-VCAM-1 antibody and VCAM-1 overexpressed at plaque sites.
3.4. Cancer cell membranes
Cancer cells can be easily cultured in vitro, where they can rapidly multiply to provide abundant membranes that can be exploited to endow biomimetic nanodelivery systems with immune-evasive and host-like properties (Rao et al., Citation2016; Li et al., Citation2022c). Membrane proteins on the isolated cancer cell membranes, such as tissue factor-antigen, galectin-3 and E-cadherin can recognize other cancer cells to drive targeted drug delivery () (He et al., Citation2020; Janiszewska et al., Citation2020). In addition, the transmembrane protein CD47 on cancer cell membranes can bind to SIRP-α on macrophages and trick the immune cells into thinking that the cancer cell-coated nanoparticle is endogenous and should not be phagocytosed (Fan et al., Citation2021).
Table 5. Examples of drug-loaded nanoparticles coated with cancer cell membranes for disease treatment.
3.4.1. Polymer-modified nanoparticles coated with cancer cell membranes
Nanoparticles coated with cancer cell membranes extracted from patient cancer cells are commonly used for the research of targeted cancer treatment. In order to prolong circulation of polymer-modified nanoparticles, they have been coated with membranes from 4T1 or C6 cancer cells (Han et al., Citation2020a; Fan et al., Citation2021). The resulting bionic nanocarriers avoided phagocytosis by macrophages and promoted drug accumulation in tumors, thereby reducing adverse reactions and enhancing therapeutic efficacy. The modification of the polymer also protects the loaded substance from enzymatic degradation and creates antigen and adjuvant deposition for controlled release (Liu et al., Citation2023a). In another study, PLGA nanoparticles co-loaded with PTX and small interfering RNA were camouflaged with HeLa cell membranes to achieve drug colocalization and synergistic antitumor effects (Xu et al., Citation2020). Similarly, negative charged small interfering RNA was condensed on the outer drug-loaded nanoparticles by electrostatic interaction and further protected by the cancer cell membranes also showed an excellent synergistic chemotherapy ability (Wang et al., Citation2023b). Modifying cancer cell membranes with PEG can further prolong drug circulation in the blood and reduce nonspecific binding between nanoparticles and serum proteins, thereby reducing systemic toxicity (Gao et al., Citation2021).
3.4.2. Targeted modification of cancer cell membrane-coated nanoparticles
Recent studies have focused on the modification of cancer cell membrane surfaces to enhance the targeting capability of coated nanoparticles. For example, SMMC-7721 cell membranes were modified with folic acid and used to coat PTX-loaded nanocrystals. The functionalized nanoplatform released PTX in a sustained manner and showed better targeting effects and biocompatibility than unmodified nanocrystals (Shen et al., Citation2022). In another study, the LWSW quorum sensing peptide was used to prepare a nanoplatform that selectively crossed the BBB and targeted glioma and neovascular endothelial cells (Ran et al., Citation2017). However, the nanoplatform was easily degraded by proteases in vivo, so the LWSW peptide was replaced by the DWSW peptide, which was more stable in serum and therefore preserved glioma targeting. In fact, adding DWSW to the C6 glioma membrane coating stabilized the drug delivery system in the brain of glioma-bearing mice (Fan et al., Citation2021).
3.4.3. Comprehensive therapy using cancer cell membrane-coated nanoparticles
Breast cancer poses a serious threat to women worldwide, as current treatments cannot achieve complete recovery (Hare et al., Citation2017; Wilkinson & Gathani, Citation2022). In one study, MDA-MB-231 cell membrane-coated mesoporous silica nanoparticles were co-loaded with superparamagnetic ferroferric oxide and PTX to combine chemotherapy and magnetic hyperthermia against breast cancer. When mesoporous silica particles were exposed to an alternating magnetic field, they oscillated to convert magnetic field energy to heat energy. High temperatures interfered with the biological regulatory processes in tumor cells, such as proliferation and metabolism, inducing cell necrosis or apoptosis, while the MDA-MB-231 cell membrane coating helped nanoparticles avoid immune rejection (Cai et al., Citation2019). In a similar study, PLGA nanoparticles co-loaded with Cur and chlorin e6 were encapsulated in MCF-7 cell membranes, which significantly increased their uptake by MCF-7 cells, while ROS generated by chlorin e6 under near-infrared light killed tumor cells (Zhang et al., Citation2021b).
To improve the therapeutic efficacy of PDT, Cur-loaded MnO2 nanoparticles were coated with cancer cell membrane. The resulting nanosystem evaded immune clearance and supported Cur-based sonodynamic therapy in parallel with the compound’s ability to stimulate apoptosis and inhibit proliferation (Yang et al., Citation2021). In addition, the MnO2 nanoparticles acted as catalase to alleviate hypoxia in the acidic tumor microenvironment.
These results indicate that coating nanodelivery systems with cancer cell membranes can help them evade immune clearance and target tumors, which cause increased the long circulation, self-binding capacity, and recognition functionalities of the cancer cells, thereby improving drug biodistribution and enhancing antitumor effects. Nevertheless, the use of patient-derived tumor cells to develop nanocarriers for specific antitumor drugs requires further investigation.
3.5. Hybrid membranes
Hybrid membrane-coated biomimetic nanocarriers are prepared by fuzing two different cell membranes and combining the functions and biomarker activities of both cell types () (Berta Esteban-Fernández et al., Citation2018; Raza et al., Citation2021; Li et al., Citation2022d). In a recent study, murine J774A.1 cells and head and neck tumor HN12 cells were used to design a composite cell membrane-camouflaged biomimetic nanoplatform (He et al., Citation2018). Coating the nanoparticles with the hybrid membrane favored their selective internalization by tumor cells, while their uptake by WBCs was significantly reduced, improving the toxic effects of drugs on cancer cells. In vivo studies in a mouse xenograft model of head and neck cancer also showed that hybrid membrane prolonged the circulation of nanoparticles and significantly enhanced their ability to target tumors () (He et al., Citation2018). In another studies, formulated a hybrid biomimetic camouflage delivery system by fuzing breast cancer cell membranes and activated fibroblast membranes possess outstanding dual-targeting ability, which addressing the microenvironment of breast cancer immunosuppression (Zang et al., Citation2022; Wang et al., Citation2023a).
Figure 7. Illustration of the schematic presentation of composite leukocyte and tumor cell membrane-camouflaged liposome and potential application in cancer treatment and diagnosis (He et al., Citation2018). © 2018. The Author(s). All rights reserved. Reproduced with permission.
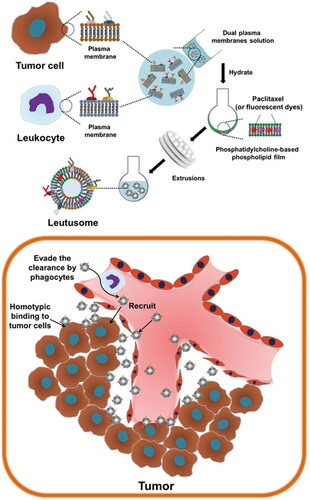
Table 6. Examples of drug-loaded nanoparticles coated with hybrid membranes for disease treatment.
The tumor microenvironment of osteosarcoma comprises various types of stromal cells, which secrete inflammatory cytokines and other humoral factors to establish an inflammatory environment (Lei et al., Citation2020; Yu et al., Citation2020). Hence, a hybrid membrane prepared from macrophages and 143B osteosarcoma cells was previously used to coat PLGA nanoparticles for targeted osteosarcoma chemotherapy (Cai et al., Citation2022). Similarly, the long functional life of platelet membranes was combined with the homologous targeting ability of human liver cancer Huh-7 cell membrane to treat hepatocellular carcinoma. The hybrid membrane was used to coat lyotropic liquid crystalline lipid nanoparticles co-loaded with sorafenib and triptolide (Li et al., Citation2021b). A similar strategy was used to coat Cur-loaded gold nanoparticles with hybrid RBC/platelet membrane, allowing the particles to evade phagocytosis, induce weaker immune responses, and support PDT (Kim et al., Citation2020).
Overall, hybrid cell membranes, by combining characteristics from two cell types, provide a potentially more flexible approach to targeted cancer treatment than single membrane-coated nanodelivery systems. However, several challenges remain to be addressed in the functionalization of nanodelivery systems with multiple cell membranes. For example, how to determine the incorporation ratio of the two cell membranes and ensure successful hybridization of the two cell membranes without damaging the cell membrane surface proteins.
4. Conclusions
Natural products have unique values and advantages in disease treatment and health care. The present manuscript has reviewed the recent scientific literature covering the last five years, in the attempt to evaluate the development of a cell-based biomimetic technology related to the delivery of natural products. Some compounds such as PTX, Cur, gambogic acid, resveratrol, triptolide and celastrol, possess myriads of pharmacological activities in vitro, although their bioavailability is virtually nil (Huang et al., Citation2023a). Cell membrane-based biomimetic nanosystems have emerged to solve this problem. First, these formulation nanodelivery strategies, such as liposomes, solid lipid nanoparticles, nanoemulsions, micelles and polymer nanoparticles, which can dramatically improve the efficacy of the natural active compounds by increasing of the bioavailability of these substances. Modification with PLGA, polylactic acid, PCL-PEG, polycaprolactone and other polymer could enhance permeability and retention effect of nanocarriers. With respect to cell membrane-based biomimetic technology, the central idea is to attach cell membrane to nanosystems superficially, before directing them inside the human body. Due to the preserved membrane compositions and antigens, some unique features and functions are inherited in these biomimetic systems, including specific neutralization of pathological molecules, immune escape capability, prolonged blood circulation, and homing to disease lesions. Additionally, further modifying folic acid, antibodies, proteins, fatty acids, and other substances by inserting lipids on the cell membrane’s surface can not only improve the bionic agent’s active targeting capability but also give it the ability to respond to stimuli. Hence, by employing cell-based biomimetic nanosystems, the drug solubility and stability can be significantly upregulated. Unwanted and adverse effects related to drug payload, particularly strong off-target toxicity, in addition to improve short half-life and low bioavailability, were prohibited.
Despite cell membrane-camouflaged nanoparticles are quite prospective in delivering natural products (), they are still in their infancy stage and many challenges need to be overcome for successful translation into the clinic. There is no clinically applicable way to guarantee the controllability, efficacy, and safety of cell membrane biomimetic nanodelivery system preparation. For instance, membrane extraction is currently performed through repeated freeze-thawing, ultrasonication or grinding-up in order to minimize the use of organic reagents or strong acids or bases, which could inactivate membrane proteins. Nevertheless, the loss of some membrane proteins during extraction is inevitable. In addition, the methods used for the fusion of cell membranes and nanodelivery systems are inefficient and can lead to substantial batch-to-batch variation, making scale-up difficult. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and western blotting can confirm the presence, but not function, of the appropriate surface components of the source cell membranes in biomimetic preparations. Therefore, additional research is required to develop more effective extraction and preparation methods to promote the application of natural products in the emerging era of nanoparticle-driven personalized medicine. The current studies have demonstrated that only application of PTX and Cur were extensively recovered, while other active components such as gambogic acid and resveratrol were not well researched. In addition, the current combined therapy of natural products nanosystems mainly focuses on magnetic hyperthermia, PDT and sonodynamic therapy. In the future studies, more emphasis can be placed on combining with other combination therapy strategies, such as radiation therapy and immunotherapy. Since these years are living an increasing interest toward natural products. Whereas, the current research on natural products nanosystems mainly focuses on monomer drugs, and there are relatively few studies on the effects of two or more drugs co-contained in the nanosystems. In future studies, more emphasis can be placed on compound drugs to achieve the reasonable collocation of different natural products to enhance the efficacy.
Table 7. Summary of cell membrane types and their characteristics.
Altogether, it can be expected that by developing the research of properties and additional embellishment of the different cell membranes, choosing the suitable drug delivery system and studding the surface of these formulations with cell membranes, the development of an efficient and safe drug delivery system could be achieved.
Authors’ contributions
Writing—original draft preparation, Yifeng Zhang and Qian Zhang; writing—review and editing, Chunhong Li. Ziyun Zhou and Hui Lei.; visualization and supervision, Minghua Liu and Dan Zhang. All authors agree to be accountable for all aspects of the work.
| Abbreviations | ||
| PTX | = | paclitaxel |
| Cur | = | curcumin |
| PEG | = | polyethylene glycol |
| RBCs | = | red blood cells |
| WBCs | = | white blood cells |
| PDT | = | photodynamic therapy |
| CD47 | = | integrin-associated protein |
| RBCMs, RBC | = | membranes |
| RES | = | reticuloendothelial system |
| PLGA | = | poly(lactic-co-glycolic acid) |
| PCL-PEG | = | poly(ε-caprolactone)–poly(ethylene glycol) |
| BBB | = | blood-brain barrier |
| AuNPs | = | gold nanoparticles |
| VCAM-1 | = | vascular cell adhesion molecule-1 |
| PPiP | = | cationic 2-aminoethyldiisopropyl |
| ROS | = | reactive oxygen species |
| JAMS | = | Janus aminated mesoporous silica |
| PDLLA | = | poly(D,L-lactic acid) |
| RVG29 | = | rabies virus glycoprotein |
| TPP | = | triphenylphosphine cation |
| PNM | = | paclitaxel-nanoparticles-erythrocyte membrane |
| NIR | = | near infrared radiation |
| PDN | = | paclitaxel/DiR hybrid nanoparticles |
| PDNM | = | paclitaxel/DiR-nanoparticles-erythrocyte membrane |
| pPLGA | = | porous poly(lactic-co-glycolic acid) |
| PDA | = | polydopamine. |
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- Bajracharya R, Song JG, Patil BR, et al. (2022). Functional ligands for improving anticancer drug therapy: current status and applications to drug delivery systems. Drug Deliv 29:1–23. doi: 10.1080/10717544.2022.2089296.
- Barclay AN. (2009). Signal regulatory protein alpha (SIRPalpha)/CD47 interaction and function. Curr Opin Immunol 21:47–52.
- Barclay AN, Van den Berg TK. (2014). The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol 32:25–50.
- Berger S. (1970). Platelet function a review. I. Normal function. Can Med Assoc J 102:1271–4.
- Berta Esteban-Fernández DÁ, Pavimol A, Ramírez-Herrera DE, et al. (2018). Hybrid biomembrane–functionalized nanorobots for concurrent removal of pathogenic bacteria and toxins. Sci Robot 3(18):eaat0485. doi: 10.1126/scirobotics.aat0485.
- Bian X, Wu P, Sha H, et al. (2016). Anti-EGFR-iRGD recombinant protein conjugated silk fibroin nanoparticles for enhanced tumor targeting and antitumor efficiency. Onco Targets Ther 9:3153–62.
- Cai D, Liu L, Han C, et al. (2019). Cancer cell membrane-coated mesoporous silica loaded with superparamagnetic ferroferric oxide and paclitaxel for the combination of chemo/magnetocaloric therapy on MDA-MB-231 cells. Sci Rep 9:14475. doi: 10.1038/s41598-019-51029-8.
- Cai JX, Liu JH, Wu JY, et al. (2022). Hybrid cell membrane-functionalized biomimetic nanoparticles for targeted therapy of osteosarcoma. Int J Nanomed 17:837–54.
- Cao H, Dan Z, He X, et al. (2016). Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano 10:7738–48. doi: 10.1021/acsnano.6b03148.
- Cao X, Hu Y, Luo S, et al. (2019). Neutrophil-mimicking therapeutic nanoparticles for targeted chemotherapy of pancreatic carcinoma. Acta Pharm Sin B 9:575–89.
- Cao X, Tan T, Zhu D, et al. (2020). Paclitaxel-loaded macrophage membrane camouflaged albumin nanoparticles for targeted cancer therapy. Int J Nanomed 15:1915–28.
- Chen M, Du ZY, Zheng X, et al. (2018b). Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease. Neural Regen Res 13:742–52. doi: 10.4103/1673-5374.230303.
- Chen H, Sha H, Zhang L, et al. (2018a). Lipid insertion enables targeted functionalization of paclitaxel-loaded erythrocyte membrane nanosystem by tumor-penetrating bispecific recombinant protein. Int J Nanomed 13:5347–59.
- Chowdhury P, Bhusetty Nagesh PK, Hollingsworth TJ, et al. (2022). Coating a self-assembly nanoconstruct with a neutrophil cell membrane enables high specificity for triple negative breast cancer treatment. ACS Appl Bio Mater 5:4554–66. doi: 10.1021/acsabm.2c00614.
- Chu D, Dong X, Shi X, et al. (2018). Neutrophil-based drug delivery systems. Adv Mater 30:e1706245.
- Chu Y, Luo Y, Su B, et al. (2023). A neutrophil-biomimic platform for eradicating metastatic breast cancer stem-like cells by redox microenvironment modulation and hypoxia-triggered differentiation therapy. Acta Pharm Sin B 13:298–314.
- Crintea A, Dutu AG, Sovrea A, et al. (2022). Nanocarriers for drug delivery: an overview with emphasis on vitamin D and K transportation. Nanomaterials (Basel) 12:1376. doi: 10.3390/nano12081376.
- Dadwal A, Baldi A. Kumar Narang R. (2018). Nanoparticles as carriers for drug delivery in cancer. Artif Cells Nanomed Biotechnol 46:295–305.
- Dash P, Piras AM, Dash M. (2020). Cell membrane coated nanocarriers – an efficient biomimetic platform for targeted therapy. J Control Release 327:546–70.
- Din FU, Aman W, Ullah I, et al. (2017). Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomedicine 12:7291–309.
- Do Carmo S, Spillantini MG, Cuello AC. (2021). Editorial: Tau pathology in neurological disorders. Front Neurol 12:754669. doi: 10.3389/fneur.2021.754669.
- Du J, Sun J, Liu X, et al. (2023). Preparation of C6 cell membrane-coated doxorubicin conjugated manganese dioxide nanoparticles and its targeted therapy application in glioma. Eur J Pharm Sci 180:106338. doi: 10.1016/j.ejps.2022.106338.
- Fan Y, Cui Y, Hao W, et al. (2021). Carrier-free highly drug-loaded biomimetic nanosuspensions encapsulated by cancer cell membrane based on homology and active targeting for the treatment of glioma. Bioact Mater 6:4402–14.
- Felsher DW. (2003). Cancer revoked: oncogenes as therapeutic targets. Nat Rev Cancer 3:375–80. doi: 10.1038/nrc1070.
- Feng Y, Tang F, Li S, et al. (2022). Mannose-modified erythrocyte membrane-encapsulated chitovanic nanoparticles as a DNA vaccine carrier against reticuloendothelial tissue hyperplasia virus. Front Immunol 13:1066268. doi: 10.3389/fimmu.2022.1066268.
- Gabizon A, Martin F. (1997). Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs 54 Suppl 4:15–21. doi: 10.2165/00003495-199700544-00005.
- Gao C, Chu X, Gong W, et al. (2020a). Neuron tau-targeting biomimetic nanoparticles for curcumin delivery to delay progression of Alzheimer’s disease. J Nanobiotechnol 18:71. doi: 10.1186/s12951-020-00626-1.
- Gao C, Huang Q, Liu C, et al. (2020b). Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat Commun 11:2622. doi: 10.1038/s41467-020-16439-7.
- Gao C, Wang Y, Sun J, et al. (2020c). Neuronal mitochondria-targeted delivery of curcumin by biomimetic engineered nanosystems in Alzheimer’s disease mice. Acta Biomater 108:285–99.
- Gao Y, Zhu Y, Xu X, et al. (2021). Surface PEGylated cancer cell membrane-coated nanoparticles for codelivery of curcumin and doxorubicin for the treatment of multidrug resistant esophageal carcinoma. Front Cell Dev Biol 9:688070. doi: 10.3389/fcell.2021.688070.
- Guo C, Hou X, Liu Y, et al. (2021). Novel Chinese angelica polysaccharide biomimetic nanomedicine to curcumin delivery for hepatocellular carcinoma treatment and immunomodulatory effect. Phytomedicine 80:153356. doi: 10.1016/j.phymed.2020.153356.
- Han Y, Chu X, Cui L, et al. (2020b). Neuronal mitochondria-targeted therapy for Alzheimer’s disease by systemic delivery of resveratrol using dual-modified novel biomimetic nanosystems. Drug Deliv 27:502–18.
- Han L, Xu Y, Guo X, et al. (2020a). Cancer cell membrane-coated biomimetic platform for targeted therapy of breast cancer in an orthotopic mouse model. J Biomater Sci Polym Ed 31:1538–51.
- Han Y, Zhao R, Xu F. (2018). Neutrophil-based delivery systems for nanotherapeutics. Small 14:e1801674. doi: 10.1002/smll.201801674.
- Hare JI, Lammers T, Ashford MB, et al. (2017). Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv Drug Deliv Rev 108:25–38.
- Harris JM, Martin NE, Modi M. (2001). Pegylation: a novel process for modifying pharmacokinetics. Clin Pharmacokinet 40:539–51. doi: 10.2165/00003088-200140070-00005.
- Hatami E, Jaggi M, Chauhan SC, et al. (2020). Gambogic acid: a shining natural compound to nanomedicine for cancer therapeutics. Biochim Biophys Acta Rev Cancer 1874:188381. doi: 10.1016/j.bbcan.2020.188381.
- He H, Guo C, Wang J, et al. (2018). Leutusome: a biomimetic nanoplatform integrating plasma membrane components of leukocytes and tumor cells for remarkably enhanced solid tumor homing. Nano Lett 18:6164–74.
- He Z, Zhang Y, Feng N. (2020). Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: a review. Mater Sci Eng C Mater Biol Appl 106:110298.
- Holinstat M. (2017). Normal platelet function. Cancer Metastasis Rev 36:195–8. doi: 10.1007/s10555-017-9677-x.
- Hu CM, Fang RH, Wang KC, et al. (2015a). Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526:118–21. doi: 10.1038/nature15373.
- Hu C, Lei T, Wang Y, et al. (2020). Phagocyte-membrane-coated and laser-responsive nanoparticles control primary and metastatic cancer by inducing anti-tumor immunity. Biomaterials 255:120159. doi: 10.1016/j.biomaterials.2020.120159.
- Hu Q, Sun W, Qian C, et al. (2015b). Anticancer platelet-mimicking nanovehicles. Adv Mater 27:7043–50.
- Hu CM, Zhang L, Aryal S, et al. (2011). Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci USA 108:10980–5.
- Huang Y, Li T, Gao W, et al. (2020b). Platelet-derived nanomotor coated balloon for atherosclerosis combination therapy. J Mater Chem B 8:5765–75.
- Huang X, Wang L, Guo H, et al. (2023b). Macrophage membrane-coated nanovesicles for dual-targeted drug delivery to inhibit tumor and induce macrophage polarization. Bioact Mater 23:69–79.
- Huang T, Wang Y, Shen Y, et al. (2020a). Preparation of high drug-loading celastrol nanosuspensions and their anti-breast cancer activities in vitro and in vivo. Sci Rep 10:8851. doi: 10.1038/s41598-020-65773-9.
- Huang J, Zhu Y, Xiao H, et al. (2023a). Formation of a traditional Chinese medicine self-assembly nanostrategy and its application in cancer: a promising treatment. Chin Med 18:66. doi: 10.1186/s13020-023-00764-2.
- Janiszewska M, Primi MC, Izard T. (2020). Cell adhesion in cancer: Beyond the migration of single cells. J Biol Chem 295:2495–505.
- Ji J, Lian W, Zhang Y, et al. (2023). Preoperative administration of a biomimetic platelet nanodrug enhances postoperative drug delivery by bypassing thrombus. Int J Pharm 636:122851. doi: 10.1016/j.ijpharm.2023.122851.
- Jin H, Luo R, Li J, et al. (2022). Inhaled platelet vesicle-decoyed biomimetic nanoparticles attenuate inflammatory lung injury. Front Pharmacol 13:1050224. doi: 10.3389/fphar.2022.1050224.
- Jin K, Luo Z, Zhang B, et al. (2018). Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sin B 8:23–33.
- Kang T, Zhu Q, Wei D, et al. (2017). Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano 11:1397–411. doi: 10.1021/acsnano.6b06477.
- Karakoti AS, Das S, Thevuthasan S, et al. (2011). PEGylated inorganic nanoparticles. Angew Chem Int Ed Engl 50:1980–94. doi: 10.1002/anie.201002969.
- Katharina Buerger MD, Raymond Zinkowski PD, Stefan J, Teipel MD, et al. (2003). Differentiation of geriatric major depression from Alzheimer’s disease with CSF tau protein phosphorylated at threonine 231. Brief Report.
- Kattoor AJ, Pothineni NVK, Palagiri D, et al. (2017). Oxidative stress in atherosclerosis. Curr Atheroscler Rep 19:42. doi: 10.1007/s11883-017-0678-6.
- Kaur A, Tiwari R, Tiwari G, et al. (2022). Resveratrol: a vital therapeutic agent with multiple health benefits. Drug Res (Stuttg) 72:5–17. doi: 10.1055/a-1555-2919.
- Kim MW, Lee G, Niidome T, et al. (2020). Platelet-like gold nanostars for cancer therapy: the ability to treat cancer and evade immune reactions. Front Bioeng Biotechnol 8:133. doi: 10.3389/fbioe.2020.00133.
- Kocaadam B, Sanlier N. (2017). Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr 57:2889–95.
- Kunde SS, Wairkar S. (2021). Platelet membrane camouflaged nanoparticles: biomimetic architecture for targeted therapy. Int J Pharm 598:120395.
- Lai P-Y, Huang R-Y, Lin S-Y, et al. (2015). Biomimetic stem cell membrane-camouflaged iron oxide nanoparticles for theranostic applications. RSC Adv 5:98222–30. doi: 10.1039/C5RA17447C.
- Lanao JM, Gutiérrez-Millán C, Colino CI. (2020). Cell-based drug delivery platforms. Pharmaceutics 13:2. doi: 10.3390/pharmaceutics13010002.
- Lefrançais E, Ortiz-Muñoz G, Caudrillier A, et al. (2017). The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544:105–9. doi: 10.1038/nature21706.
- Lei Y, Junxin C, Yongcan H, et al. (2020). Role of microRNAs in the crosstalk between osteosarcoma cells and the tumour microenvironment. J Bone Oncol 25:100322.
- Li J, Gong C, Chen X, et al. (2023b). Biomimetic liposomal nanozymes improve breast cancer chemotherapy with enhanced penetration and alleviated hypoxia. J Nanobiotechnol 21:123. doi: 10.1186/s12951-023-01874-7.
- Li Y, Guo C, Chen Q, et al. (2022b). Improvement of pneumonia by curcumin-loaded bionanosystems based on platycodon grandiflorum polysaccharides via calming cytokine storm. Int J Biol Macromol 202:691–706. doi: 10.1016/j.ijbiomac.2022.01.194.
- Li R, He Y, Zhang S, et al. (2018). Cell membrane-based nanoparticles: a new biomimetic platform for tumor diagnosis and treatment. Acta Pharm Sin B 8:14–22.
- Li S-D, Huang L. (2010). Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J Control Release 145:178–81.
- Li H, Qiao W, Shen Y, et al. (2023a). Biomimetic boron nitride nanoparticles for targeted drug delivery and enhanced antitumor activity. Pharmaceutics 15:1269. doi: 10.3390/pharmaceutics15041269.
- Li Y, Ruan S, Guo J, et al. (2022c). B16F10 cell membrane-based nanovesicles for melanoma therapy are superior to hyaluronic acid-modified nanocarriers. Mol Pharm 19:2840–53.
- Li Z, Song Z, He C, et al. (2022d). Aspirin curcumin ester loaded biomimetic nanodrug improves cognitive deficits in a mouse model of Alzheimer’s disease by regulating M1/M2 microglial polarization. Mater Today Adv 16:100321. doi: 10.1016/j.mtadv.2022.100321.
- Li B, Tan T, Chu W, et al. (2022a). Co-delivery of paclitaxel (PTX) and docosahexaenoic acid (DHA) by targeting lipid nanoemulsions for cancer therapy. Drug Deliv 29:75–88.
- Li Z, Yang G, Han L, et al. (2021b). Sorafenib and triptolide loaded cancer cell-platelet hybrid membrane-camouflaged liquid crystalline lipid nanoparticles for the treatment of hepatocellular carcinoma. J Nanobiotechnol 19:360. doi: 10.1186/s12951-021-01095-w.
- Li C, Zhao Z, Luo Y, et al. (2021a). Macrophage-disguised manganese dioxide nanoparticles for neuroprotection by reducing oxidative stress and modulating inflammatory microenvironment in acute ischemic stroke. Adv Sci 8(20):e2101526.. doi: 10.1002/advs.202101526.
- Liu R, An Y, Jia W, et al. (2020a). Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. J Control Release 321:589–601.
- Liu Q, Hu Y, Zheng P, et al. (2023a). Exploiting immunostimulatory mechanisms of immunogenic cell death to develop membrane-encapsulated nanoparticles as a potent tumor vaccine. J Nanobiotechnol 21:326. doi: 10.1186/s12951-023-02031-w.
- Liu Y, Luo J, Chen X, et al. (2019d). Cell membrane coating technology: a promising strategy for biomedical applications. Nanomicro Lett 11:100.
- Liu Y, Luo J, Liu Y, et al. (2022b). Brain-targeted biomimetic nanodecoys with neuroprotective effects for precise therapy of Parkinson’s disease. ACS Cent Sci 8:1336–49.
- Liu R, Luo C, Pang Z, et al. (2023b). Advances of nanoparticles as drug delivery systems for disease diagnosis and treatment. Chin Chem Lett 34:107518. doi: 10.1016/j.cclet.2022.05.032.
- Liu X, Sun Y, Xu S, et al. (2019c). Homotypic cell membrane-cloaked biomimetic nanocarrier for the targeted chemotherapy of hepatocellular carcinoma. Theranostics 9:5828–38. doi: 10.7150/thno.34837.
- Liu Y, Wen N, Li K, et al. (2022c). Photolytic removal of red blood cell membranes camouflaged on nanoparticles for enhanced cellular uptake and combined chemo-photodynamic inhibition of cancer cells. Mol Pharm 19:805–18.
- Liu Y, Xie X, Chen H, et al. (2020b). Advances in next-generation lipid-polymer hybrid nanocarriers with emphasis on polymer-modified functional liposomes and cell-based-biomimetic nanocarriers for active ingredients and fractions from Chinese medicine delivery. Nanomedicine 29:102237.
- Liu S, Xu J, Liu Y, et al. (2022a). Neutrophil-biomimetic “nanobuffer” for remodeling the microenvironment in the infarct core and protecting neurons in the penumbra via neutralization of detrimental factors to treat ischemic stroke. ACS Appl Mater Interf 14:27743–61.
- Liu J, Yang Y, Liu X, et al. (2021). Macrophage-biomimetic anti-inflammatory liposomes for homing and treating of aortic dissection. J Control Release 337:224–35.
- Liu C, Zhang W, Li Y, et al. (2019a). Microfluidic sonication to assemble exosome membrane-coated nanoparticles for immune evasion-mediated targeting. Nano Lett 19:7836–44.
- Liu G, Zhao X, Zhang Y, et al. (2019b). Engineering biomimetic platesomes for pH-responsive drug delivery and enhanced antitumor activity. Adv Mater 31:e1900795.
- Liu Z, Zhou X, Li Q, et al. (2023c). Macrophage-evading and tumor-specific apoptosis inducing nanoparticles for targeted cancer therapy. Acta Pharm Sin B 13:327–43.
- Lu J, Gao X, Wang S, et al. (2023). Advanced strategies to evade the mononuclear phagocyte system clearance of nanomaterials. Exploration 3(1):20220045. doi: 10.1002/EXP.20220045.
- Ma J, Dai L, Yu J, et al. (2023). Tumor microenvironment targeting system for glioma treatment via fusion cell membrane coating nanotechnology. Biomaterials 295:122026. doi: 10.1016/j.biomaterials.2023.122026.
- Mantovani A, Allavena P, Sica A, et al. (2008). Cancer-related inflammation. Nature 454:436–44. doi: 10.1038/nature07205.
- Mei D, Gong L, Zou Y, et al. (2020). Platelet membrane-cloaked paclitaxel-nanocrystals augment postoperative chemotherapeutical efficacy. J Control Release 324:341–53.
- Najafi M, Hashemi Goradel N, Farhood B, et al. (2019). Macrophage polarity in cancer: a review. J Cell Biochem 120:2756–65.
- Nguyen DX, Bos PD, Massagué J. (2009). Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9:274–84. doi: 10.1038/nrc2622.
- Oroojalian F, Beygi M, Baradaran B, et al. (2021). Immune cell membrane-coated biomimetic nanoparticles for targeted cancer therapy. Small 17:e2006484. doi: 10.1002/smll.202006484.
- Parodi A, Quattrocchi N, van de Ven AL, et al. (2013). Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol 8:61–8.
- Patel SR, Hartwig JH, Italiano JE.Jr. (2005). The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest 115:3348–54. doi: 10.1172/JCI26891.
- Patra JK, Das G, Fraceto LF, et al. (2018). Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology 16:71. doi: 10.1186/s12951-018-0392-8.
- Pei Q, Hu X, Zheng X, et al. (2018). Light-activatable red blood cell membrane-camouflaged dimeric prodrug nanoparticles for synergistic photodynamic/chemotherapy. ACS Nano 12:1630–41. doi: 10.1021/acsnano.7b08219.
- Pierige F, Serafini S, Rossi L, et al. (2008). Cell-based drug delivery. Adv Drug Deliv Rev 60:286–95.
- Puglia C, Lauro MR, Tirendi GG, et al. (2017). Modern drug delivery strategies applied to natural active compounds. Expert Opin Drug Deliv 14:755–68.
- Ran D, Mao J, Zhan C, et al. (2017). D-retro-enantiomer of quorum sensing peptides-modified polymeric micelles for brain tumor targeted drug delivery. ACS Appl Mater Interf 9(31):25672–25682.
- Rao L, Cai B, Bu LL, et al. (2017). Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano 11:3496–505. doi: 10.1021/acsnano.7b00133.
- Rao L, Bu L-L, Cai B, et al. (2016). Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging. Adv Mater 28(18):3460–3466.
- Raza F, Zafar H, Zhang S, et al. (2021). Recent advances in cell membrane-derived biomimetic nanotechnology for cancer immunotherapy. Adv Healthc Mater 10:e2002081.
- Safarpour F, Kharaziha M, Emadi R. (2022). Inspiring biomimetic system based on red blood cell membrane vesicles for effective curcumin loading and release. Int J Pharm 613:121419. doi: 10.1016/j.ijpharm.2021.121419.
- Saigusa R, Winkels H, Ley K. (2020). T cell subsets and functions in atherosclerosis. Nat Rev Cardiol 17:387–401. doi: 10.1038/s41569-020-0352-5.
- Sanchez Armengol E, Unterweger A, Laffleur F. (2022). PEGylated drug delivery systems in the pharmaceutical field: past, present and future perspective. Drug Dev Ind Pharm 48(4):129–139.
- Sha H, Li R, Bian X, et al. (2015a). A tumor-penetrating recombinant protein anti-EGFR-iRGD enhance efficacy of paclitaxel in 3D multicellular spheroids and gastric cancer in vivo. Eur J Pharm Sci 77:60–72.
- Sha H, Zou Z, Xin K, et al. (2015b). Tumor-penetrating peptide fused EGFR single-domain antibody enhances cancer drug penetration into 3D multicellular spheroids and facilitates effective gastric cancer therapy. J Control Release 200:188–200.
- Shen W, Ge S, Liu X, et al. (2022). Folate-functionalized SMMC-7721 liver cancer cell membrane-cloaked paclitaxel nanocrystals for targeted chemotherapy of hepatoma. Drug Deliv 29:31–42.
- Shen LM, Li MC, Wei WJ, et al. (2021). In vitro neuroprotective effects of macrophage membrane-derived curcumin-loaded carriers against 1-methyl-4-phenylpyridinium-induced neuronal damage. ACS Omega 6:32133–41. doi: 10.1021/acsomega.1c04894.
- Song M, Dong S, An X, et al. (2022). Erythrocyte-biomimetic nanosystems to improve antitumor effects of paclitaxel on epithelial cancers. J Control Release 345:744–54.
- Swierczewska M, Crist RM, McNeil SE. (2018). Evaluating nanomedicines: obstacles and advancements. Methods Mol Biol 1682:3–16.
- Tang Z, Meng S, Song Z, et al. (2023). Neutrophil membrane fusogenic nanoliposomal leonurine for targeted ischemic stroke therapy via remodeling cerebral niche and restoring blood-brain barrier integrity. Mater Today Bio 20:100674.
- Viegas JSR, Praca FG, Kravicz M, et al. (2020). Therapeutic applications and delivery systems for triptolide. Drug Deliv Transl Res 10:1584–600.
- Villa CH, Seghatchian J, Muzykantov V. (2016). Drug delivery by erythrocytes: “Primum non nocere. Transf Apheresis Sci 55:275–80. doi: 10.1016/j.transci.2016.10.017.
- Wan S, Fan Q, Wu Y, et al. (2023). Curcumin-loaded platelet membrane bioinspired chitosan-modified liposome for effective cancer therapy. Pharmaceutics 15:631. doi: 10.3390/pharmaceutics15020631.
- Wang S, Duan Y, Zhang Q, et al. (2020d). Drug targeting via platelet membrane-coated nanoparticles. Small Struct 1(1):2000018.
- Wang P, Jiang F, Chen B, et al. (2020c). Bioinspired red blood cell membrane-encapsulated biomimetic nanoconstructs for synergistic and efficacious chemo-photothermal therapy. Colloids Surf B Biointerf 189:110842.
- Wang S, Jiang H, Wang J, et al. (2021b). Superior in vitro anticancer effect of biomimetic paclitaxel and triptolide co-delivery system in gastric cancer. J Biomed Res 35:327–38. doi: 10.7555/JBR.35.20210102.
- Wang H, Liu Y, He R, et al. (2020a). Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater Sci 8:552–68.
- Wang Z, Tang XL, Zhao MJ, et al. (2023b). Biomimetic hypoxia-triggered RNAi nanomedicine for synergistically mediating chemo/radiotherapy of glioblastoma. J Nanobiotechnol 21:210. doi: 10.1186/s12951-023-01960-w.
- Wang D, Wang S, Zhou Z, et al. (2022). White blood cell membrane-coated nanoparticles: recent development and medical applications. Adv Healthc Mater 11:e2101349.
- Wang H, Williams GR, Xie X, et al. (2020b). Stealth polydopamine-based nanoparticles with red blood cell membrane for the chemo-photothermal therapy of cancer. ACS Appl Bio Mater 3:2350–9.
- Wang L, Wu M, Pan Y, et al. (2023a). Sequential targeting biomimetic nano platform for enhanced mild photothermal therapy and chemotherapy of tumor. Comput Struct Biotechnol J 21:2780–91.
- Wang H, Zang J, Zhao Z, et al. (2021a). The advances of neutrophil-derived effective drug delivery systems: a key review of managing tumors and inflammation. Int J Nanomed 16:7663–81.
- Wilkinson L, Gathani T. (2022). Understanding breast cancer as a global health concern. Br J Radiol 95:20211033.
- Wolf D, Ley K. (2019). Immunity and inflammation in atherosclerosis. Circ Res 124:315–27.
- Wu L, Chen J, Wu Y, et al. (2017a). Precise and combinatorial PEGylation generates a low-immunogenic and stable form of human growth hormone. J Control Release 249:84–93.
- Wu MY, Li CJ, Hou MF, et al. (2017b). New insights into the role of inflammation in the pathogenesis of atherosclerosis. Int J Mol Sci 18(10):2034.
- Wu Y, Zhu R, Zhou M, et al. (2023). Homologous cancer cell membrane-camouflaged nanoparticles target drug delivery and enhance the chemotherapy efficacy of hepatocellular carcinoma. Cancer Lett 558:216106. doi: 10.1016/j.canlet.2023.216106.
- Xie X, Wang H, Williams GR, et al. (2019). Erythrocyte membrane cloaked curcumin-loaded nanoparticles for enhanced chemotherapy. Pharmaceutics 11:429. doi: 10.3390/pharmaceutics11090429.
- Xie L, Zhang C, Liu M, et al. (2023). Nucleus-targeting manganese dioxide nanoparticles coated with the human umbilical cord mesenchymal stem cell membrane for cancer cell therapy. ACS Appl Mater Interf 15:10541–53.
- Xinyue D, Dafeng C, Zhenjia W. (2018). Neutrophil-mediated delivery of nanotherapeutics across blood vessel barrier. Ther Deliv 9:29–35.
- Xu Z, Huang J, Zhang T, et al. (2023b). RGD peptide modified RBC membrane functionalized biomimetic nanoparticles for thrombolytic therapy. J Mater Sci Mater Med 34:18. doi: 10.1007/s10856-023-06719-1.
- Xu J, Li D, Kang L, et al. (2023a). Systematic evaluation of membrane-camouflaged nanoparticles in neutralizing Clostridium perfringens epsilon-toxin. J Nanobiotechnol 21:95. doi: 10.1186/s12951-023-01852-z.
- Xu C, Liu W, Hu Y, et al. (2020). Bioinspired tumor-homing nanoplatform for co-delivery of paclitaxel and siRNA-E7 to HPV-related cervical malignancies for synergistic therapy. Theranostics 10:3325–39. doi: 10.7150/thno.41228.
- Yang Y, Hua S, Suo W, et al. (2021). A novel bionic catalyst-mediated drug delivery system for enhanced sonodynamic therapy. Front Bioeng Biotechnol 9:699737. doi: 10.3389/fbioe.2021.699737.
- Ying K, Zhu Y, Wan J, et al. (2023). Macrophage membrane-biomimetic adhesive polycaprolactone nanocamptothecin for improving cancer-targeting efficiency and impairing metastasis. Bioact Mater 20:449–62.
- Yu Y, Zhang H, Ren T, et al. (2020). Development of a prognostic gene signature based on an immunogenomic infiltration analysis of osteosarcoma. J Cell Mol Med 24:11230–42.
- Zang S, Huang K, Li J, et al. (2022). Metabolic reprogramming by dual-targeting biomimetic nanoparticles for enhanced tumor chemo-immunotherapy. Acta Biomater 148:181–93.
- Zhai Z, Xu P, Yao J, et al. (2020). Erythrocyte-mimicking paclitaxel nanoparticles for improving biodistributions of hydrophobic drugs to enhance antitumor efficacy. Drug Deliv 27:387–99.
- Zhang Y, Cai K, Li C, et al. (2018a). Macrophage-membrane-coated nanoparticles for tumor-targeted chemotherapy. Nano Lett 18:1908–15.
- Zhang Y, Feng X, Jia X, et al. (2021a). Biomimetic Ca(2+) nanogenerator based on ions interference strategy for tumour-specific therapy. J Drug Target 29:1094–101.
- Zhang Y, He Z, Li Y, et al. (2021b). Tumor cell membrane-derived nano-Trojan horses encapsulating phototherapy and chemotherapy are accepted by homologous tumor cells. Mater Sci Eng C Mater Biol Appl 120:111670.
- Zhang Z, Ji Y, Hu N, et al. (2022). Ferroptosis-induced anticancer effect of resveratrol with a biomimetic nano-delivery system in colorectal cancer treatment. Asian J Pharm Sci 17:751–66.
- Zhang Z, Qian H, Huang J, et al. (2018b). Anti-EGFR-iRGD recombinant protein modified biomimetic nanoparticles loaded with gambogic acid to enhance targeting and antitumor ability in colorectal cancer treatment. Int J Nanomed 13:4961–75.
- Zhang Y, Xia Q, Wu T, et al. (2021c). A novel multi-functionalized multicellular nanodelivery system for non-small cell lung cancer photochemotherapy. J Nanobiotechnol 19:245. doi: 10.1186/s12951-021-00977-3.
- Zheng Y, Wang Y, Xia M, et al. (2022). The combination of nanotechnology and traditional Chinese medicine (TCM) inspires the modernization of TCM: review on nanotechnology in TCM-based drug delivery systems. Drug Deliv Transl Res 12:1306–25.
- Zheng J, Yang N, Wan Y, et al. (2023). Celastrol-loaded biomimetic nanodrug ameliorates APAP-induced liver injury through modulating macrophage polarization. J Mol Med (Berl) 101:699–716. doi: 10.1007/s00109-023-02321-8.
- Zhou J, Guo B, Zhu W, et al. (2021). Novel biomimetic nanostructured lipid carriers for cancer therapy: preparation, characterization, and in vitro/in vivo evaluation. Pharm Dev Technol 26:81–91.
- Zhu F, Huang C, Lin Y, et al. (2023). Self-delivery of a metal-coordinated anti-angiogenic nanodrug with GSH depleting ability for synergistic chemo-phototherapy. Biomater Sci 11:7132–45. doi: 10.1039/d3bm00994g.
- Zhu DM, Xie W, Xiao YS, et al. (2018). Erythrocyte membrane-coated gold nanocages for targeted photothermal and chemical cancer therapy. Nanotechnology 29:084002. doi: 10.1088/1361-6528/aa9ca1.