Abstract
Background: HBOC-201 is an ultra purified bovine hemoglobin solution. It has already been used in clinical phase II/III trials for emergency treatments. Animal experiments have shown that HBOC-201 is highly effective in tissue oxygenation. The study was performed in order to assess the potential of low dose HBOC-201 to improve tumor oxygenation.
Methods: 30 rats with a subcutaneously growing rhabdomyosarcoma R1H tumor were randomly assigned either to be ventilated with carbogen (n = 10), or to receive an IV injection of 0.3 g/kg HBOC-201 (n = 10) or a combination of 0.3 g/kg HBOC-201 and carbogen breathing (n = 10). Under general anesthesia the effects of the respective treatment on the tissue oxygen tension (tpO2) of the tumor were determined using a flexible stationary probe at baseline (b) and 15 and 60 min after application of the respective medication.
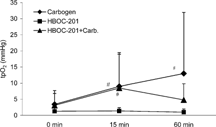
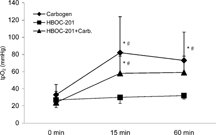
Results: HBOC-201 alone failed to improve tumor tpO2 (b: 1.3 ± 1.2 mmHg; 15 min: 1.4 ± 1 mmHg; 60 min: 1 ± 1 mmHg). In combination with carbogen the mean tpO2 of the tumor raised in comparison to baseline values (b: 3.1 ± 4.6 mmHg; 15 min: 8.5 ± 11* mmHg; 60 min: 4.8 ± 5 mmHg; *p < 0.05 vs. b), but this effect was less pronounced than the increase in tpO2 by carbogen alone (b: 3.4 ± 3.4 mmHg; 15 min: 9 ± 10* mmHg; 60 min: 13 ± 19* mmHg; *p < 0.05 vs. b).
Conclusion: The application of low dose hemoglobin solution HBOC-201 does not result in improvement of tissue oxygenation in the rat rhabdomyosarcoma R1H.
INTRODUCTION
Hypoxia decreases the radiocurability of tumors and is a general marker for malignancy, i.e. hypoxic tumors show a poorer treatment outcome in terms of local tumor control and overall survival [Citation[1], Citation[2]]. The potential to improve local control and survival by hypoxia modification was demonstrated by a meta-analysis of 83 clinical trials [Citation[3]] and a number of therapeutic strategies have been established to overcome tumor hypoxia by improving oxygen supply either by oxygen or carbogen breathing or by increasing the hemoglobin level and oxygen delivery [Citation[4], Citation[5]].
Alternatively to transfusions or the treatment with recombinant human erythropoietin, the oxygen delivery might be improved by using hemoglobin based oxygen carrier (HBOC). HBOC-201 (Biopure, Cambridge, MA, USA) is a cell-free ultra purified polymerized bovine hemoglobin solution without significant side effects on liver, kidney and coagulation [Citation[6]]. Oxygen is molecularly bound and transported in the plasma. The low oxygen affinity (p50 = 36 mmHg), which is regulated by chloride ions rather than by 2, 3-diphosphoglycerate, and a pronounced Bohr effect are the main advantages of bovine over human hemoglobin [Citation[7], Citation[8]]. In comparison to red blood cells, HBOC-201 provided higher muscular tissue oxygen tensions after profound hemodilution [Citation[9]]. The higher oxygen extraction ratio from HBOC results in a relative tissue oxygenation potential that is three–to four-fold higher than that of stored autologous red cells [Citation[10]]. The favorable oxygen binding curve of HBOC-201 with a low oxygen affinity results in facilitated oxygen release to oxygen deprived tissue, e.g. in post-stenotic areas [Citation[10], Citation[11]]. The enhanced diffusive oxygen transport may be beneficial in increasing tissue oxygenation in a hypoxic tumor and thus improving the therapeutic results after fractionated irradiation or chemotherapy.
The present animal study was performed to investigate the influence of the hemoglobin solution HBOC-201 on tumor oxygenation of a rat rhabdomyosarcoma in comparison with a healthy skeletal muscle. HBOC-201 was additionally combined with carbogen (95% O2 + 5% CO2), which has been shown to be an effective and non-toxic radio sensitizer, both in clinical and experimental settings [Citation[12-14]].
MATERIALS AND METHODS
The study was approved by the local Animal Care Committee and supervised according to the German Law for Animal Protection from 1987.
All experiments were performed with the rat rhabdomyosarcoma R1H. This transplantable solid tumor, with a doubling time of 4 days at a size of 1.6 cm3, was derived from the BA 1112 tumor [Citation[15]]. The tumor shows no signs of specific immunogencity neither when induced by serial transplantation of cryoconserved tumor tissue [Citation[16]] nor when induced by injection of viable tumor cells obtained from in vitro culture.
Cryoconserved R1H-tumor pieces from the same source tumor were transplanted subcutaneously (s.c.) to the back of WAG/Rij-rats. The originating tumors were passaged in the back of a second group of animals before transplantation into experimental animals. In order to guarantee utmost homogeneity of the tumor system, not more than ten passages were allowed. Source tumors were excised, cleaned from necrotic tissue, cut into small pieces (about 1 mm3 in size) and transplanted s.c. into the back (laterally) of syngenic male WAG/Rij albino rats. For transplantation the animals were anesthetized with intramuscular (i.m.) 0.5 mg/100 g xylazine and 4.5 mg/100 g ketamine hydrochloride.
The animals were housed under conventional conditions, the room was maintained at an ambient temperature of 23 ± 2°C (mean ± SD) and the relative humidity was 55 ± 5% (mean ± SD). Animals were provided with food and water ad libitum and were kept at periods of 12 h lights on and 12 h lights off.
Thirty animals were randomly assigned either to be ventilated with carbogen (n = 10), to receive an i.v. injection of 0.3 g/kg HBOC-201 (n = 10) or to receive 0.3 g/kg HBOC-201 in combination with carbogen breathing (n = 10). After induction of anesthesia (0.5 mg/100 g xylazine and 4.5 mg/100 g ketamine hydrochloride i.m.), a tracheotomy was performed and animals were ventilated via a 16 G cannula with an FIO2 of 0.21 (tidal volume 7 ml kg−1, respiratory frequency 80 min−1). A central venous catheter was placed in the left or right jugular vein, an arterial line for withdrawal of blood and blood pressure measurement was placed in the left or right carotid artery. Heart rate was measured using an intracutaneous needle. Oxygen tensions were measured with the flexible Licox silicon catheter (Licox Medical Systems, GMS, Kiel, Germany) with a diameter of 0.8 mm placed in the center of the tumor and in a skeletal muscle on the opposite lower extremity using an introducer needle. The employed latest version of the Licox system spared the calibration of the device, since the catheters are calibrated by the manufacturer. The standard measures were transferred to the monitor during every measurement. After insertion in the respective tissue the catheter remained at the same place during the whole time of the measurements. The microelectrode averages the tissue oxygen tensions near the tip of the electrode in a tissue layer located concentrically around the long axis of the microelectrode [Citation[17]]. 120 single tpO2 values (1 every 5 sec) over a period of 10 min were determined and displayed on the monitor of the connected computer at every time of measurement. Resulting histograms consequently do not represent a variety of values corresponding with the distribution of tpO2 values in the tissue, but a variety of tpO2 values in an outlined area over a period of 10 min. The actual value of the tpO2 is generated from the reduction of oxygen and is displayed continuously as a number expressed in millimeters of mercury. The accuracy of measurement has been demonstrated in different animal experiments [Citation[17], Citation[18]]. Body temperature was measured using a flexible subcutaneous probe and maintained constant.
After an equilibration time of 30 minutes a baseline measurement was performed. Arterial blood pressure, blood gases, heart rate, temperature and oxygen tensions in the tumor and skeletal muscle were recorded. Fifteen and 60 min after start of the respective therapy hemodynamics, tissue oxygen tensions, blood gases, plasma hemoglobin concentrations and temperature were recorded.
Statistical analyses were performed using the software SPSS 9.0 (SPSS Inc., Chicago, IL, USA) with Man-Whitney-U-Test and Wilcoxon-Test. P < 0.05 was considered to be statistically significant.
Sample size calculation was performed using the program Instat (Graphpad, CA, USA) and was based on the expected increase in muscle oxygen tension. Based on measurements in previous studies [Citation[9], Citation[10]], we expected a baseline tpO2 in the skeletal muscle of around 25 ± 5 mmHg. After application of HBOC-201 we expected an increase to values of 40 mmHg with an anticipated pooled standard deviation of 10 mmHg. We would permit a type I error of α = 0.05, and with the alternate hypothesis, the null hypothesis would be retained with a type II error of β = 0.2. This analysis reaches a power of 0.8 and indicated that a sample size of at least 8 animals per group was required. To compensate for missing data or potential dropouts, we set the group size to 10 animals in each group.
RESULTS
Animals of all groups were comparable with respect to body weight and tumor volume (Carbogen-group: 1.2 ± 0.5 cm3, HBOC-group: 1.3 ± 0.6 cm3,HBOC + Carbogen-group: 1.4 ± 0.9 cm3; p = 0.7). Measurements of body temperature showed no differences between groups over the whole study period.
The effect of HBOC-201, carbogen or the combination of both on mean arterial pressure, arterial pO2, paCO2 and plasma hemoglobin concentrations are summarized in .
Table 1. Mean arterial blood pressure (MAP) and oxygenation parameters of animals treated with 0.3 g/kg HBOC-201, carbogen alone, and a combination of HBOC-201 and carbogen
Fifteen and 60 minutes after application of HBOC-201 a significant increase of the mean arterial pressure was observed. The same effect was seen when HBOC-201 was combined with carbogen.
No significant changes of arterial pO2 was observed neither 15 nor 60 minutes after application of HBOC-201, whereas carbogen breathing alone or in combination with HBOC-201 resulted in a significant increase in arterial pO2 at each time of measurement.
The paCO2 increased only in animals ventilated with carbogen. HBOC-201 treatment resulted in an increase of the plasma hemoglobin concentration ().
Oxygen partial pressures in the tumor increased significantly 15 and 60 min after the onset of carbogen breathing. An increase was also seen 60 min after treatment with HBOC-201 + Carbogen, but no change of tpO2 could be detected after application of HBOC-201 alone (). A significant increase in oxygen tensions, 15 and 60 minutes after carbogen breathing alone or in combination with HBOC-201, was also seen in the skeletal muscle on the opposite leg (), whereas HBOC-201 alone had no effect on the skeletal muscle tpO2.
DISCUSSION
For socio-economic reasons the development of safe and effective synthetic oxygen carriers as an alternative to homologous red blood cell transfusion was an important issue in perioperative medicine during the last decades [Citation[19]]. Currently, two types of artificial oxygen carriers are experimentally and clinically investigated for their ability to replace red blood cells and to ensure adequate tissue oxygenation in case of acute anemia: cell-free human or bovine hemoglobin solutions and synthetic perflourocarbone emulsions. Since bovine hemoglobin solutions are free of significant side effects on liver, kidney and coagulation [Citation[7], Citation[20]], and proved to be very effective tissue oxygenators [Citation[9], Citation[10]], their application for sensitizing tumors in radiation and chemotherapy by improving tissue oxygenation was expectable.
Animal studies have shown that HBOC-201 is a potent oxygen carrier with a high oxygenation potential after profound hemodilution [Citation[9]]. Its physical and chemical properties, such as the low oxygen affinity independent of 2, 3-DPG and a high oxygen extraction ratio, makes HBOC-201 a promising substance for the improvement of tumor oxygenation. Already ten years ago an improved tumor response to radiation as well as to chemotherapeutic treatment after the application of hemoglobin solutions was shown. Studies on different mouse tumors [Citation[21]] demonstrated an effect on growth delay and cell survival in the in vivo-in vitro excision assay after single dose irradiation or fractionated treatment applying five fractions, respectively.
In contrast to our expectation, the oxygenation of tumor tissue and skeletal muscle, determined by the microelectrode measurements was only significantly increased by carbogen breathing, but was not changed after HBOC-201 treatment. The lacking effect on the tpO2 after HBOC-201 treatment might be caused by a vasoconstrictive side effect of cell free hemoglobin, which is consistent with the significant increase in mean arterial pressures in these groups (). A possible explanation could be scavenging of nitric oxide by cell free hemoglobin in the plasma, which might lead to vasoconstriction of the rare tumor feeding vessels. A pronounced vasoconstrictive effect as a consequence of NO scavenging by free hemoglobin was demonstrated for former generations of cell free hemoglobin. Although this effect is minimized by the polymerization with glutaraldehyde [Citation[22]] a vasoconstrictive effect of HBOC-201 on the vessels and by that a worsening of tumor perfusion cannot be ruled out in our study.
Our results of lacking increase of tpO2 in the tumor are disappointing in the light of our previous studies where HBOC-201 provided tissue oxygenation, even at low dosages in areas with restricted blood flow [Citation[10]] and in poststenotic tissues [Citation[11]]. The improved diffusive oxygen transport should have made HBOC-201 a promising agent for tumor oxygenation.
Moreover, our results are in conflict with the literature [Citation[21], Citation[23]], where an increase in tpO2 as well as an improvement of radiation response of experimental murine tumors after treatment with hemoglobin solutions is reported. However, it has to be noted that the hemoglobin dosage used in these studies was about fivefold higher than in the present study. With the dosage of 0.3 g/kg HBOC-201 we were able to achieve a plasmatic hemoglobin concentration of 0.8 ± 0.1 g/dl, which we considered to be high enough for the improvement of tissue oxygenation according to previous studies [Citation[9], Citation[10]]. Possibly the selected dosage only improves tissue oxygenation in normal tissue but not in hypoxic tissue with inadequate and disturbed vascularisation. However, the applied amount of HBOC-201 also failed to increase muscle tpO2 in the healthy rat skeletal muscle, which only can be explained by an extensive increase in systemic vascular resistance, which is supplied by the increase in blood pressure after application of HBOC-201 in this study.
Using a higher dosage in patients to improve tissue oxygenation in tumors needs a much higher volume to infuse and might be associated with a dose-dependent increase in side effects, especially with repetitive application for fractionated tumor irradiation. It has to be noted that a higher concentration of HBOC does not necessarily lead to improved radio responsiveness. Teicher reports for the Lewis lung carcinoma a decrease of the dose-modifying factor with increasing dosage of a hemoglobin solution when combined with carbogen breathing [Citation[24]]. Additionally, the effect of increasing oxygen tension in the tumor by application of HBOC-201 is probably dependent on the type of tumor.
In conclusion, we were not able to show increased oxygenation by a low dose of the cell free hemoglobin solution HBOC-201 in the rat R1H tumor. However, since HBOC-201 also failed to increase the tpO2 in the healthy skeletal muscle, more studies with different tumors in different species have to be done before this new therapeutic approach to improve tumor oxygenation can be finally assessed.
Note: Both first authors contributed equally to this publication.
REFERENCES
- Fyles, A.W., Milosevic, M., Wong, R., Kavamagh, M.C., Pintilie, M., Sun, A., Cahpman, W., Levin, W., Manchul, L., Keane, T.J., Hill, R.P. (1998). Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother. Oncol. 48(2): 149–156. [PUBMED], [INFOTRIEVE], [CSA]
- Sundfor, K., Lyng, H., Tropé, C.G., Rofstad, E.K. (2000). Treatment outcome in advanced squamous cell carcinoma of the uterine cervix: relationships to pre-treatment tumor oxygenation and vascularisation. Radiother. Oncol. 54(2): 101–107. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Overgaard, J., Horsman, M.R. (1996). Modification of hypoxia-induced radio resistance in tumors by the use of oxygen and sensitizers. Semin. Radiat. Oncol. 6(1): 10–21. [PUBMED], [INFOTRIEVE], [CSA]
- Bernier, J., Denekamp, J., Rojas, A., Minatel, E., Horiot, J., Hamers, H., Antognoni, P., Dahl, O., Richaud, P., Van Glabbeke, M., Pierart, M. (2000). ARCON: accelerated radiotherapy with carbogen and nicotinamide in head and neck squamous cell carcinomas. The experience of the Cooperative Group of Radiotherapy of the European Organization for Research and Treatment of Cancer (EORTC). Radiother. Oncol. 55(2): 111–119. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kaanders, J.H., Bussing, J., Van der Kogel, A.J. (2002). ARCON: a novel biology-based approach in radiotherapy. Lancet. Oncol. 3(12): 728–737. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lee, R., Atsumi, N., Jacobs, E.E., Austen, W.G., Vlahakes, G.J. (1989). Ultrapure, stroma-free, polymerized bovine hemoglobin: evaluation of renal toxicity. J. Surg. Res. 47(5): 407–411. [PUBMED], [INFOTRIEVE], [CSA]
- Manning, J.M. (1994). Random chemical modification of hemoglobin to identify chloride binding sites in the central dyad axis: their role in control of oxygen affinity. Artif. Cells Blood Substit. Immobil. Biotech. 22(2): 199–205. [CSA]
- Fronticelli, C., Bucci, E., Ort, C. (1984). Solvent regulation of oxygen affinity in hemoglobin. Sensitivity of bovine haemoglobin to chloride ions. J. Biol. Chem. 259(17): 10841–10844. [PUBMED], [INFOTRIEVE], [CSA]
- Standl, T., Horn, E.P., Wilhelm, S., Greim, C., Freitag, M., Freitag, U., Sputtek, A., Jacobs, E.E., Schulte am Esch, J. (1996). Bovine hemoglobin is more potent than autologous red blood cells in restoring muscular tissue oxygenation after profound isovolaemic haemodilution in dogs. Can. J. Anesth. 43(7): 714–23. [CSA]
- Standl, T., Freitag, M., Burmeister, M.A., Horn, E.P., Wilhelm, S., Schulte am Esch, J. (2003). Hemoglobin based oxygen carrier HBOC-201 provides higher and faster increase in oxygen tension in the sceletal muscle of anemic dogs than stored red cells. J. Vasc. Surg. 37(4): 859–865. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Horn, E.P., Standl, T., Wilhelm, S., Jacobs, E.E., Freitag, U., Freitag, M., Schulte am Esch, J. (1997). Bovine hemoglobin increases skeletal muscle oxygenation during 95% artificial arterial stenosis. Surgery 121(4): 411–418. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Zaricksson, B., Franzen, L., Henriksson, R., Littbrand, B., Stratford, M., Dennis, M., Rojas, A., Denekamp, J. (1994). Acute effects of accelerated radiotherapy in combination with carbogen and nicotinamide (ARCON). Acta. Oncol. 33(4): 377–381. [CSA]
- Hartmann, K.A., Van der Klej, A.J., Carl, U.M., Hulshof, M.C.C.M., Willers, R., Sminia, P. (2001). Effects of hyperbaric oxygen and normobaric carbogen on the radiation response of the rat rhabdomyosarcoma R1H. Int. J. Radiat. Oncol. 51(4): 61–73. [CSA]
- Rojas, A., Hodgekiss, R.J., Stratfort, M.R.L., Dennis, M.F., Johns, H. (1993). Pharmocokinetics of varying doses of nicotinamide and tumor radio sensitization with carbogen and nicotinamide: clinical considerations. Brit. J. Cancer. 68(6): 1115–21. [PUBMED], [INFOTRIEVE], [CSA]
- Reinhold, H.S. (1965). A cell dispersion technique for use in quantitative transplantation studies with solid tumors. Eur. J. Cancer. 1(1): 67–71. [PUBMED], [INFOTRIEVE], [CSA]
- Raabe, A., Quaester, S., Dubben, H.H., Zieron, J.O., Krull, A., Alberti, W., Beck-Bornholdt, H.P. (2001). Impact of treatment acceleration and its timing on the response of the Rhabdomyosarcoma R1H of the rat to fractionated irradiation. Strahlenther. Oncol. 77(7): 362–366. [CSA]
- Knudson, M.M., Lee, S., Erikson, V., Morabito, D., Derugin, N., Manley, G.T. (2003). Tissue oxygen monitoring during hemorrhagic shock and resuscitation: a comparison of lactated ringer's solution, hypertonic saline dextran, and HBOC-201. J. Trauma. 54(2): 242–252. [PUBMED], [INFOTRIEVE], [CSA]
- Lee, S.K., Morabito, D., Hemphill, J.C., Erikson, V., Holcroft, J.J., Derugin, N., Knudson, M.M., Manley, G.T. (2002). Small volume resuscitation with HBOC-201: Effects on cardiovascular parameters and brain tissue oxygen tension in an out of hospital model of hemorrhage in swine. Acad. Emerg. Med. 9(10): 969–976. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Goodnough, L.T., Shander, A., Brecher, M.E. (2003). Transfusion medicine: looking to the future. Lancet. 361(9352): 161–9. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lipfert, B., Standl, T., Dullmann, J., Helmchen, U., Schulte am Esch, J., Lorke, D.E. (1999). Histology and ultrastructure of liver and kidney following blood exchange with ultrapurified, polymerized bovine hemoglobin in comparison with hydroxyethyl starch. Lab. Invest. 79(5): 573–82. [PUBMED], [INFOTRIEVE], [CSA]
- Teicher, B.A., Dupuis, N.P., Emi, Y., Ikebe, M., Kakeji, Y., Menon, K. (1995). Increased efficacy of chemo- and radiotherapy by a hemoglobin solution in the 9L gliosarcoma. In vivo 9(1): 11–18. [PUBMED], [INFOTRIEVE], [CSA]
- Gilroy, D., Shaw, C., Parry, E., Olding Smee, W. (1988). Detection of a vasoconstrictor factor in stroma-free hemoglobin solutions. J. Trauma. 28(9): 1312–1316. [PUBMED], [INFOTRIEVE], [CSA]
- Teicher, B.A., Holden, S.A., Dupuis, N.P., Kusomoto, T., Liu, M., Liu, F., Menon, K. (1994). Oxygenation of the rat 9L gliosarcoma and the rat 13672 mammary carcinoma with various doses of a hemoglobin solution. Artif. Cells Blood Substit. Immobil. Biotechnol. 22(3): 827–33. [PUBMED], [INFOTRIEVE], [CSA]
- Teicher, B.A., Terence, T.S., Hopkins, R.E., Menon, K. (1991). Effect of tumor response to radiation by perfluorochemical emulsions or a bovine hemoglobin preparation. Int. J. Radiat. Oncol. Biol. Phys. 21(4): 969–974. [PUBMED], [INFOTRIEVE], [CSA]