Abstract
Short term cell cultures are usually grown in contact with biomaterials to assess cytocompatibility. Depending on the rate of material degradation or corrosion, the time of culture can be a key- point in the method which, if too short, may not show any effect of the released material on the cells.
A long term culture was therefore carried out with L929 fibroblast cells in contact with PLLA/PDLA samples for up to eight months. The degradation was measured in terms of shear-strength properties, intrinsic viscosity of the material and its cristallinity. The effect of the material on the cells was evaluated by measuring the growth rate of the cells.
A significant decrease in the shear strength of the material was measured after three months. The rate of modification of the intrinsic viscosity was regular and decreased progressively throughout the culture period. Differential scanning calorimetry showed that the samples were initially essentially amorphous and that contact with the cell culture and its medium did not change its crystallinity level. The growth rate of the cells was not modified by the presence of the material when compared to the control. This study showed this material to be cytocompatible for a long period of time, even after detection of modifications of its physico-chemical properties.
INTRODUCTION
Biodegradable polymers whose hydrolysis and/or biodegradation leads to degradation by-products of the Krebs cycle represent a great interest as they are unlikely to exacerbate recipient's reactions, because all the debris are supposed to be physiologically eliminated after degradation. The family of polylactide polymers ranks amongst the most attractive biodegradable polymers as they are easy to process for the manufacture of pegs, screws and plates used in orthopedics and maxillo-facial surgery [Citation[1]]. Numerous studies have been published concerning the in vitro cell response to polylactic acid polymers. None of them reported any side effect in vitro after a short incubation period following the standard procedures [Citation[2], Citation[3]]. However, after 4 weeks incubation in water, polylactic acid was found to be toxic [Citation[4]]. The implantation of those materials in vivo and their following degradation caused the apparition of a foreign body reaction associated to the hydrolysis of the polymer [Citation[5-7]]. Debris of high molecular PLLA originating from plates and screws still caused exacerbated tissue reactions more than 5 years after implantation. The inflammatory reaction surrounding the implant depends upon many factors, most of them related to the device characteristics, i.e. size, cristallinity, and molecular weight. Several mechanisms have been suggested to explain this biocompatibility alteration [Citation[8]]. All are related to the degradation process occurring after several weeks of implantation and particularly to the release of acidic products by the material.
In that regard, the aim of the present work is to evaluate the degree of hydrolysis of poly-L-lactide samples with their toxicity by using an established fibroblast cell line [Citation[9-13]]. The cell culture was observed for a total period of eight months. The cell growth rate was measured throughout the culture period and the samples were subjected monthly to mechanical testing and to an evaluation of polymer viscosity, as well as its crystallinity, in order to assess the material degradation.
MATERIALS AND METHODS
1. Material
The polymer tested consisted of a copolymer, PLA 96 (Purac Biochem, Netherlands), made of 96% L-polylactide (PLLA) and 4% D-polylactide (PDLA), and was received in granules. The viscosity of the copolymer was 6–7 dl/g. The samples were prepared by injection under a controlled atmosphere using an injection press (Babyplast - Mecaplast, Spain). The samples had the shape of a rectangular slide with the following dimensions length: 102±0.2 mm, width 9±0.2 mm, thickness 2±0.1 mm, and weighed between 7.5±0.23 g.
For the purpose of this study, three samples were tested for each condition. At the end of the incubation period, the samples were dried at room temperature, weighed, tested mechanically. Their fragments were used for the evaluation of the remaining viscosity and cristallinity of the material.
2. Cell Culture
L929 mouse fibroblasts were seeded at a density of 105 cells/ml and grown to confluence in 80 cm2 polystyrene cell flasks containing 10 ml of culture medium. This culture medium was composed of DMEM medium supplemented with L-glutamine (20 mM) and 10% fetal calf serum. The medium was changed every two days and the culture grown in a 5% CO2 atmosphere at 37°C. Three controls were used. The first one consisted of a flask with cells only in order to evaluate the effect of the material on the cell growth. The second control consisted of a flask containing samples without cells in order to evaluate the possible effect of the cells on the degradation of the material. The third control consisted of a flask with the medium only.
When the cells reached a confluent state, they were suspended in the culture medium using a trypsin-EDTA solution. Half of the cell suspension was removed at each subculture and the cells were counted with a Malassez hemocytometer. The growth rate was determined as:where n is the cell number at the time of the subculture, n0 is the initial cell number at t0, and t = t1 − t0. The cell culture was maintained in this way for up to 8 months.
The culture medium pH was measured at each subculture using a pH probe (Mettler-Toledo, USA).
3. Implant Weight and Gross Observations
After the incubation, the samples were carefully removed from the flask, rinsed with demineralized water, and allowed to dry at room temperature. They were then weighed, and the weight loss was reported using this equation:where Wt is the dry weight after incubation and W0 is the initial weight.
4. Mechanical Testing
The shear strength and the Young's modulus of the material were evaluated by using the three point flexural test. The test was performed using an INSTRON tensile tester. One sample from each period of incubation was used for the determination of the test parameters. A total of three to five trials were performed for each time period.
The shear strength was determined using the following equation:where F is the load applied (N), L is the distance between the contact points (mm), b is the sample width (mm), and h is the sample thickness (mm).
5. Differential Scanning Calorimetry
The DSC thermograms were recorded with a Perkin Elmer DSC-7 thermal analyzer. Experiments were carried out in triplicate on samples weighing between 5 and 10 mg. The samples were heated from 30°C to 200°C at 10°C/min. Indium and tin were used to calibrate the system. The crystallinity level was determined using a value of 93.4 J/g for the melting enthalpy of a 100% crystalline PLLA sample.
6. Viscosity Measurement
The samples were dissolved in chloroform at different concentrations (0.02, 0.04, 0.06, 0.08 g/dl). The intrinsic viscosity (IV) was determined using a Ubbelohde viscosimeter at 25°C. The average viscometric molecular weight, Mw, was calculated from the IV measurement using the Mark-Houwink equation:where the values of K and a are constancies, 5.45.10−4 and 0.73, respectively, and M is the molecular mass.
RESULTS
1. Cell Culture
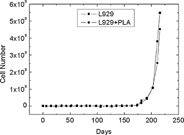
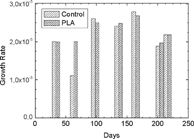
The cells generally covered the material. No significants differences in cell numbers were observed, in absence or in presence of the material (). In addition, the examination under light microscopy did not reveal any anomalies of the cell morphology. Their growth rate was constant all along the culture period. No significant variation of the growth rate between cells grown in the presence of samples and that of control cultures was reported for the duration of the experiment. Furthermore, no significant variation was observed in the growth rate between the different periods of culture, whether the cells were grown in the presence of the material or not ().
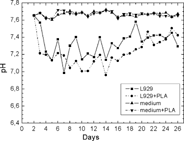
The pH of the culture medium containing cells grown with the material did not differ significantly from the one containing the control culture. However, it was significantly lower than the pH in the medium containing the control samples only ().
2. Gross Observation and Weight Variations
All the specimens were initially translucent. They progressively became more opaque, with the first traces of white appearing after 45 days of culture. At day 120, all the samples were white. The weights of the samples progressively increased by more than 1% after 180 days of incubation compared to the initial weights. The increases were similar for the two series of samples, i.e., without cells vs with cells.
3. Mechanical Properties
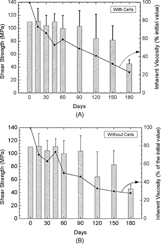
The mechanical properties did not change significantly during the first two months for the material incubated without cells, and for three months for the material incubated with cells. After 6 months, the value of the flexural resistance decreased from 110 MPa initially to 45 MPa for both materials. The presence of cells did not affect the degradation of the material in regard to this parameter ( and ).
4. Inherent Viscosity
The variations of the inherent viscosity did not follow the same trend as the mechanical characteristics. The decrease of the inherent viscosity was regular throughout the culture period. The presence of cells did not affect this property ( and ).
5. Differential Scanning Calorimetry
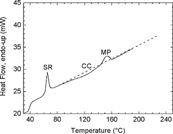
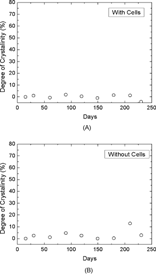
The results of the crystallinity measurements are summarized in . It can be seen that the crystallinity level for each sample is very low. The peak at 65°C is due to the occurrence of stress relief, the polymer chains having enough mobility at this temperature to release the strain induced on them by the manufacturing process. The downward curve between 80°C and 140°C represents cold crystallization and creates additional crystalline zones in the sample. The area under the fusion peak at around 150°C is therefore proportional to the sum of the initial crystallinity in the sample and that added by cold crystallization when the sample is heated to measure the thermogram. The crystallinity level of a sample is therefore determined by measuring the area under the fusion peak (around 150°C) and subtracting from it the contribution resulting from the cold crystallization (around 120°C). The results obtained for samples incubated with and without cells indicate that the samples were initially essentially amorphous (< 97%) and the effect of the cell culture or their culture media on the crystallinity level remained marginal ( and ).
DISCUSSION
The culture of an established cell line at the surface of the polymer showed that the cytocompatibility of the device, based on cell proliferation, remained satisfactory throughout the culture period, although hydrolysis of the material was occurring. The compounds released by the material during this period did not significantly modify the pH of the culture medium.
The very low initial crystallinity can most likely be explained by the manufacturing process. If subsequent cooling occurs rapidly, it can strongly limit the formation of crystalline domains in the sample because the polymer chains are unable to arrange themselves in orderly fashion before the temperature becomes so low as to make their movements very slow. The results presented here also indicate that the PLLA samples were unable to significantly modify their crystallization state in the presence of the cells and their culture media. This observation is in agreement with the one made [Citation[14]].
The buffering ability of the culture medium suggests that, in vitro, the release rate of the acid compounds constituting the polymer is low during the culture period. The polymer is degraded when implanted in an animal for the same period and a fragmentation of the material occurs accompanied by a foreign body reaction.
Degradation in vivo occurs more rapidly than in vitro. This suggests that the nature of the cells in contact with the material, the possible release of enzymes, such as α-chymotrypsin [Citation[15]], may have a role in implant degradation. The pH around the implant is another factor that would need to be addressed. In the present study, the incubation solution was replaced regularly in order to avoid any major pH changes. However, in vivo, the degradation of the device could be associated with a local decrease of this pH. Since the degradation reaction is autocatalytic at lower pH, the degradation of the device is then enhanced by the new conditions [Citation[16]].
The mechanical characteristics did not vary significantly during the first 3 months of culture. The subsequent decrease in the mechanical characteristics was then very rapid. It is important to note that during the degradation period, there was no loss of weight. After 7–8 months, the samples had no mechanical resistance, but no modification of the shape could be noted.
Comparison of the mechanical characteristics and the inherent viscosity confirmed that IV is a good indicator of the degradation state of the specimen. It is very important to note that the decreases in IV and the mechanical characteristics did not match. The decrease in IV was regular suggesting a decrease in Mw due to hydrolysis of the polymer. The same trend was previously reported by Tsuji [Citation[17]].
CONCLUSION
All the results and observations from this study confirm that PLA96 retains its initial mechanical characteristics during the first three months, even if the measure of the intrinsic viscosity showed a degradation of the material after a few days of incubation.
This study was also able to confirm the non toxicity of PLA96, even though any discrepancy between the results induced in vivo would indicate that the cytotoxicity tests based on cell proliferation should be interpreted with caution.
REFERENCES
- Vert, M. (2004). Med. J. Malaysia 59: B, 73–74. [PUBMED], [INFOTRIEVE], [CSA]
- Chu, C.R., Monosov, A.Z., Amiel, D. (1995). Biomaterials 16: 1381–1384. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Park, A., Griffith Cima, L.G. (1996). J. Biomed. Mater. Res. 31: 117–130. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Taylor, M.S., Daniels, A.U., Andriano, K.P., Heller, J. (1994). J. Appl. Biomater. 5: 151–157. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Van der Elst, M., Kuiper, I., Klein, C.P.A.T., Patka, P., Haarman, HJ.Th.M. (1996). J. Biomed. Mater. Res. 30: 139–143. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lam, K.H., Schackenraad, J.M., Esselbrugge, H., Feijen, J., Nieuwenhuis, P. (1993). J. Biomed. Mater. Res. 27: 1569–1577. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gibbons, D.F., Gysberg, J.E., Kato, K.H., Parks, P.J. (1994). Biodegradable Plastics and Polymers, Y. Doi, K. Fukuda, Eds., Elsevier Science B.V.: Amsterdam, pp. 470–477.
- All, S.A.M., Doherty, P.J., Williams, D.F. (1993). J. Biomed. Mater. Res. 27: 1409–1418. [CSA], [CROSSREF]
- Biological evaluation of medical devices-part 5: tests for cytotoxicity: in vitro methods. 1999 International Standards 10993-5.
- Standard practice for direct contact cell culture evaluation of materials for medical devices. ASTM F 813-83 (reapproved 1988).
- Standard test method for agar diffusion cell culture screening for cytotoxicity. 2001 ASTM F895-84.
- Materiel medico-chirurgical: evaluation in vitro de la cytotoxicite des materiaux et dispositifs medicaux. 1988 Normalisation française S90-702.
- Bordji, K., Jouzeau, J.Y., Mainard, D., Payan, E., Netter, P., Rie, K.T., Stucky, T., Hage-Ali, M. (1996). Biomaterials 17: 929–940. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kader, A., Jalil, R. (1998). Drug Dev. Ind. Pharm. 24: 527–534. [PUBMED], [INFOTRIEVE], [CSA]
- Tsitlanadze, G., Kviria, T., Katsarava, R., Chu, C.C. (2004). J. Mat. Sci.: Mat. Med. 15: 185–190. [CSA], [CROSSREF]
- Li, S.M., Garreau, H., Vert, M. (1990). J. Mater. Sci.: Mater. Med. 1: 123–130. [CSA], [CROSSREF]
- Tsuji, H. (2003). Biomaterials 24: 537–547. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]