Abstract
Epineurium or perineurium neurorrhaphy to recover the nerve continuity was the choice of peripheral nerve mutilation. The nerve selective regeneration theory was put forth by Cajal et al. As this theory was gradually accepted, many researchers had focused on it and its possible application. Our labs had centered on the small gap sleeve bridging fields for about 30 years, using autogeneic vein, artery and biogradable chitin conduits. Our goal was to improve the nerve regeneration effect by means of nerve selective regeneration theory and degradable biomaterials. This serial experiment was to confirm the possibilities of using conduit small gap sleeve bridging to substitute the traditional epineurium neurorrhaphy.
Keywords:
INTRODUCTION
Epineurium or perineurium neurorrhaphy to recover the nerve continuity was the choice of peripheral nerve mutilation [Citation[1]]. But the functional recovery was unsatisfactory in many cases. The influencing factor in the postoperative functional recovery was the correct matching of the proximal sensory or motor tracts with the corresponding tracts in the distal stumps. When there was a small gap between the ruptured mixed nerve, the proximal sensory and motor nerve fibers would grow through the gap and selectively find their counterpart in the distal nerve stumps. This was the nerve selective regeneration theory, which was put forth by [Citation[2]]. As this theory was gradually accepted, many researchers had focused on it and its possible application [Citation[3-5]]. Our labs had centered on the small gap sleeve bridging fields for about 30 years, using autogeneic vein, artery and biogradable chitin conduits. These serials experiments were to confirm the possibilities of using conduit small gap sleeve bridging to substitute the traditional epineurium neurorrhaphy.
EXPERIMENT ONE: AUTOGENEIC ARTERY SMALL GAP SLEEVE BRIDGING PERIPHERAL NERVE INJURY
Materials and Methods
1. Materials: autogenic right common carotid artery (5 mm).
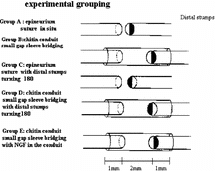
2. Groups: Build the sciatic rat injury model at left side with cut breakpoint locating at 10 mm above sciatic nerve crotch. 48 Wistar female rats(weight 200 ∼ 250 g) were divided into four groups according to the operation methods, with 12 rats each group. All the operations on animals were allowed by the animal operation experiment committee of Peking University. Group A: Small gap artery conduit bridging sciatic nerve in situ after cutoff (with 5 mm gap between the two stumps). Group B Epineurium neurorrhaphy was applied in situ after sciatic cutoff. Group C: Small gap conduit bridging sciatic nerve with distal stump turning 180° after cutoff (2 mm gap between the two stumps). Group D: Epineurium neurorrhaphy was applied with distal stump turning 180° after sciatic cutoff.
Experiment Methods
SD rats were anaesthesized with 5% ketamine hydrochloride (0.2–0.3 ml/100 g) by intraperitoneal injection. Sciatic nerve injury models were constructed by cutting the right sciatic nerve at 10 mm above sciatic nerve crotch.
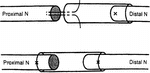
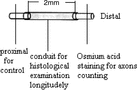
1. Artery conduit sture methods: Artery conduit was put between the two stumps at 4x microsurgery. 9-0 nylon suture penetrated the conduit wall from external side to inner side at the site 1 mm from the conduit stump fringe. After suturing the epineurium, suture needle penetrated the conduit wall from inner side to external side at the corresponding paralleling site with the needling point. There was 2 mm between the two nerve stumps and nerve stumps were inserted into conduit about 1 mm. The suture model is shown in .
2. Epineurium neurorrhaphy methods: Epineurium neurorrhaphy was applied using 9-0 nylon suture at 4x microsurgery with 2 stitches one stump.
Detection Items
1. Observation in general: Observation on stitch site and conduit morphology were applied at postoperation 2nd week and 8th week, respectively, as shown in and .
2. Electrophysiology examination: 6 SD rats were subjected to electrophysiology examination postoperation 2nd week and 8th week, respectively. Electrodes were placed at the two sides of nerve suture site or conduit. Receiving electrode was placed at peripheral side. We measured the distance between two electrodes and calculated the nerve conduction velocity and calculated the motor nerve conduction velocity.
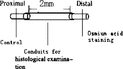
3. Light microscope observation: Experiment animals were killed after electrophysiology examination at postoperation 2nd week and 8th week, respectively. Artery conduit group: Regenerated nerve fibers were observed on the conduit longitude sections; Nerve segment 5 mm distal of conduit was taken for nerve fiber counting after osmium tetroxide staining. Nerve segment 5 mm proximal of conduit served as controlled. Non-conduit group. Nerve segment 5 mm distal suture site was taken for nerve fiber counting after osmium tetroxide staining. Nerve segment 5 mm proximal of conduit served as controlled. The section methods are shown in .
4. Immunohistochemistry staining of the regenerated nerve fibers: The histological samples were carried out for immunohistochemistry staining (S100 for Schwann cells and SMI-31 for myelinated sheath). Specimes were fixed by 10% formaldehyde and carried out for osmium tetroxide staining. 5 µm transactions were obtained after paraffin imbedding. Myelinated fibres counting per unit vision field was conducted under 10 × 40 light microscope. 6 nerve transactions of every time point of 5 groups were taken to the counting. We got the center point of the vision field and chose up left, up right, down left, down right and the center as 5 unit vision fields. The unit vision fields frame were fixed under the microscope for counting. Distal myelinated fibres cross section area was calculated by equating 100 myelinated fibres cross section area using pathological picture software of Beijing Aviation Aerospace University.
5. Statistics analysis: Data was diaplayed by x¯±s and analysed by software SPSS 10.0. “q” test was used to compare the mean value of multi-groups, and “t” test was used to compare the mean value of two groups. p < 0.05 was considered to be statistical difference.
Results
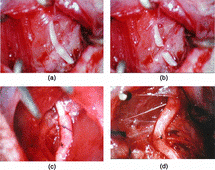
1. Observation in general: Artery conduit outlines were smooth and can still retain the cave continuity for regenerated nerve fibers at 2nd week. The regenerated nerve fibers had grown through the artery conduits at 8th week.
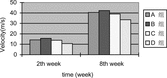
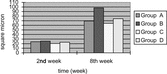
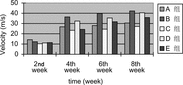
2. Motor nerve conduction velocity: The motor nerve conduction velocity of every time point of 4 groups can be seen in . Seen from the motor nerve conduction velocity recovery tendency of 4 group, the recovery tendency of small gap artery conduit bridging sciatic nerve (Group B) was the best. There were no statistical differences between small gap artery conduit bridging sciatic nerve group (Group A) and epineurium neurorrhaphy group (Group B) at the two time points (2nd week and 8th week). There were no statistical differences between small gap artery conduit bridging sciatic nerve with distal stump turning 180° group (Group C) and epineurium neurorrhaphy with distal stump turning 180° group (Group D) at 2nd week time point; (p > 0.05); while there were significant statistical differences at 8th week time point (p < 0.05).
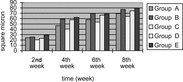
3. Myelinated fibers counting per unit vision field: The number of myelinated fibers counting per unit vision field of 2nd week and 8th week time point of 4 group can be seen in . Seen from the myelinated fibers counting per unit vision field recovery tendency of 4 group, the recovery tendency of epineurium neurorrhaphy with distal stump turning 180° group (Group C) was the worst. There were no significant statistical differences between small gap artery conduit bridging sciatic nerve group (Group A) and epineurium neurorrhaphy group (Group B) at 2nd week and 8th week time point (p > 0.05). There were no significant statistical differences between epineurium neurorrhaphy with distal stump turning 180° group (Group D) and small gap artery conduit bridging sciatic nerve with distal stump turning 180° group (Group C) at 2nd week (p > 0.05), while there were significant statistical differences at 8th week (p < 0.05).
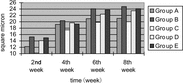
4. Distal myelinated fibers axons cross section area: The distal myelinated fibers axons cross section area of 2nd week and 8th week time point of every group was calculated respectively. Seen from the myelinated fibers axons cross section area recovery tendency of 4 group, the recovery tendency of epineurium neurorrhaphy group (Group A) was the best, and that of small gap artery conduit bridging sciatic nerve with distal stump turning 180° group (Group C) was better, and that of epineurium neurorrhaphy with distal stump turning 180° group (Group D) was the worst. There were no significant statistical differences between small gap artery conduit bridging sciatic nerve group (Group A) and epineurium neurorrhaphy group (Group B) at 2nd week and 8th week time point (p > 0.05) There were no significant statistical differences between epineurium neurorrhaphy with distal stump turning 180° group (Group D) and small gap artery conduit bridging sciatic nerve with distal stump turning 180° group (Group C) at 2nd week and 8th week time point, respectively (p < 0.05), as can be seen in .
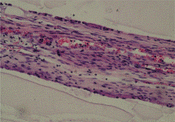
5. Histological observation: S100 immunohistochemistry staining demonstrated that regenerated nerve fibers grow through the conduit of group A and group C at 2nd week, as can seen in . The SMI-38 immunohistochemistry staining demonstrated that the quality of the regenerated nerve axons of group A and group B were of almost the same, as can be seen in .
Figure 8 Regenerated nerve fibers grow through the conduit (2nd week, S100 immunohistochemistry staining).
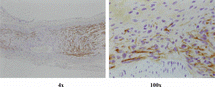
Figure 9 SMI-38 immunohistochemistry staining for the regenerated nerve axons of the distal segments (100 ×).

Nerve conduit made good use of the relative for nerve axons to regeneration. Many scholars had tried autogenic vein to bridge the dissected two stumps. But the vein wall was not forceful enough to retain the containing cave for regeneration as autogenic artery conduit. Our autogenic artery conduit bridging peripheral nerve data demonstrated that the recovery effect of autogenic artery conduit bridging group was almost the same as that of the epineurium neurorrhaphy group. But the limited resource of autogenic artery limited the further animal experiments and clinical applications.
Recently, with the development of biomaterials and biology, new biogradable biomaterials have been invented. Peking University and Chinese Spinning and Weaving Institute co-invented a kind of biomaterials named de-acetyl chitin conduit, which can retain the conduit structure for at least 6 weeks in vivo and biodegrade completely as shown in .
EXPERIMENT TWO: BIODEGRADABLE CHITIN CONDUIT SMALL GAP SLEEVE BRIDGING PERIPHERAL NERVE INJURY
Materials and Methods
1. Materials: medium altitude circular cylinder conduits a kind of de-acetyl chitin conduit patented by Peking University and China Spinning and Weaving Insititute, National Patent Number: 01136314.2). Specification of manufactured conduit: conduit length 8 mm, conduit wall thickness: 0.1 mm internal diameter: 1.5 mm.
2. Groups: All the operations on animals were allowed by the animal operation experiment committee of Peking University. 120 healthy adult SD rats (weight 200 ∼ 250 g, respectively) were chosen to build the right sciatic nerve injury model. The cut breakpoint was located at 10 mm above sciatic nerve crotch. Animals were divided into 5 groups according to the operation methods, with 24 in one group. Group A: Epineurium neurorrhaphy was applied in situ after sciatic cutoff. Group B: Small gap conduit bridging sciatic nerve in situ after cutoff (with 5 mm gap between the two stumps). Group C: Epineurium neurorrhaphy was applied with distal stump turning 180° after sciatic cutoff. Group D: Small gap conduit bridging sciatic nerve with distal stump turning 180° after cutoff (5 mm gap between the two stumps). Group E: Small gap conduit bridging sciatic nerve in situ after cutoff (with 5 mm gap between the two stumps), NGF (produced by Chinese Military Science Institute) was added into the conduit with 1000 U/conduit, as can be seen in .
Experiment Methods
SD rats were anaesthesized with 5% ketamine hydrochloride (0.2–0.3 ml/100 g) by intraperitoneal injection. Sciatic nerve injury models were constructed by cutting the right sciatic nerve at 10 mm above sciatic nerve crotch. SD rats were anaesthesized with 5% ketamine hydrochloride (0.2–0.3 ml/100 g) by intraperitoneal injection. Sciatic nerve injury models were constructed by cutting the right sciatic nerve at 10 mm above sciatic nerve crotch.
1. Conduit sture methods: Conduit was put between the two stumps at 4x microsurgery. 9-0 nylon suture penetrated the conduit wall from external side to inner side at the site 1 mm from the conduit stump fringe. After suturing the epineurium, suture needle penetrated the conduit wall from inner side to external side at the corresponding paralleling site with the needling point. There was 2 mm between the two nerve stumps and nerve stumps were inserted into conduit about 1 mm. The suture model was shown in .
2. Epineurium neurorrhaphy methods: Epineurium neurorrhaphy was applied using 9-0 nylon suture at 4x microsurgery with 2 stitches one stump.
Detection Items
1. Observation in general: Observation on stitch site and conduit morphology were applied at postoperation 2 weeks, 4 weeks, 6 weeks, 8 weeks, respectively.
2. Electrophysiology examination: 6 SD rats were subjected to electrophysiology examination postoperation 2 weeks, 4 weeks, 6 weeks, 8 weeks, respectively. Electrodes were placed at the two sides of nerve suture site or conduit. Receiving electrode was placed at peripheral side. Measured the distance between two electrodes and calculated the nerve conduction velocity using Oxford Electromyologram.
3. Light microscope observation: Experiment animals were killed after electrophysiology examination at postoperation 2 weeks, 4 weeks, 6 weeks, 8 weeks, respectively. Conduit group: Regenerated nerve fibers were observed on the conduit longitude sections; Nerve segment 5 mm distal of conduit were taken for nerve fiber counting after osmium tetroxide staining. Nerve segment 5 mm proximal of conduit served as controlled. Non-conduit group: Nerve segment 5 mm distal suture site were taken for nerve fiber counting after osmium tetroxide staining. Nerve segment 5 mm proximal of conduit served as controlled. The section methods are shown in .
Specimens were fixed by 10% formaldehyde and carried out for osmium tetroxide staining. 5 µm transactions were obtained after paraffin imbedding. Myelinated fibres counting per unit vision field was conducted under 10 × 40 light microscope. 6 nerve transactions of every time point of 5 groups were taken to the counting. We got the center point of the vision field and chose up left, up right, down left, down right and the center as 5 unit vision fields. The unit vision fields frame were fixed under the microscope for counting. Distal myelinated fibres cross section area was calculated by equating 100 myelinated fibres cross section area using pathological picture software of Beijing Aviation Aerospace University.
4. Statistics Analysis: Data was diaplayed by x¯±s and analyzed by software SPSS 11.0. “q” test was used to compare the mean value of multi-groups. and “t” test was used to compare the mean value of two groups. p < 0.05 was considered to be statistical difference.
Results
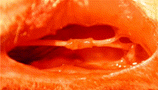
1. Observation in general: No obvious adhesions were found 6th week and 8th week after operation. Chitin conduit outline became crude and absorbed partial but still can retain the cave continuity for regenerated nerve fibers. Blood capillary vasoganglion can be seen on the chitin conduit, as can be seen in .
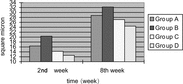
2. Motor nerve conduction velocity: The motor nerve conduction velocity of every time point of 5 groups can be seen in . Seen from the motor nerve conduction velocity recovery tendency of 5 group, the recovery tendency of small gap conduit bridging sciatic nerve (Group B) was the best. There were no statistical differences between small gap conduit bridging sciatic nerve group (Group B) and epineurium neurorrhaphy group (Group A) at the four time point (2nd weeks, 4th weeks, 6th weeks and 8th weeks). There were no statistical differences between small gap conduit bridging sciatic nerve with distal stump turning 180° group (Group D) and epineurium neurorrhaphy with distal stump turning 180° group (Group C) at 2nd week time point, (p > 0.05), while there were significant statistical differences at 4th week, 6th week and 8th week time point (p < 0.05). There were significant statistical differences between small gap conduit bridging sciatic nerve group (Group B) and small gap conduit bridging sciatic nerve in situ with NGF group (Group E) at 4th week, 6th week and 8th week time point (p < 0.05), while there were no statistical differences at 2nd week time point (p > 0.05), as can be seen in .
3. Myelinated fibres counting per unit vision field: The number of myelinated fibres counting per unit vision field of every time point (2nd week, 4th week, 6th week and 8th week) of 5 group can be seen in . Seen from the myelinated fibres counting per unit vision field recovery tendency of 5 group, the recovery tendency of small gap conduit bridging sciatic nerve in situ with NGF group (Group E) was the best; the simple small gap conduit bridging sciatic nerve group (Group B) was the better; while epineurium neurorrhaphy with distal stump turning 180° group (Group C) was the worst. There were no significant statistical differences between small gap conduit bridging sciatic nerve group (Group B) and epineurium neurorrhaphy group (Group A) at 2nd week, 4th week, 6th week and 8th week time point (p > 0.05). There were no significant statistical differences between epineurium neurorrhaphy with distal stump turning 180° group (Group C) and small gap conduit bridging sciatic nerve with distal stump turning 180° group (Group D) at 2nd week, (p > 0.05), while there were significant statistical differences at 4th week, 6th week and 8th week respectively (p < 0.05). There were no significant statistical differences between simple small gap conduit bridging sciatic nerve group (Group B) and small gap conduit bridging sciatic nerve in situ with NGF group (Group E) at 2nd week, 4th week, 6th week and 8th week time point (p > 0.05), but the myelinated fibers counting per unit vision field of group E exceeded that of group B as shown in .
4. Distal myelinated fibers axons cross section area: The distal myelinated fibres axons cross section area of every time point (2nd week, 4th week, 6th week and 8th week) of every group was calculated respectively. Seen from the myelinated fibres axons cross section area recovery tendency of 5 group, the recovery tendency of simple small gap conduit bridging sciatic nerve group (Group B) was the best; that of small gap conduit bridging sciatic nerve with distal stump turning 180° group (Group D) was better, and that of epineurium neurorrhaphy with distal stump turning 180° group (Group C) was the worst. There were no significant statistical differences between simple small gap conduit bridging sciatic nerve group (Group B) and epineurium neurorrhaphy group (Group A) at 2nd week time point (p > 0.05), while there were significant statistical differences at 4th week, 6th week and 8th week time point respectively (p < 0.05). There were no significant statistical differences between epineurium neurorrhaphy with distal stump turning 180° group (Group C) and small gap conduit bridging sciatic nerve with distal stump turning 180° group (Group D) at 2nd week (p > 0.05), while there were significant statistical differences 4th week, 6th week and 8th week time point respectively (p < 0.05). There were no significant statistical differences between between simple small gap conduit bridging sciatic nerve group (Group B) and small gap conduit bridging sciatic nerve in situ with NGF group (Group E) at 2nd week, 4th week, 6th week and 8th week time point (p > 0.05), as can be seen in .
5. Histological observation: No regenerated nerve fibers grow through the conduit of group B and group D at 2nd week. Regenerated nerve fibers grow through the conduit of group B and group D at 4th week and 8th week, as can seen in and .
DISCUSSION
Peripheral nerve injure is very common in the clinical practice and the most commonly used way to repair it is the epineurium suture or fascicular suture under the microscope in order to reach the accurate connection of the proximal stump and the distal stump. Epineurium or perineurium anastomosis is the most used repair method for peripheral nerve mutilation. As known to all, multifold factors can impact the peripheral nerve repair therapeutic effect, while the effective regenerated nerve axons and the effective innervationto distal organs were the most important two sides. Over the last couple of years, people have done a lot of research on the apposition of functionally corresponding fascicules by means of anatomy and immunohistochemistry in order to achieve the accurate apposition but most of them failed to find application in the clinical practice. Nerve selected regeneration theory was put forward by Cajal et al. [Citation[2]] and was confirmed by some researchers in the last decade [Citation[6-9]]. Cajal demonstrated in the neural regenerative chamber model that a great deal of sprouts grow from the proximal stumps and preferentially into the distal corresponding degenerated and regenerated myelinated sheaths composed by Schwann cells, namely sensory nerve fibers grow into distal sensory nerve myelinated sheaths and motor nerve fibers grow into distal motor nerve myelinated sheaths. This is termed preferential or selective regeneration. Small gaps between stumps can form a micro-environment suitable for the chemotaxis factors from the distal stumps imposing effects on the proximal stumps so it is plausible to figure out that reserving a special cavity between the proximal and the distal stumps that is called small gap bridging or entubulation will be beneficial to the regeneration of mixed nerve fibers. The peripheral nerve selective regeneration theory provided new applicable ways for peripheral nerve effective regeneration [Citation[10]]. Many scholars had utilized autogeneic epineurium [Citation[11], Citation[12]], or normal nerve trunks [Citation[13]] sautogeneic vein and autogeneic small artery and even the muscle fibers [Citation[14], Citation[15]] to allow peripheral nerve selected regeneration [Citation[16], Citation[17]]. The satisfactory regeneration effect was observated in SD rat sciatic nerve injury model but the limited resource of autogeneic epineuriumautogeneic, vein and autogeneic small artery confined their possible clinical applicable feasibility. The autogeneic epineurium and autogeneic vein conduit may also collapse when the gap between two stumps was a little longer than 2 mm. Our animal experiment compared the results of epineurium anastomosis and autogeneic small artery or biodegradable chitin conduit with 2 mm gap and found that: (1) The regeneration results of 2 mm small gap bridging nerve stumps were better than that of epineurium anastomosis when nerve distal segments were rotated. (2) The suitable regeneration gap for SD rat nerve was 2 mm, and it can not facilitate the nerve regeneration effects when the gap exceeded 5 mm. (3) No neuromas were found in conduit. We concluded that the repair effect of bridging nerve stumps with small gap can be compared to that of epineurium anastomosis. We were seeking a kind of new material with better biocompatibility that can facilitate the nerve regeneration effects. With the development of biomaterial science, several kinds of biodegradable materials were found and used in biomedical fields [Citation[18], Citation[19]]. Some scholars even demonstrated that adding Schwann cells [Citation[20-23]] or bone marrow stromal cells [Citation[24]] in the conduit cavity can improve the nerve fibers regeneration effect. In this experiment, the chitin conduits were invented by Chinese Spinning and Weaving Science Institute. The conduits were made from natural marine shell life with partial de-acetyl procedure. Animal experiment had proved that its degradation residue had no toxicity to imbedding body and can be absorbed completely [Citation[25]], Chitin conduits possess satisfactory biocompatibility and can retain conduit cave frame in vivo for 6 ∼ 8 weeks to allow axons regeneration. Related data about this chitin conduit had obtained the patent of Chinese National Patent Bureau.
Seen from the results of this experiment, the motor nerve conduction velocity and myelinated fibres counting per unit vision field of group B and group A were of no significant differences at all the four time point. The regenerated nerve fibers quality guidelines (distal myelinated fibres axons cross section area; myelinated fibres counting per unit vision field) of group B (small gap conduit bridging sciatic nerve with 5 mm gap) was better than that of group A (epineurium neurorrhaphy), which implied that the regeneration effect of small gap (2 mm) bridging nerve stumps was better than that of epineurium neurorrhaphy. The regenerated nerve fibers quality guidelines (motor nerve conduction velocity, myelinated fibres counting per unit vision field and distal myelinated fibres axons cross section area) of group D (small gap conduit bridging sciatic nerve with distal stump turning 180°) and group C (epineurium neurorrhaphy with distal stump turning 180°) were of no significant differences at 2nd week time point, while of significant differences at 4th week, 6th week and 8th week time point, which implied that the regeneration effect of group D was better than that of group C. The regenerated nerve fibers quality guidelines (motor nerve conduction velocity and distal myelinated fibres axons cross section area) were no significant differences at four time points between group B (small gap conduit bridging sciatic nerve with 2 mm gap) and group E (small gap conduit bridging sciatic nerve in situ with NGF), excepting the myelinated fibres counting dominance in group E. The explanation accounted for this results may be that: NGF mainly promoted the neurosensory regeneration effect, while the promotion of motorial nerve was not obviously.
Myelinated fibres counting of distal segments was higher than that of proximal segments norml controls; but the distal myelinated fibres axons cross section mean values exceeded that of proximal segments controls in all five groups. A great quantity of neurite lateral branchs during nerve axons regeneration accounted for the above reason. Only a little neurite lateral branchs matured and function to innerve the distal targeted organs. This process may include the regenerated axons wither process. We devised the distal segments 180° rotated group to imitated the clinical common cases with distal segments rotated for the reason of microsurgery technique and local tissue condition limitations. Our research results revealed the regeneration effects of epineurium anastomosis in situ group and small gap conduit bridging nerve stumps groups were better than that of rotated epineurium anastomosis, which implied the potential clinical application value.
Our study provided powerful evidence for the effectiveness of the small gap sleeve bridging or entubulation methods. De-acetyl chitin biogradable conduit small gap bridging peripheral nerve injury can substitute the common used epineurium anastomosis methods in SD rats.
REFERENCES
- Belkas, J.S., Shoichet, M.S., Midha, R. (2004). Peripheral nerve regeneration through guidance tubes. Neurol. Res. 26(2): 151–160. [PUBMED], [INFOTRIEVE], [CSA]
- Cajal, R.S. (1928). Degeneration and Regeneration of the Nervous System, Oxford University Press: New York.
- Dahlin, L.B., Lundborg, G. (2001). Use of tubes in peripheral nerve repair. Neurosurg Clin N Am. 12(2): 341–352. [PUBMED], [INFOTRIEVE], [CSA]
- Mligiliche, N.L., Tabata, Y., Ide, C. (1999). Nerve regeneration through biodegradable gelatin conduits in mice. East Afr Med J. 76(7): 400–406. [PUBMED], [INFOTRIEVE], [CSA]
- Strauch, B. (2000). Use of nerve conduits in peripheral nerve repair. Hand Clin. 16(1): 123–130. [PUBMED], [INFOTRIEVE], [CSA]
- Danielsson, P., Dahlin, L., Povlsen, B. (1996). Tubulization increases axonal outgrowth of rat sciatic nerve after crush injury. Exp Neurol. 139(2):238–243. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kuffler, D.P. (1994). Promoting and directing axon outgrowth. Mol Neurobiol. 9: 233–243. [PUBMED], [INFOTRIEVE], [CSA]
- Lundborg, G. (2000). A 25-year perspective of peripheral nerve surgery: Evolving neuroscientific concepts and clinical significance. J Hand Surg. 25: 391–414. [CSA]
- Rodrigues, Ade, C., Silva, M.D. (2001). Inside-out versus standard artery graft to repair a sensory nerve in rats. Microsurgery 21(3): 102–107. [CSA], [CROSSREF]
- Chiu, D.T., Smahel, J., Chen, L., Meyer, V. (2004). Neurotropism revisited. Neurol Res. 26(4): 381–387. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ayhan, S., Yavuzer, R., Latifoglu, O., Atabay, K. (2000). Use of the turnover epineurial sheath tube for repair of peripheral nerve gaps. J. Reconstr. Microsurg 16(5): 371–378. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Karacaoglu, E., Yuksel, F., Peker, F., Guler, M.M. (2001). Nerve regeneration through an epineurial sheath: Its functional aspect compared with nerve and vein grafts. Microsurgery 21(5): 196–201. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Yuksel, F., Ulkur, E., Baloglu, H., Celikoz, B. (2002). Nerve regeneration through a healthy peripheral nerve trunk as a nerve conduit: A preliminary study of a new concept in peripheral nerve surgery. Microsurgery 22(4): 138–143. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Geuna, S., Tos, P., Battiston, B., Giacobini-Robecchi, M.G. (2004). Bridging peripheral nerve defects with muscle-vein combined guides. Neurol Res. 26(2): 139–144. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Varejao, A.S., Cabrita, A.M., Meek, M.F., Fornaro, M., Geuna, S. (2003). Nerve regeneration inside fresh skeletal muscle-enriched synthetic tubes: A laser confocal microscope study in the rat sciatic nerve model. Ital J Anat Embryol. 108(2): 77–82. [PUBMED], [INFOTRIEVE], [CSA]
- Baoguo, Jiang, Shuhuan, Wang, Chuanhan, Feng. (1994). The comparison of small gap autogenic artery and epineurium anastomosis bridging peripheral nerve. Journal of Beijing Medical University 26: 249–250. [CSA]
- Barcelos, A.S., Rodrigues, A.C., Silva, M.D., Padovani, C.R. (2003). Inside-out vein graft and inside-out artery graft in rat sciatic nerve repair. Microsurgery 23(1): 66–71. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Bini, T.B., Gao, S., Xu, X., Wang, S., Ramakrishna, S., Leong, K.W. (2004). Peripheral nerve regeneration by microbraided poly (L-lactide-co-glycolide) biodegradable polymer fibers. J Biomed Mater Res A. 68(2): 286–295. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gamez, E., Ikezaki, K., Fukui, M., Matsuda, T. (2003). Photoconstructs of nerve guidance prosthesis using photoreactive gelatin as a scaffold. Cell Transplant. 12(5): 481–90. [PUBMED], [INFOTRIEVE], [CSA]
- Fornaro, M., Tos, P., Geuna, S., Giacobini-Robecchi, M.G., Battiston, B. (2001). Confocal imaging of Schwann-cell migration along muscle-vein combined grafts used to bridge nerve defects in the rat. Microsurgery 21(4): 153–155. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Komiyama, T., Nakao, Y., Toyama, Y., Vacanti, C.A., Vacanti, M.P., Ignotz, R.A. (2004). Novel technique for peripheral nerve reconstruction in the absence of an artificial conduit. J Neurosci Methods 134(2):133–140. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mosahebi, A., Fuller, P., Wiberg, M., Terenghi, G. (2002). Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration. Exp Neurol. 173(2): 213–223. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Tseng, C.Y., Hu, G., Ambron, R.T., Chiu, D.T. (2003). Histologic analysis of Schwann cell migration and peripheral nerve regeneration in the autogenous venous nerve conduit (AVNC). J Reconstr Microsurg. 19(5): 331–340. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Peixun, Zhang, Xiangjun, He, Kaiyan, Liu, Fuqiang, Zhao, Zhongguo, Fu, Dianying, Zhang, Qi, Zhang, Baoguo, Jiang. (2004). Bone marrow stromal cells differentiated into functional schwann cells in injured rats sciatic nerve. Artificial Cells, Blood Substitutes, & Biotechnology 32(4): 509–518. [INFOTRIEVE], [CSA], [CROSSREF]
- Chen, Y.S., Chang, J.Y., Cheng, C.Y., Tsai, F.J., Yao, C.H., Liu, B.S. (2005). An in vivo evaluation of a biodegradable genipin-cross-linked gelatin peripheral nerve guide conduit material. Biomaterials 26(18): 3911–3918. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]