Abstract
To explore the adhesion and proliferation characteristics of bone marrow mesenchymal cells (MSCs) to chitosan conduit membrane, MSCs were induced by a sequential composition Beta-mercaptoethanol, retinoic acid, forskolin, basic-FGF, PDGF and heregulin. Schwann Cell markers, namely S-100 and GFAP, were used to discriminate the induced MSCs' properties by immunofluorescent staining. These results suggested that MSCs can take on Schwann cell's phenotype in vitro and the induce MSCs were gifted with good biocompatibility to biogradable chitosan conduit membrane. The results provided the possibilities to using the induced MSCs with chitosan conduit membrane in artificial peripherial nerve fields to promote nerve regeneration.
INTRODUCTION
Bone marrow mesenchymal cells (MSCs) are regarded as multi-potential stem cells, which can differentiate into several cell types such as osteoblasts, adipocytes chondrocytes and even muscle cells [Citation[1]]. Some researchers even induced the mesenchymal cells into neuronal lineage. Pedro Cuevas [Citation[2]] and Peixun [Citation[3]] had injected MSCs directly into the nerve injury site and verified its abilities to facilitate peripheral nerve regeneration. To explore the possibilities of bone marrow mesenchymal cells (MSCs) to take on Schwann cell's phenotype in vitro and the adhesion and proliferation characteristic of bone marrow mesenchymal cells (MSCs) to chitosan conduit membrane, MScs were induced by a sequential composition Bet-mercaptoethanol, retinoic acid, forskolin, basic-FGF, PDGF and heregulin. Schwann cell markers, namely S-100 and GFAP, were used to discriminate the induced MSCs' properties by immunofluorescent staining. It had been reported that the artificial biogradable chitosan conduit with Schwann cells seeded in can effectively promote peripheral nerve regeneration. Our experiment intends to use induced functional Schwann cells in conduit to promote the peripheral nerve regeneration because of limited Schwann cells resources. This experiment was carried out to confirm the induced MSCs' biocompatibility to biogradable chitosan conduits membrane in order to evaluate the growth and proliferation characteristic in conduits. These results suggested that MSCs can take on Schwann cell's phenotype in vitro and the induced MSCs were gifted with good biocompatibility to biogradable chitosan conduit membrane. The results provided the possibilities to using the induced MSCs with chitosan conduit membrane in artificial peripherial nerve fields to promote nerve regeneration.
MATERIALS AND METHODS
Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals from the National Academy of Science, People's Republic of China.
Isolation of MSCs
Isolation of adult SD rat MSCs was performed according to the procedures provided by Mari Dezawa [Citation[2]] and Pedro Cuevas [Citation[4]]. The tibia and femur bone marrow were extruded with α-MEM (Gibco) after the rats were sacrificed with overdose pentobarbital (40 mg/kg). The exudation fluid is cultured in α-MEM supplemented with 10% fetal bovine serum (Gibco) and 200 mg/ml Kanamycin, incubated at 37°C, humidity 95% and CO25%. 10 ng/ml leukemia inhibitory factor (LIF, Sigma, US) was added to maintain the MSCs' differentiation potential [Citation[5]]. To get rid of the hematic cells, the culture medium was removed twice by virtue of their adherence characteristics to plastic flask after 48 hours. The adhered cells were cultured and passaged [Citation[6]].
Induction of MSCs
Subcultured MSCs were incubated in α-MEM medium supplemented with 1 mmol/L β-mercaptoethanol (β-ME, Sigma) for 24 hours. After the medium was removed and washed by PBS, MSCs were cultured with 10% fetal bovine serum and 35 ng/ml all-trans-retinoic acid (RA, Sigma) for 3 days. Subsequently, MSCs were incubated in α-MEM supplemented with 10% fetal bovine serum and compound containing 5 mmol/L forskolin (FSK, Sigma), 10 ng/ml recombinant human basic-fibroblast growth factor (bFGF, Peprotech, London, UK), 5 ng/ml recombinant human platelet derived growth factor-AA (PDGF-AA), Peprotech, London, UK), and 200 ng/ml recombinant human heregulin-β (HRG-β, R&D System, Minneapolis, MN) for 7 days without any passage.
Immunofluorescence Staining
Induced MSCs were washed in PBS and fixed with 4% formaldehyde in PBS at room temperature for 20 minutes. Immunocytochemistry was performed to discriminate the differentiation characteristics as described using the following primary antibodies against S-100 and GFAP (rabbit anti rat, Sigma). Primary antibodies were detected with Rhodamine (TRITC)-conjugated, affinipure labeled, goat anti rabbit 1gG(H + L) antibody (Jackson Immunoresearch, US). A double-stranded DNA specific fluorescent dye, 4′, 6′-diamidino-2-phenylindole hydrochloride (DAPI), was used for cell nuclei counterstaining.
Coculture of Induced MSCs with Chitosan Conduit Membrane
Induced MSCs were cultured and passaged to enrichment. The enriched induced MSCs were digested by 0.25% trypsin and were centrifugated at 1500 rpm/m for about 20 minutes. Thrashed and scattered induced MSCs to prepare uniform cells suspension, and then seeded them into 96 wells cells culture plate at concentration of 2 × 104/ml. Grouping as this:
1◯ 96 wells cells culture plate-No.1 (detection at 5th day after seeding)
Group A: (control group): 10 wells with 0.8 ml cells suspension (2 × 104/ml) for each well.
Group B: (Simple conduit membrane group): 10 wells were all tiled by biogradable chitosan conduit membrane, 0.8 ml cells suspension (2 × 104/ml) for each well.
Group C: (Rat tail collagen covered chitosan membrane group): 10 wells were all tiled by rat tail collagen covered chitosan membrane group biogradable chitosan conduit membrane, 0.8 ml cells suspension (2 × 104/ml) for each well.
2◯ 1◯ 96 wells cells culture plate-No.2 (detection at 7th day after seeding). Grouping methods were the same as No. 1.
Morphology Observation
Induced MSCs were cultured and observed under inversion phase contrast microscope each day after seeding into 96 wells cells plate.
Cell Proliferation Activity Detection
The fifth day after seeding, discarded the culture medium of 96 wells culture plate-No.1, added α-MEM without serum (0.4 ml/well), MTT (Mono-nuclear cell direc cytotoxicity assay) solution 20 ul/well. Incubated in cell culture cabinet at 37°C for 4 hours. Added formazan solution 0.4 ml/well and incubated at 37°C for 2 hours. Extracted 0.5 ml solution from every well and transferred it to another 96 well cells culture plate for spectroscopical instrument detection absorption value. The 96 wells cells culture plate-No. 2 was evaluated as the same methods described before.
Statistical Analysis
ANOVA (analysis of variance) test was applied between the three groups using SAS System Software Packet. “t” test was applied between two groups and the statistical difference standard was defined by p < 0.05.
RESULTS
Cultured MSCs Morphological Characteristics Before Induction and After Induction
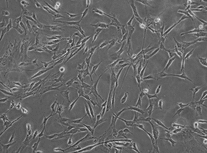
The morphological characteristic of un-induced MSCs were considerably disarrayed, with slight loose body shape. The induced MSCs morphology was much more regular than that of un-induced MSCs under phase-contrast microscopy ().
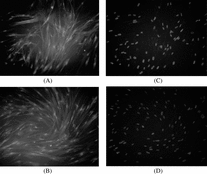
Immunofluorescence Staining Discrimination of Induced MSCs
Immunofluorescence staining was used to discriminate the cell phenotype induced MSCs. Schwann cell markers, GFAP and S 100, were used to be primary antibody. With red Rhodamine labeled second antibody, the positive reaction was red color region in cell body, as can be seen in .
Co-culture Observation of Induced MSCs with Chitosan Conduit Membrane
Induced MSCs of control group adhered to the culture wall and the growth proliferation condition was quite good. After 7 days culture, induced MSCs converged and spread to the whole well wall. The morphology and the cells proliferation velocity was almost the same as primary cultured MSCs. Induced MSCs adhered to the chitosan conduit membrane tightly and the growth condition was as satisfied as control group (commonly used cell culture medium). But the induced MSCs growth and proliferation condition to Group C (Rat tail collagen covered chitosan membrane group) were quite better than that of Group B (simple conduit membrane group). The cell growth condition can be seen in .
Induced MSCs Proliferation Detection
MTT (Mono-nuclear cell direc cytotoxicity assay) was used to detect the induced MSCs proliferation condition. MTT was the effect substrate of mitochondria succinodeydrogenase, and can become purple colour crystallization after intaking by live cells. After this cystallization resolve, there was a maxium absorption peak at 560–610 nm and its concentration can be evaluated and calculated by spectroscopical instruments. There was a quite satisfied linear correlation between the absorption value and the living cells quantities [Citation[7]]. The detection results implied that the cells quantities of group B (simple conduit membrane group) was fewer than that of control group (common used cell culture medium) at fifth day, while the cells quantities of group C (Rat tail collagen covered chitosan membrane group) was more than that of control group (common used cell culture medium) and group B (simple conduit membrane group) at fifth day with statistical difference respectively (P < 0.05).
There were no statistical differences between group B (simple conduit membrane group) and control group (common used cell culture medium), and no statistical difference between group C (Rat tail collagen covered chitosan membrane group) and control group (common used cell culture medium) at 7th day (P > 0.05, respectively). But the cells quantities of group C (Rat tail collagen covered chitosan membrane group) was more that that of group B (simple conduit membrane group) with statistical difference at 7th day (P > 0.05).
DISCUSSION
Adult bone marrow stem cells (BMCs) include two populations of bone marrow stem cells (BMCs): hematopoietic stem cells (HSCs), which give rise to all mature lineages of blood, and mesenchymal stem cells (MSCs), which can differentiate into bone, cartilage, and fate [Citation[8], Citation[9]]. The increasing recognition of the properties of marrow mesemchymal cells had spawned a major switch in our perception of their nature, and the ramifications of their potential therapeutic application have been envisioned and implemented. In addition, they may be experimentally induced to undergo unorthodox differentiation, possibly forming neural and myogenic cells [Citation[10]]. As such, they represent an important paradigm of post-natal nonthematopoietic stem cells, and an easy source for potential therapeutic use.
Table 1. Absorption value of three groups (x ± s)
Schwann cells display a number of characteristics that suggest their role in facilitating peripheral nerve regeneration [Citation[11]], but the limited Schwann cell resource and the xenogenic graft immunorejection reaction hindered its further application. MSC can take on several cell phenotypes after being induced in vitro by different inducer factors. Considering all these facts, we induced the MSC in vitro using sequential administration of inducer factors and discriminated the biological characteristics of MSCs phenotype. We also examined the biocompatibility to biogradable chitosan conduit membrane. In our experiment presumption, we intend to use the induced MSCs with biogradable chitosan conduit to promote the peripheral nerve regeneration effect. The result implied the induced MSCs were gifted with good biocompatibility to biogradable chitosan conduit membrane, especially to rat tail collagen covered chitosan conduit membrane. These results hinted that we can make good use of the chitosan conduit in artificial peripheral nerve fields to promote nerve regeneration. The rat tail collagen covered chitosan conduit membrane was the best material for induced MSCs growth and proliferation.
The sequential administration of various factors such as β-ME, RA, followed by a mixture of FSK, bFGF, PDGF-AA and HRG, effectively induced MSCs into cells with Schwann cells phenotype, expressing GFAP and S-100. β-ME and RA are presumed to work as triggering factors, inducing changes in the morphological and transcriptional characteristics of MSCs. The sequential administration of β-ME and RA, followed by a mixture of FSK, bFGF, PDGF-AA and HRG, had a cumulative effect for the MSCs' turning into Schwann cell phenotype.
This experiment proved the good biocompatibility to rat tail collagen covered biogradable chitosan conduit membrane. As a next step, we would center on the induced MSCs and rat tail collagen covered chitosan conduit in vivo to confirm its possible effect on promote peripheral nerve regeneration. Further, a 3D structure artificial chitosan conduit with induced MSCs may be constructed to bridge longer nerve defects.
This research was supported by Chinese National 863 Project Grant (2002AA205071) and Chinese National Nature Science Grant (30271306).
REFERENCES
- Brazelton, T.R., Rossi, F.M., Keshet, G.I., Blau, H.M. (2000). From marrow to brain: Expression of neuronal phenotypes in adult mice. Science 290: 1775–1779. [PUBMED], [INFOTRIEVE], [CSA]
- Cuevas, P., Carceller, F., Dujovny, M., Garcia-Gomez, I., Cuevas, B., Gonzalez-Corrochano, R., Diaz-Gonzalez, D., Reimers, D. (2002). Peripheral nerve regeneration by bone marrow stromal cells. Neurological Research 24: 634–638. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Peixun, Zhang, Xiangjun, He, Kaiyan Liu, Fuqiang, Zhao, Zhongguo, Fu, Dianying, Zhang, Qi, Zhang, Baoguo, Jiang. (2004). Bone marrow stromal cells differentiated into functional Schwann cells in injured rats sciatic nerve. Artificial Cells, Blood Substitutes, and Biotechnology 4: 509–518. [CSA]
- Dezawa, M., Takahashi, I., Esaki, M., Takano, M., Sawada, H. (2001). Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. European Journal of Neuroscience 14: 1771–1776. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Jiang, Yuehua, Jahagirdar, Balkrishna, N., Reinhardt, R. Lee, Schwartz, Robert, E., Keene, C., Dirk, Ortiz-Gonzalez, Xilma, R., Reyes, Morayma, Lenvik, Todd, Lund, Troy, Blackstad, Mark, Du, Jingbo, Aldrich, Sara, Lisberg, Aaron, Low, Walter, C., Largaespada, David, A. (2002). Pluripoency of meschymal stem cells derived from adult marrow. Nature 418: 41–49. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Bosnakovski, D., Mizuno, M., Kim, G., Takagi, S., Okumura, M., Fujinaga, T. (2005). Isolation and multilineage differentiation of bovine bone marrow mesenchymal stem cells. Cell Tissue Res. 319(2): 243–53. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxity assays. J Immunol methods 65: 55–63. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Aldrich, S., Lisberg, A., Low, Walter C., Largaespada, David A. (2002). Pluripoency of meschymal stem cells derived from adult marrow. Nature 418: 41–49. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Lee, K.D., Kuo, T.K., Whang-Peng, J., Chung, Y.F., Lin, C.T., Chou, S.H., Chen, J.R., Chen, Y.P., Lee, O.K. (2004). In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 40(6): 1275–1284. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kim, B.J., Seo, J.H., Bubien, J.K., Oh, Y.S. (2002). Differentiation of adult bone marrow stem cells into neuroprogenitor cells in vitro. Neuroreport 13(8): 1003–1004. [CSA]
- Dezawa, M., Adachi-Usami, E. (2000). Role of Schwann cells in retinal gsnglion cell axon regeneration. Prog. Retin. Eye Res. 19: 171–204. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]