Abstract
Carboxyl modified PEG spacer was synthesized and linked covalently to cellulose beads. L-lysine ligand was coupled to the spacer and its selective affinity for low-density lipoprotein-cholesterol (LDL-C) was determined. It was found that the adsorption capacity and the efficiency of the ligand for adsorption of LDL-C was increased when PEG spacer was used. Experimental results showed that with the chain length of PEG spacers increased from 1000 Da to 6000 Da, the average adsorption capacity of LDL-C was enhanced from 0.242 mg/ml to 0.903 mg/ml.
Above results indicate that PEG spacers are conducible to the selective removal of LDL-C from human plasma. In addition, LDL-C adsorbent with PEG spacers has a low adsorption capacity for high-density lipoprotein (HDL-C).
INTRODUCTION
Familial hypercholesterolemia is characterized by a high concentration of plasma cholesterol in the form of low-density lipoprotein-cholesterol (LDL-C). In order to decrease the LDL-C level in patients, drugs and surgical intervention were reported [Citation[1]]. Hemoperfusion treatment is currently employed when the reduction of LDL-C level appears impossible to be achieved by drug therapeutic methods. Since the late 1970s, scientists have engaged in developing different kinds of cellulose adsorbents to remove pathogenic substances [Citation[2-4]]. Such adsorbents are required to possess high adsorption capacity, high selectivity, good biocompatibility and adequate mechanical and physical properties [Citation[5]]. According to this rationale, we selected cellulose beads as the support matrix and L-lysine as the ligand.
The density of ligands on the carrier and the effect of steric hindrance are both important factors in specific adsorption. When the target substance is a small molecule, there may be no steric hindrance, so the enhancement of the density of ligands can improve the adsorption capacity. H. M. Wang et al. used poly-α-vinyl-pyridine porous resin to adsorb bilirubin in buffer solution [Citation[6]], which attained high adsorption capacity and no steric hindrance was found. But when the target substance is a large molecule, due to the presence of steric hindrance, a high density of ligands linked may display a low adsorption capacity of target protein. In our previous work, Yang Yi et al. employed epoxy-activated agarose resin to adsorb IgMRF and IgARF via L-tryptophan ligand. When the density of L-tryptophan ligands increased, the adsorption capacity reached a maximum and then decreased [Citation[7]], which showed that the steric hindrance caused by the fairly dense layer of ligands prevented the adsorption. Spacers were used to solve the steric problem: Kenneth C. Hou et al. [Citation[8]] and Adrian Murza et al. [Citation[9]] employed short spacers to reduce the steric hindrance and improve the adsorption capacity for target proteins. J. Q. Chu et al. used glutaraldehyde spacer and exerted good adsorption capacity for IgARF [Citation[10]]. In order to facilitate adsorption interaction between the ligand and target protein, long spacers were also introduced [Citation[11]].
PEG is widely used as a pharmacological product because of its preferable hydrophilicity, non-biodegradability, very low toxicity, excellent solubility in aqueous solutions [Citation[12]], biological compatibility and ease of chemical modification [Citation[13]]. Covalent attachment of PEG to the surface of a number of different materials has been used to improve the biocompatibility of such surfaces [Citation[14-16]]. Soltys et al. employed PEG in ion-exchange membranes for the immobilization of IgG to adsorb apoB [Citation[14]], which exerted a limited beneficial effect on the removal capacity of LDL in human plasma, but the adsorption capacity for apoB showed no increase when different spacer length was used.
In order to reduce the steric hindrance of the adsorption of high molecular weight pathogenic toxins by adsorbents, the present paper describes the effects of different molecular weight PEG spacers to the adsorption capacity for LDL-C. Cellulose adsorbents containing various lengths of PEG spacers and L-lysine as ligand were synthesized. The adsorption capacity of the adsorbents for LDL-C was compared with non-spacer adsorbent in view of the reduction of steric hindrance and effect of different length of PEG spacer on adsorption capacity was studied.
MATERIALS AND METHODS
Materials
PEG (chemical grade) with different molecular weights (MW = 1000, 2000, 4000, 6000 Da) was purchased from Tianjin TianTai Fine Chemical Reagents Co., Ltd. and 1,6-Diisocyanatohexane (analytical grade) was purchased from Tokyo Kasei Kogyo Co., Ltd. Succinic anhydride (analytical grade) was purchased from Beijing Chemical Reagents Co., Ltd. and N, N′—Dicyclohexylcarbodiimide (DCC) from Tianjin Chemical Reagents Factory; L-lysine (analytical grade, BBI, Japan) was purchased from Shanghai Shenggong Bioengineering Co., Ltd.; Dimethyl sulfoxide (DMSO), pyridine and all other reagents were of analytical grade and made in China. Plasma was taken from several patients aged between 45 to 65 years old suffering from hypercholesterolemia in Tianjin General Hospital, who were not taking lipid-lowering drugs or medicine that could alter the metabolism of lipid for at least two months.
Preparation of Cellulose Beads [Citation[17]]
Thirty grams cotton (of medical use) was soaked in 19% NaOH solution at room temperature and placed in a flask for 3 days. 13 ml carbon disulfide was reacted with the aged cellulose for 5 h to convert it into a viscose solution, which was then diluted to 300.0 g with 6% NaOH solution to make the 10% viscous solution of cellulose. In a reactor equipped with a stirrer, a mixture of 800 ml chlorobenzene, 200 ml carbon tetrachloride and 2.0 g potassium oleate were stirred for 30 min at 300 rpm under room temperature. Then 300.0 g 10% viscose solution was added to the reactor and we continued stirring for 30 min until the liquid particles were dispersed uniformly. Thereupon, the temperature was slowly raised to 90°C and kept for 2.5 h to solidify the liquid particles into resins. The mixture was then cooled to room temperature. Cellulose beads were filtered (20–40 mesh) and washed thoroughly with alcohol and distilled water to remove all the solvents.
Preparation of Adsorbent without PEG Spacers
Ten grams of cellulose beads was activated with 10 ml epichlorohydrin and 20 ml 2 mol/L sodium hydroxide was added to the mixture and stirred at 40°C for 4 h. Then the epoxy-activated cellulose beads were washed excessively with distilled water.
Two milliliter 5% L-lysine (w/w) in sodium carbonate/sodium bicarbonate buffer (pH = 10.0) was added to 1.0 g epoxy-activated cellulose beads and stirred at 50°C. After overnight incubation, the adsorbent without PEG spacers was washed with distilled water. The elute was collected and tested with ninhydrin until the unreacted L-lysine was eliminated thoroughly. Then the adsorbent was dried to constant weight. The content of L-lysine ligands was determined by Perkin-Elmer-2400C element analytical meter and calculated according to equation 1 (data listed in ).
Table 1. Elemental analytical data of cellulose adsorbent
Equation 1. Calculation of the amount of L-lysine linked to cellulose beads without PEG spacerswhere Dlysine stands for the amount of immobilized L-Lysine, Wc stands for the weight of adsorbent, N stands for the nitrogen weight percentage, Mnitrogen stands for the mol weight of nitrogen, Vc stands for the volume of the adsorbent, and Mlysine stands for the molar weight of L-lysine.
Unreacted epoxy groups were quenched by immersion in 1.0 mol/l ethanolamine blocking solution (4 ml) for 2 h with agitation. Then the adsorbent was washed with 0.9% sodium chloride to pH 7.0, and stored at 4°C for further use.
Synthesis of Cellulose Beads with PEG Spacers
One hundred and seventy milliliter DMSO, 25.7 ml 1,6-Diisocyanatohexane and 4 ml pyridine were added to 4.2 g dried cellulose beads and stirred at 80°C for 10 h. After cooling to room temperature, the product was washed with toluene, and then sucked dry.
One hundred and fifty milliliter toluene, 70.0 g PEG2000 and 4.0 ml pyridine were added to the product of the first step and reacted for 48 h at 80°C. After cooling to room temperature, the mixture was washed with toluene to yield 5.0 g cellulose beads with PEG2000 as spacer. All the reagents used above must be dried in advance.
L-lysine was immobilized on the cellulose beads following the same preparation method for beads without PEG spacers. Then the adsorbent was washed with distilled water. The elute was collected and tested with ninhydrin until the unreacted L-lysine was eliminated thoroughly. Then the adsorbent was dried to constant weight. From the elemental analysis, the amount of L-lysine ligands linked was calculated according to Equation 2:
Equation 2. Calculation of the amount of L-lysine linked to cellulose beads via PEG spacer.where Dlysine stands for the density of L-lysine linked, Mnitrogen stands for the molar weight of nitrogen, Mlysine stands for the mol weight of L-lysine, N0 and N stands for the nitrogen weight percentage before and after L-lysine linkage respectively. Vc stands for the volume of cellulose beads, and Wc stands for the weight of the adsorbent. (Data shown in .)
Unreacted sites were quenched by immersion in 1.0 mol/l ethanolamine solution (4 ml) for 2 h with agitation. Then the adsorbent was washed with 0.9% sodium chloride to pH 7.0, and stored at 4°C for further use.
Synthesis of Carboxyl PEG
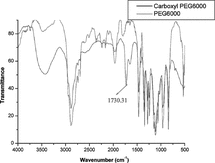
Five grams of PEG6000 dried under vacuum at 40°C for 4 h was dissolved in 10 ml dried dimethyl sulfoxide. Five ml dried pyridine and 1.5 g succinic anhydride were added to the solution and the mixture was stirred at 40°C in a nitrogen atmosphere for 18 h [Citation[18]]. The product was precipitated from the solution by dropwise addition of ether (400 ml) with rapid stirring. The precipitate was filtered and washed several times with ether, and dried under vacuum to yield 4.7 g white powder. IR spectra analysis was conducted on a Bio-Rad FTS 135 IR spectrometer ().
Covalent Linking of PEG to Carrier
Four milliliter ethylenediamine and 6 ml water were added to 5.0 g epoxy-activated cellulose beads and stirred at 40°C for 6 h to introduce amino groups, then the amino-cellulose beads were washed excessively and sucked dry.
Ten grams of amino-cellulose beads, 2 ml water and 5 ml pyridine were added to a solution of carboxyl PEG6000 (4.0 g) in 8 ml DMSO. Eight hundred mg of DCC in 5 ml pyridine was then added to the solution and the reaction mixture was stirred at 20°C for 48 h [Citation[19]]. A precipitate was separated and the modified adsorbent was washed with distilled water ( indicates the nitrogen content of the cellulose beads with PEG spacers).
Covalent Linking of L-lysine to Carrier via PEG Spacer
L-lysine (5% in sodium carbonate/sodium bicarbonate buffer, pH = 10.0) was reacted with PEG spacers following the above method. Then the adsorbent (cellulose beads as carrier, PEG as spacer and L-lysine as ligand) was washed excessively with distilled water and dried to constant weight. From elemental analysis, the amount of L-lysine ligands linked was calculated ().
Unreacted sites were quenched by immersion in 1.0 mol/l ethanolamine solution (4 ml) for 2 h with agitation. Then the adsorbent was washed with 0.9% sodium chloride to pH 7.0, and stored at 4°C for further use.
Evaluation of Adsorption Capacity [Citation[5], Citation[20]]
Total cholesterol (TC), LDL-C, and high-density lipoprotein-cholesterol (HDL-C) were determined by enzymatic assay. (Commercial test kits, Zhongsheng High Tech Bioengineering Company, Beijing, P. R. China.) One gram L-lysine adsorbent was incubated in 3.0 ml plasma from hyperlipidemia patients with agitation for 3 h at 37°C.
Adsorption percentage and adsorption capacity were calculated according to the following equations:where AP and AC stand for adsorption percentage and adsorption capacity (mg/ml), respectively, [C]B is the concentration before adsorption, [C]A is the concentration after adsorption, and Vp is the volume of plasma used during adsorption.
RESULTS
Infrared Spectroscopy
The characteristic adsorption at 1730.31 cm−1 (vco) indicates that carboxyl group was introduced successfully ().
Effect of PEG Spacer on Adsorption Capacity
According to the analytical data of cellulose adsorbents, the amount of L-lysine ligand could be calculated. Although the amount of L-lysine linked to the adsorbent with PEG spacers (10.5, 9.8, 9.0, 8.6 mg per ml cellulose adsorbent, respectively) was lower than those without PEG spacers (121.6 mg per ml cellulose adsorbent) (), the average adsorption capacity for LDL-C per ml cellulose adsorbent increased from 0.130 mg/ml to 0.903 mg/ml after the introduction of PEG spacers (), and consequently the adsorption capacity for LDL-C per unit ligand increased significantly from 0.001 mg/mg L-lysine to 0.105 mg/mg L-lysine ().
Table 2. Adsorption capacity of TC, and LDL-C of cellulose beads with different length of PEG as a spacer
Table 3. Adsorption capacity of LDL-C by L-lysine ligand
Effect of PEG Chain Length on Adsorption Capacity
To examine the effect of PEG chain length on adsorption capacity, different lengths of PEG spacers were synthesized and linked to cellulose beads. As the chain length increased from 1000 Da to 6000 Da, the amount of L-lysine linked per ml cellulose beads decreased from 10.5 mg/ml to 8.6 mg/ml, but the average adsorption capacity for LDL-C increased from 0.242 mg/ml to 0.903 mg/ml. With the chain length increased from 1000 Da to 6000 Da, the adsorption percentage reached a maximum of 44.76 ± 0.36% with a capacity for LDL-C and TC of 0.903 ± 0.003 mg/ml and 1.028 ± 0.011 mg/ml, respectively.
Effect of the Adsorbent on HDL-C Adsorption
The adsorption of cellulose beads with PEG 6000 spacers was tested for its adsorption for HDL-C. Results showed that the HDL-C adsorption was only 0.009 ± 0.001 mg/ml (the total amount of HDL-C in the test plasma was 1.79 mg), less than 5%, which indicated that the adsorbent has a good selectivity.
DISCUSSION
The density of ligands is an important factor in specific adsorption, which may determine the intensity of the adsorption for target protein. To enhance adsorption capacity, a large amount of ligand linked is required. But the effect of steric hindrance which could be promoted by the interaction between each individual ligand and the corresponding binding site on the target protein, a fairly dense layer of ligands linked covering the matrix surface could prevent the interaction between the ligands linked and partially bury the protein-binding sites, thus resulting in a low capacity for adsorption of the target protein [Citation[9]]. In order to reduce the steric hindrance and facilitate the interactions between the ligands and the protein binding sites, spacers were generally introduced, which could enhance the adsorption capacity.
We introduced PEG to the cellulose beads as a flexible spacer. In agreement with the result of previous work, the presence of PEG spacers increased the distance between the binding site of LDL-C and the surface of cellulose beads, which reduced steric hindrance, thus facilitating the binding interaction. By using PEG spacer, the adsorption capacity for LDL-C was enhanced despite the amount of ligands linked decreased. For the adsorbent with PEG spacers (MW = 2000), the amount of L-lysine ligands linked (9.8 mg/ml) was much lower than that without PEG spacers (121.6 mg/ml), but the adsorption of LDL-C (0.263 ± 0.013 mg/ml) was higher than that without PEG spacer (0.130 ± 0.013 mg/ml). Consequently, the adsorption capacity of LDL-C per unit L-lysine ligand (0.027 mg LDL-C/mg L-lysine) was much higher than that without PEG spacers (0.001 mg LDL-C/mg L-lysine), which indicated that in the presence of PEG spacers, the adsorption efficiency of L-lysine ligands linked was enhanced significantly (). It is postulated that appropriate enhancement of the L-lysine ligands linked and the use of PEG spacers can improve the adsorption capacity for LDL-C.
To investigate the effect of the chain length on adsorption capacity, short length PEG spacers of different molar weights were introduced. When the chain length increased from 1000 Da to 6000 Da, the amount of L-lysine ligand linked decreased while the adsorption capacity for LDL-C increased, which showed that in such a range of chain length, a longer PEG spacer would increase the adsorption capacity and also increase the adsorption efficiency of L-lysine. That is to say, the amount of effective ligand was enhanced (). It has been reported that covalently linked PEG spacer to carrier to a large extend can enhance the adsorption capacity significantly, so it is possible that the adsorption capacity for LDL-C may be increased when more spacers are introduced, which will correspondingly increase the amount of ligand linked. But it is difficult to covalently link more PEG spacers to cellulose adsorbent. In order to demonstrate our view point, chloromethylated polystyrene(CPS) was selected as carrier because it is easy to covalently link more PEG spacers to CPS carrier. PEG with MW1000 was linked to the carrier, then L-lysine was immobilized on CPS beads modified by PEG following the same method mentioned above. Experimental results show that CPS LDL adsorbent with PEG spacers could improve the adsorption capacity significantly for LDL-C compared to CPS LDL adsorbent without PEG spacers ().
Table 4. Adsorption capacity of TC, and LDL of CPS beads with PEG as spacer and without PEG spacer
CONCLUSION
Adsorbent with PEG spacers and L-lysine as ligand has many advantages, which signifies that is a promising adsorbent for removal of LDL with good selectivity. Introduction of PEG spacer could increase the adsorption capacity for TC and LDL-C and enhance the adsorption efficiency of the ligand linked, which can exert a beneficial effect in therapeutic application. For PEG spacers, the adsorption capacity and percentage of adsorption increased when the chain length increased.
The support of The National Key Project of Fundamental Research and Advances (G1999064707), the Project 863 (No. 2002AA326060) and Tianjin-Nankai University Co-Construction Foundation are acknowledged.
REFERENCES
- Armstrong, V.W., Windisch, M., Wieland, H., Fuchs, C., Rieger, J., Kostering, H., Nebendahl, K., Scheler, F., Seidel, D. (1983). Selective continuous extracorporal ekimination of low-density lipoprotein with heparin at acidic pH. Trans. Am Soc. Artif. Intern Organs 29: 323–327. [PUBMED], [INFOTRIEVE], [CSA]
- Kojima, S., Harada-Shiba, M., Toyota, Y., Kimura, G., Tsushima, M., Kuramochi, M., Sakata, T., Uchida, K., Yamamoto, A., Omae, T. (1992). Changes in coagulation factors by passage through a dextran sulfate cellulose column during low-density lipoprotein apheresis. Int. J. Artif. Organs 15(3): 185–190. [PUBMED], [INFOTRIEVE], [CSA]
- Sato, Y., Agishi, T. (1996). Low-density lipoprotein adsorption for arteriosclerotic patients. Artif. Organs 20(4): 324–327. [PUBMED], [INFOTRIEVE], [CSA]
- Behm, E., Ivanovich, P., Klinkmann, H. (1989). Selective and specific adsorbents for medical therapy. Int. J. Artif. Organs 121: 1–10. [CSA]
- Cheng, Y., Wang, S.-Q., Yu, Y.-T., Yuan, Y. (2003). In vitro, in vivo studies of a new amphiphilic adsorbent for the removal of low density lipoprotein. Biomaterials 24: 2189–2194. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Wang, H.-M., Chen, C.-Z., Tong, M.-R., Zhen, J.-T., Yu, Y.-T. (1989). In vitro studies on removal of middle molecules by macroporous resins. Acta Poly. Sinica. 2: 171–175. [CSA]
- Yang, Y. (2002). Agarose resin for removal of rheumatoid arthritis factors. Master thesis. [CSA]
- Hou, Kenneth, C., Zaniewski, R. (1991). Protein A immobilized cartridge for immunoglobulin purification. Biotechnol. Appl. Biochem. 13: 257–268. [CSA]
- Adrian, M., Roberto, F.-L., José, M.G. (2000). Essential role of the concentration of immobilized ligands in affinity chromatography: Purification guanidinobenzoatase on an ionized ligand. J. Chromatography B. 740: 211–218. [CSA]
- Chu, J.-Q., Zhang, W.-H., Yu, Y.-T., Zhu, B.-R., Cheng, C.-Z. (2004). In vitro studies of the immunoadsorbent for removal of IgA-nephropathy (III). Chemical Journal of Chinese Universities 25(8): 1454–1457. [CSA]
- Leckband, D.E., Kuhl, T., Wang, H.K., Herron, J., Muller, W., Ringsdorf, H. (1995). 4-4-20 anti-fluorescyl IgG Fab' recognition of membrane bound hapten:direct evidence for the role of protein and interfacial structure. Biochem. 34: 11467–11478. [CSA], [CROSSREF]
- Zalipsky, S. (1995). Chemistry of polyethylene glycol conjugates with biological active molecules. Advanced Drug Delivery Reviews 16: 157–182. [CSA], [CROSSREF]
- Harris, M.J., Struck, C.E., Case, G.M., Paley, S.M., Yalpanic, M., Van Alstine, M.J., Brooks, E.D. (1984). Synthesis and characterization of poly(ethylene glycol) derivatives. J. Polymer Sci. 22: 341–352. [CSA]
- Soltys, J.P., Etzel, R.M. (2000). Equilibrium adsorption of LDL and gold immunoconjugates to affinity membranes containing PEG spacers. Biomaterials 21: 37–48. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Desai, N.P., Hubbell, J.A. (1991). Biological responses to polyethylene oxide modified polyethylene terephthalate surfaces. J Biomed. Mater. Res. 25: 829–843. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gombotz, W.R., Wang, G.H., Horbett, T.A. (1991). Protein adsorption to poly(ethylene oxide) surfaces. J. Biomed. Mater. Res. 25: 1547–1562. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Fang, H., Wei, J., Yu, Y.-T. (2004). In vivo studies of endotoxin removal by lysine-cellulose adsorbents. Biomaterials 25(23): 5433–5440. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Cao, N.-N., Chen, C.-Z., Yu, Y.-T. (2002). In vitro study of a novel low density lipoprotein adsorbent. Artif. Cells, Blood Subs. and Immob. Biotech. 30(1): 53–61. [CSA], [CROSSREF]
- Klyashchitsky, B.A., Mitina, V.K. (1981). Affinity adsorbents with polysaccharide spacers preparation and properties. J. Chromatogr. 210: 55–65. [CSA], [CROSSREF]
- Wang, S.-Q., Yu, Y.-T., Cui, T., Cheng, Y. (2002). Cellulose adsorbent for the removal of low density lipoprotein. Artif. Cells, Blood Subs. and Immob. Biotech. 30(4): 285–292. [CSA], [CROSSREF]