Abstract
Background: Hemoglobin based oxygen carrying solutions (HBOC) have been designed to combine the beneficial effects of colloidal solutions with oxygen carrying capacity. Clinical trials in humans using HBOCs have had variable results.
Methods: We used a rodent 50% exchange model to compare HemolinkTMand HemopureTM HBOC to autologous blood and Pentastarch solution. We monitored hemodynamic parameters, hemoglobin clearance, weight gain and hematocrit over a five-day period.
Results: Acute hemodynamic effects between the two HBOCs were similar with mild vasoconstriction. Cardiac output, systemic vascular resistance and renal function were similar to that seen with blood. HBOC's were associated with hemoglobinuria with a half-life in the circulation of 13.8 hrs for Hemolink and 19.2 hrs for Hemopure. Animals resuscitated with HBOCs exhibited delayed weight gain.
Conclusion: Hemodynamic effects in rodents exchange-transfused with blood, HemolinkTM, or HemopureTM were similar. The delayed weight gain observed with the HBOCs must be investigated.
INTRODUCTION
Trauma remains a leading cause of mortality and morbidity. Large volume fluid replacement is frequently required after major trauma. The ideal replacement solution should restore hemodynamic parameters, be retained within the vascular space, and deliver oxygen to ischemic tissues without adverse effects, but the ideal resuscitative or replacement solution has yet to be identified [Citation[1-3]].
Autologous blood is considered the best replacement solution and is frequently used as the benchmark against which other resuscitative fluids are measured. Since autologous blood is seldom available in practice, allogeneic transfusions of whole blood or components of blood are frequently used as an alternative. Despite the recent advances in the testing of blood products, the requirement for low temperature storage and cross-matching, and the risk of transmission of bacterial, parasitic, and viral pathogens continue to make blood transfusion potentially dangerous. This is particularly true in areas of the world where the prevalence of blood-borne pathogens is high or where testing centers are not readily available.
Crystalloid solutions, like Ringer's lactate or saline, have the advantage of stability at room temperature and do not require cross-matching. However, these replacement fluids have poor retention in the circulation and have been associated with rapid distribution into the extravascular space and adult respiratory distress syndrome [Citation[4]]. This is especially true when large volume replacement is required. Colloidal solutions like pentastarch, hetastarch and albumin, on the other hand, have longer retention in the circulation, although in some studies they were reported to be toxic [Citation[4]]. Elevated partial prothrombin times have been reported with hetastarch and retention in the extravascular space has been documented in patients that had increased vascular permeability [Citation[5]]. Colloidal solutions do not contribute to oxygen delivery and their use has not led to improved survival in clinical trials comparing colloidal solutions to inexpensive crystalloid solutions like saline [Citation[2], Citation[3]].
Hemoglobin based oxygen carrying solutions (HBOCs) have been developed in the last several decades in an attempt to produce resuscitative fluids that solve some of the problems identified above. HBOCs carry oxygen and they are retained in the circulation for about 24 hours. While unmodified, cell free hemoglobin induces severe vasoconstriction, is filtered at the glomerulus, and may induce acute renal failure, HBOCs have been modified to reduce the vasoconstrictor effects [Citation[6], Citation[7]]. In this study, we used a 50% exchange transfusion in rodents to compare two cross-linked hemoglobin based oxygen carrying solutions.
METHODS
Preparation of Resuscitative Fluids
Pentastrach preparation: The characteristics of pentastarch (PentaSpan, DuPont Pharma, Wilmington, DE) are shown in . This solution was diluted with normal saline to decrease the colloid osmotic pressure to 23 mmHg, a level similar to that in the two HBOC's used. Endotoxin level was undetectable in the diluted solution by the limulus amebocyte lysate (Biowhittaker, Baltimore, MD).
Table 1 Characteristics of the exchange fluids
Preparation of HBOC: Deoxygenated o-raffinose-crosslinked human hemoglobin HemolinkTM was provided by Hemosol Inc., Toronto, Canada, and was prepared from highly purified human hemoglobin Ao [Citation[8]]. This procedure generated a polymer by inter- and intra-molecular crosslinking, which contained 35% tetramer (). Hemolink was stored in the deoxygenated state at 4°C for up to one year prior to use. Deoxygenated glutaraldehyde-crosslinked bovine hemoglobin (HemopureTM) was provided by Biopure (Cambridge, MA) [Citation[9]]. Glutaraldehyde crosslinking produced a bovine hemoglobin-glutamer, which contained <5% tetramer (). Deoxygenated Hemopure was stored at room temperature for up to one year prior to use.
Animal Protocols
Animal care: Charles-Dawley (CD) rats weighing 275–325 g were used for all studies. Rats were housed in the animal care facility at the Boston Medical Center and allowed free access to regular rat chow (Purina Mills, Chicago, IL) and water. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the Boston Medical Center.
Surgical preparation: Rats were weighed then anesthetized by intra-peritoneal injection of 55 mg/kg of sodium pentobarbital (Nembutal). Surgery was performed on a temperature controlled operating table. Rat body temperature was measured continuously during the procedures by intra-arterial thermocouple and maintained between 36°C and 38°C. A tracheostomy was performed using polyethylene tubing (PE-240). Both femoral arteries were cannulated using PE-50 tubing to allow for continuous blood pressure monitoring through one artery and blood sampling through the other. The right internal jugular vein was cannulated with two PE-50 catheters. The first jugular catheter was advanced into the vena cava and used for the infusion of resuscitative solutions and measurement of cardiac output (see below). A second jugular catheter was used for the infusion of radiolabelled para-amino-hippurate (PAH) and radiolabelled inulin used for assessing renal blood flow and function. Urine was collected via a suprapubic bladder catheter (PE-90).
Exchange transfusion: Rats were subjected to a ∼50% exchange transfusion to mimic ongoing blood loss with intermittent resuscitation. To this end, 2 ml of blood was removed from the femoral artery and replaced with the same volume of autologous blood or one of the three resuscitative solutions (Hemolink, Hemopure, or Pentastarch). Warmed heparinized blood or one of the resuscitative solutions was infused through a catheter placed in the superior vena cava (see above). During the 16 minute exchange period, this 2 ml exchange was performed eight times. Blood was removed over one minute and the resuscitation solutions infused over the next minute to allow an ∼50% isovolemic exchange (15 ml/300 g).
Acute studies: Animals were divided into four groups (n = 12/group) that received either autologous blood (control) or one of three resuscitation solutions (Hemolink, Hemopure, or Pentastarch). Measurements of blood pressure, cardiac output, renal plasma flow and glomerular filtration rate were made after completion of the exchange transfusion. Data were collected during four separate and consecutive clearance periods of 20 minutes each. Systemic vascular resistance and renal blood flow were calculated from the measured data using standard formulae [Citation[6]]. We compared the data from the four clearance periods and found no differences among them. The data reported represent the average of values obtained between one and two hours after starting the exchange transfusion.
Delayed studies: Separate cohorts of animals were subjected to the 50% exchange transfusion as described above and were resuscitated with either blood or one of the three experimental solutions. After administering analgesia (buprinorphine), these rats were allowed to regain consciousness and return to individual cages. They were allowed free access to rat chow and water. After one, two, and five days rats were weighed, then re-anesthetized for the assessment of blood pressure, renal blood flow and glomerular filtration rate (GFR) as described above. The wet:dry ratio of muscle was measured in tissue obtained from the sternomastoid muscle on the side opposite to that of the jugular vein cannulation.
Group size: Based on power calculations derived from our previous studies [Citation[6], Citation[7], Citation[10]] we used the following number of animals for the acute and delayed studies mentioned above. Acute studies n = 12/group. Follow-up studies at one day (n = 10–14/group), two days (n = 10–11/group), five days (n = 8–11/group).
Hemodynamic Measurements
Blood pressure: Intra-arterial blood pressure was measured in rats during anesthesia using a pressure transducer (Narcotrace, Houston, TX).
Cardiac output: Cardiac output was measured by thermodilution using a CardioMax IIR (Columbus Instruments Corp., Columbus, OH) as previously described [Citation[6], Citation[11-13]].
Renal plasma flow and renal blood flow: Renal plasma flow (RPF) was calculated using a standard formula derived from the clearance of PAH assuming a clearance of 80%. Renal blood flow was calculated as RPF/(1-Hct).
Statistics
Data are expressed as mean±SEM. Groups were compared using the analysis of variance (ANOVA) and unpaired students t-test with Bonferroni correction for multiple comparisons. Animals resuscitated with autologous blood were considered the control group. Statistical significance was p < 0.05.
RESULTS
Acute Hemodynamic Effects
Rats tolerated the exchange transfusion well and there was no mortality associated with the procedure. The exchange transfusion did not alter the hematocrit in those animals resuscitated with autologous heparinized blood. In contrast, exchange transfusion resulted in significant and comparable decreases in the hematocrit in groups resuscitated with either of the hemoglobin based oxygen carrying solutions (Hemolink or Hemopure), or Pentastarch (A).
Figure 1 Acute hemodynamic effects of 50% exchange transfusion. (A) Hematocrit. Hematocrit was maintained in control rats that underwent 50% exchange transfusion and received autologous heparinized blood. In contrast, rats that received Pentastarch, or either of the hemoglobin based oxygen carrying solutions (Hemolink or Hemopure), had a >50% decrease in hematocrit. (B) Intra-arterial mean arterial pressure, (C) systemic vascular resistance, and (D) cardiac output. While blood pressure, systemic vascular resistance and cardiac output were well maintained in animals that received autologous blood or either of the hemoglobin based oxygen carrying solutions, animals exchanged with Pentastarch had decreased blood pressure and systemic vascular resistance. In this latter group there was a trend toward an increase in cardiac output. *p < 0.001 vs. blood.
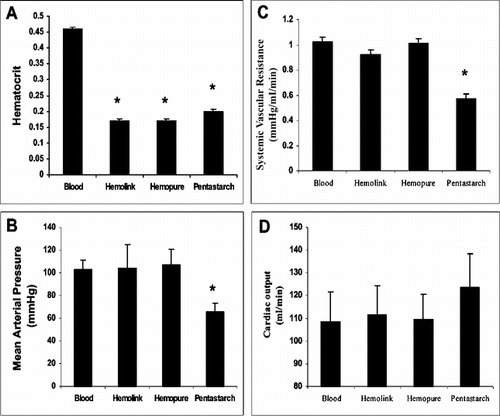
One hour after the exchange transfusion, the mean arterial pressure (MAP) and cardiac output (CO) were comparable in rats that received autologous blood or either of the hemoglobin based oxygen carriers (B and D). The hemoglobin based oxygen carrying solutions tested did not induce significant vascoconstriction and did not significantly alter systemic vascular resistance (1.02 ± 0.03 and 0.92 ± 0.05 after Hemolink or Hemopure, respectively) (C). The systemic vascular resistance for both HBOC's was similar to that observed after exchange transfusion with blood (1.01 ± 0.05 ml/min/mmHg). However, Pentastarch exchange was associated with a decrease in systemic vascular resistance (SVR 0.57 ± 0.03) that produced a significant decrease in MAP (B).
Acute Effects on Renal Function
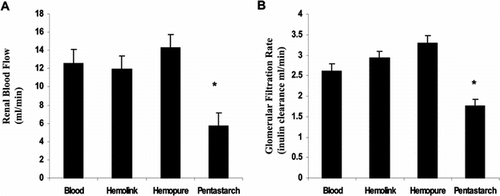
Renal blood flow was similar whether the rats were exchanged with one of the hemoglobin based oxygen carriers Hemolink and Hemopure or with autologous blood (12.57 ± 1.24, 11.89 ± 1.96, and 14.25 + 1.65 ml/min), but after exchange transfusion with Pentastarch, renal blood flow decreased significantly (5.71±0.89 ml/min) (A). In rats exchange transfused with Pentastarch, an acute drop in glomerular filtration rate was seen (1.71 ± 0.17 ml/min/300g). In contrast, glomerular filtration rate was well maintained in animals that underwent exchange with autologous blood, Hemolink or Hemopure (2.61 ± 0.62, 2.93 ± 0.2, or 3.30 ± 0.15 respectively) (B).
Figure 2 Acute effects of exchange transfusion on renal function. (A) Renal blood flow. Renal blood flow measured by clearance of para-aminohippurate one hour after completion of 50% exchange transfusion. (B) Glomerular filtration rate as measured by clearance of radio-labeled inulin one hour after exchange transfusion. *p < 0.001 vs. blood.
Plasma Hemoglobin and Urine Hemoglobin Levels
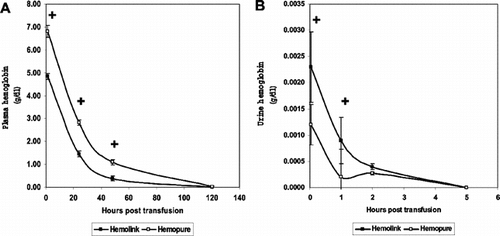
In animals exchanged with Hemolink or Hemopure, the clearance of plasma hemoglobin and hemoglobinuria were measured following the exchange. HBOC were cleared from the plasma during the first 48 hours after the exchange (). Hemolink containing 35% tetramer hemoglobin was cleared more rapidly (t1/2 = 13.8 hours) than Hemopure containing <5% tetramer hemoglobin (t1/2 = 19.2 hours) (). Hemoglobinuria was noted in animals that received either of the HBOC. Hemolink containing 35% tetrameric hemoglobin was associated with increased levels of urinary hemoglobin compared to Hemopure, which contained less than 5% tetrameric hemoglobin. Neither of the HBOC caused significant renal dysfunction ().
Figure 3 Clearance of plasma hemoglobin after 50% exchange transfusion with crosslinked hemoglobin based oxygen carrying solutions. (A) Plasma hemoglobin. Plasma hemoglobin concentration measured over the five days after exchange transfusion with either Hemolink or Hemopure. (B) Urinary hemoglobin concentration. Urinary hemoglobin concentrations measured during the five days following exchange transfusion with either Hemolink or Hemopure. +p < 0.001 Hemolink vs. Hemopure.
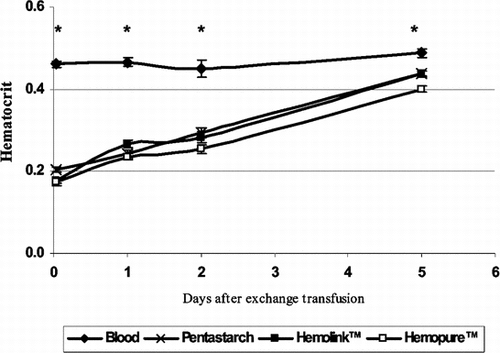
Recovery of Hematocrit Following 50% Exchange Transfusion
Rats exchanged with blood did not become anemic. In the three experimental groups that were resuscitated with Hemolink, Hemopure, or Pentastarch, hematocrit value recovered during the five days following the exchange transfusion (). There were no differences between HBOCs and pentastarch groups.
Figure 4 Recovery of hematocrit following 50% exchange transfusion with hemoglobin based oxygen carrying solutions. Recovery of hematocrit during the first five days following a 50% exchange transfusion was used as a measure of erythropoiesis. No differences were observed between HBOC solutions and Pentastarch. *p < 0.01 vs. blood.
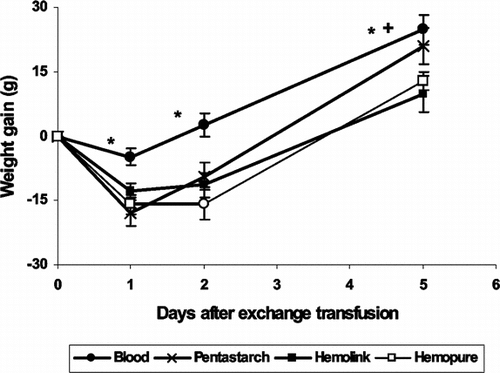
Well-being After Exchange Transfusion
We monitored rats for five days during recovery from 50% exchange with autologous blood, Pentastarch, or one of the two HBOCs (). There were no differences in wet:dry ratios of muscle tissue among the three test groups and the control group at any of the time periods after the exchange transfusion. While all the rats survived and appeared healthy, rats exchanged with autologous blood lost an insignificant amount of weight during the first 24 hours after the exchange transfusion (− 4.8 ± 2.1g), but rats resuscitated with Hemolink, Hemopure, or pentastarch lost significantly more weight during this period (Hemolink − 12.7 ± 1.7 g, Hemopure − 15.9 ± 2.2 g, pentastarch − 17.9 ± 3.1). Rats resuscitated with Hemolink, Hemopure, or pentastarch gained weight after the first 24 hours. Rats that received Hemolink or Hemopure gained less weight on day 5 than those resuscitated with pentastarch (Hemolink 9.7 ± 4.2 g, Hemopure 12.8 ± 2.1 g, vs pentastarch 20.9 ± 4.2 g) (HBOC vs. pentastarch p < 0.05).
DISCUSSION
HBOC's are currently being developed and tested as blood substitutes. The acute effects of hemodilution or resuscitation with HBOCs have been reviewed [Citation[14-17]]. In this study, we compared Hemolink and Hemopure in a controlled experiment of 50% exchange transfusion without hypotension. Despite the differences in the method of cross-linking, the percentage of tetramer, and the source of the hemoglobin (human vs bovine), we found no differences in the acute hemodynamic or renal effects of these solutions. In keeping with its <5% tetrameric hemoglobin, Hemopure was retained in the circulation longer than Hemolink with 35% tetrameric hemoglobin, but this did not affect the blood pressure or the systemic vascular resistance following the exchange. These data are similar to results in our previous study using Hemolink in two exchange transfusions performed in rats [Citation[6], Citation[7]].
Phase II and III trials using HBOC as a blood substitute for traumatic and elective surgical situations in humans when blood was not available or to avoid allogeneic RBC transfusions have demonstrated that the tetramer content of the HBOC correlated with the vasoconstrictor activity, and both diaspirinated-crosslinked hemoglobin and o-raffinose cross-linked hemoglobins were associated with increased adverse events [Citation[14], Citation[18-22]].
While the hemodynamic effects of the 50% exchange transfusion were similar in rats exchanged with HBOC or blood, rats given pentastarch became mildly hypotensive. In the pentastarch group at the 24-hour period there were no significant differences among any of the groups in terms of blood pressure, cardiac output, systemic vascular resistance, or renal function (data not shown). In this study we measured weight gain after the exchange transfusion to assess general well-being. Despite being less effective in maintaining hemodynamic parameters and renal function, rats that were exchanged with pentastarch gained weight more rapidly that those resuscitated with HBOC. Weight gain of the rats in the pentastarch group was not due to an increase in the water content of the muscle of these rats. The decrease in weight gain 5 days following the exchange transfusion with both the crosslinked hemoglobins (Hemolink and Hemopure) was in contrast to our previous experience with the o-raffinose crosslinked hemoglobin in rats subjected to a 50% exchange transfusion, which showed weight gains similar to those observed with blood [Citation[6]]. The decreased weight gain observed in this study with these two modified HBOC's is an adverse event related to HBOC's, which needs to be investigated.
Recovery of hematocrit is influenced by iron availability, erythropoietin levels and the presence of inflammation. Hemoglobin containing solutions may accelerate the recovery of the hematocrit value because of the heme moiety that replaces iron lost during the exchange transfusion; HBOCs may delay recovery of the hematocrit by increasing tissue oxygen tension, which may inhibit the erythropoietin response to anemia, or HBOC may induce an inflammatory response. To address this issue we measured the hematocrit during the first 5 days after the exchange transfusion. We were unable to observe a difference in the rate of recovery of the hematocrit value after 50% exchange with the HBOC's as compared to pentastarch.
In this study we evaluated two HBOC's in rats subjected to a 50% exchange transfusion without shock. Despite favorable acute hemodynamic effects, the exchange transfusions with both HBOCs were associated with delayed weight gains 5 days after the transfusions. The delayed weight gain observed with both HBOC's is an adverse event, which needs to be investigated.
This work was supported by the U.S. Navy (Office of Naval Research Contract N00014-02-1-0923).
The opinions or assertions contained herein are those of the authors and are not to be construed as official or reflecting the views of the Navy Department or Naval Service at large.
We would like to thank Hemosol and Biopure for supplying Hemolink and Hemopure, respectively. Neither of these companies was involved in the design or performance of the studies described in this paper, nor were they involved in the preparation of the manuscript.
REFERENCES
- Bunn, F., Alderson, P., Hawkins, V. (2001). Colloid solutions for fluid resuscitation. Cochrane Database Syst. Rev. CD001319, , [CSA]
- Alderson, P., , et al. (2004). Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst. Rev. CD001208, , [CSA]
- Roberts, I., , et al. (2004). Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst. Rev. CD000567, , [CSA]
- Ernest, D., Belzberg, A.S., Dodek, P.M. (1999). Distribution of normal saline and 5% albumin infusions in septic patients. Crit. Care Med. 27: 46–50, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Roberts, J.S., Bratton, S.L. (1998). Colloid volume expanders. Problems, pitfalls and possibilities. Drugs 55: 621–630, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Lieberthal, W., , et al. (2002). Comparison of the effects of a 50% exchange-transfusion with albumin, hetastarch, and modified hemoglobin solutions. Shock 17: 61–69, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Lieberthal, W. , et al. (1999). O-raffinose cross-linking markedly reduces systemic and renal vasoconstrictor effects of unmodified human hemoglobin. J. Pharmacol. Exp. Ther. 288: 1278–1287, [PUBMED], [INFOTRIEVE], [CSA]
- Adamson, J.G., Moore, C. (1998). Hemolink, an o-raffinose crosslinked hemoglobin-based oxygen carrier, in Blood Substitutes: Principles, Methods, Products and Clinical Trials, T.M.S. Chang, Ed., Karger Landes: New York.
- Gawryl, M., Clark, T., Rausch, C. (1991). Characterization of highly purified polymerized bovine hemoglobin, in Red Cell Substitutes: The Proceedings of the Second International Symposium of Red Cell Substitutes, S. Sekuguchi, Ed., Kindai Shuppan: Tokyo.
- Lieberthal, W., Fuhro, R., Andry, C., Valeri, C. R. (2000). Effects of hemoglobin-based oxygen-carrying solutions in anesthetized rats with acute ischemic renal failure. J. Lab. Clin. Med. 135: 73–81, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Thompson, A., Valeri, C.R., Lieberthal, W. (1995). Endothelin receptor A blockade alters hemodynamic response to nitric oxide inhibition in rats. Am. J. Physiol. 269: H743–H748, [PUBMED], [INFOTRIEVE], [CSA]
- Lieberthal, W., Thompson, A., Valeri, C.R. (1996). Effects of nitric oxide inhibition on systemic and renal hemodynamics in the hemorrhaged rat. Kidney Blood Press Res. 19: 340–346, [PUBMED], [INFOTRIEVE], [CSA]
- Thompson, A., McGarry, A.E., Valeri, C.R., Lieberthal, W. (1994). Stroma-free hemoglobin increases blood pressure and GFR in the hypotensive rat: role of nitric oxide. J. Appl. Physiol. 77: 2348–2354, [PUBMED], [INFOTRIEVE], [CSA]
- Creteur, J., Vincent, J.L. (2003). Hemoglobin solutions. Crit Care Med 31: S698–S707, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Kingma, J.G., Jr., , et al. (2002). The effects of hemodilution with Hemolink upon hemodynamics and blood flow distribution in anesthetized dogs. Artif. Cells Blood Substit. Immobil. Biotechnol. 30: 137–154, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Levy, J.H., , et al. (2002). Polymerized bovine hemoglobin solution as a replacement for allogeneic red blood cell transfusion after cardiac surgery: results of a randomized, double-blind trial. J. Thorac. Cardiovasc. Surg. 124: 35–42, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Moqattash, S., Lutton, J.D., Rosenthal, G., Abu-Hijleh, M.F., Abraham, N.G. (1997). Effect of blood substitute, recombinant hemoglobin, on in vivo hematopoietic recovery from AZT toxicity. Acta. Haematol. 98: 76–82, [PUBMED], [INFOTRIEVE], [CSA]
- Cheng, D.C. (2001). Safety and efficacy of o-raffinose cross-linked human hemoglobin (Hemolink) in cardiac surgery. Can. J. Anaesth. 48: S41–S48, [PUBMED], [INFOTRIEVE], [CSA]
- Lamy, M.L., , et al. (2000). Randomized trial of diaspirin cross-linked hemoglobin solution as an alternative to blood transfusion after cardiac surgery. The DCLHb Cardiac Surgery Trial Collaborative Group. Anesthesiology 92: 646–656, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Sloan, E.P., , et al. (1999). Diaspirin cross-linked hemoglobin (DCLHb) in the treatment of severe traumatic hemorrhagic shock: A randomized controlled efficacy trial JAMA. 282: 1857–1864, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Kerner, T., , et al. (2003). DCL-Hb for trauma patients with severe hemorrhagic shock: the European “On-Scene” multicenter study. Intensive Care Med. 29: 378–385, [PUBMED], [INFOTRIEVE], [CSA]
- Swan, S.K., , et al. (1995). Pharmacologic profile of diaspirin cross-linked hemoglobin in hemodialysis patients. Am. J. Kidney Dis. 26: 918–923, [PUBMED], [INFOTRIEVE], [CSA]