Abstract
A simple and straightforward approach to encapsulating an enzyme and preserving its function in polypeptide-based artificial cells is demonstrated. A model enzyme, glucose oxidase (GOx), was encapsulated by repeated stepwise adsorption of poly(L-lysine) and poly(L-glutamic acid) onto GOx-coated CaCO3 templates. These polypeptides are known from previous research to exhibit nanometer-scale organization in multilayer films. Templates were dissolved by ethylenediaminetetraacetic acid (EDTA) at neutral pH. Addition of polyethylene glycol (PEG) to the polypeptide assembly solutions greatly increased enzyme retention on the templates, resulting in high-capacity, high-activity loading of the enzyme into artificial cells. Assay of enzyme activity showed that over 80 mg-mL−1 GOx was retained in artificial cells after polypeptide multilayer film formation and template dissolution in the presence of PEG, but only one-fifth as much was retained in the absence of PEG. Encapsulation is a means of improving the availability of therapeutic macromolecules in biomedicine. This work therefore represents a means of developing polypeptide-based artificial cells for use as therapeutic biomacromolecule delivery vehicles.
INTRODUCTION
Proteins, peptides, and oligonucleotides can be potent therapeutic agents. Such macromolecules, however, are targets of various degradation mechanisms in vivo. Encapsulation of biomacromolecules within a biocompatible microenvironment, for extended preservation of function or controlled release, could improve availability at targeted sites [Citation[1]]. Electrostatic layer-by-layer nanoassembly is a means of preparing polyelectrolyte multilayers films and capsules of high stability and tunable permeability [Citation[2]]. Applications of this technology include nano-reactors, biosensors, artificial cells, and drug delivery vehicles. Encapsulation of protein molecules into preformed multilayer capsules has been reported and typically has been achieved by changing the surrounding environment and thereby capsule wall permeability [Citation[3-6]]. Current challenges to this method include maximizing the loading of macromolecules, reducing nonspecific adsorption of macromolecules to the capsule surface, and optimizing the physical, chemical, and biological properties of the capsules for applications in biomedicine and biotechnology [Citation[7], Citation[8]]. This work describes an alternative means of achieving direct and efficient encapsulation of a model protein in engineered biodegradable polypeptide microcapsules. Such microcapsules could also be called artificial cells [Citation[9]].
The model encapsulant was glucose oxidase (GOx). The biochemical activity of this enzyme is easily monitored in vitro. The challenge here was to develop a straightforward approach to template-based enzyme microencapsulation. Stepwise adsorption of polypeptides onto a biomacromolecule-coated core and subsequent dissolution of the template involves three practical difficulties. One is that deposition of a highly-charged or “stronger” polyion onto existing polyion layers can displace the adsorbed materials, promote their release and resolubilization, and in some cases destroy film structure [Citation[10], Citation[11]]. Another is that dissolution of the core particle employed for capsule assembly can denature the encapsulated macromolecules, reducing biochemical activity [Citation[3-6]]. A third is that because adsorbed material can be displaced by polymers from solution by mass action, it is important to protect adsorbed functional biomolecules from dislodgement during the encapsulation process. This work demonstrates achievement of high-capacity, high-activity loading of GOx by addition of polyethylene glycol 300 (PEG) as a cosolvent to aqueous solutions of poly(L-lysine) (PLL) and of polyglutamic acid (PLGA), the polypeptides used for artificial cell fabrication.
EXPERIMENTAL SECTION
GOx Adsorption on CaCO3 Particles
All chemicals were from Sigma (USA), except as indicated. In GOx adsorption experiments, 5 mg of CaCO3 particles (PlasmaChem GmbH, Germany) were mixed with a 100 µL of 37,300 units-g−1 II-S GOx from A. niger in 10 mM Tris buffer, pH 7.4. The final particle concentration was 5% (w/v). Enzyme adsorption onto microparticles was quantified by decrease in absorbance of the liquid phase at 280 nm (Jasco V-430 spectrophotometor, Japan). GOx-loaded microparticles were separated from polypeptide adsorption solution by centrifugation.
Enzyme Encapsulation
0.10 mL of 5 mg-mL−1 GOx solution was added to 5 mg of CaCO3 microparticles and mixed thoroughly for 2 h. The suspension was centrifuged for 5 min at 1000 × g for removal of the fluid phase. A polypeptide multilayer film was self-assembled on GOx-adsorbed CaCO3 microparticles as follows. In the formation of each layer, peptides adsorbed onto GOx-coated particles for 10 min under gentle shaking at 4°C, and centrifugation was used to separate particles from unbound peptide in the fluid phase. 0.1 mL of 1 mg-mL−1 solutions of PLL (MW ∼ 14.6 kDa) and of PLGA (MW ∼ 13.6 kDa) in 10 mM Tris, 0.5 M NaCl, pH 7.4 were used in succession and mixed thoroughly with the microparticles. Up to 50% (v/v) PEG 300 (Fluka, Switzerland) was present in the polypeptide solutions; higher average molecular mass PEG yields an overly viscous solution for the purpose. Two microparticle washing steps were carried out with de-ionized water at 4°C between polypeptide adsorption cycles. The process was repeated until the desired number of layers was deposited (typically 6 bilayers). After deposition of the final layer of polypeptide, coated particles were rinsed and collected by centrifugation. The supernatant of the polypeptide assembly solution was analyzed for aromatic absorbance after separation of the fluid phase from microparticles following each polypeptide adsorption step. PLL and PLGA contain no aromatic residues, so absorbance at this wavelength was assumed to be due effectively exclusively to GOx. Microparticle cores were dissolved by treatment with 0.2 M EDTA, pH 7.4 for a 10–20 min period. The resulting artificial cells were collected by centrifugation at 2000 × g for 5 min, rinsed with DI water, and re-suspended in 0.25 mL 10 mM Tris buffer. Aliquots of sample were assayed for GOx activity. The same procedure was followed for confocal fluorescence experiments except that Cy3-labeled GOx replaced GOx.
RESULTS AND DISCUSSION
Characterization of CaCO3 Particles
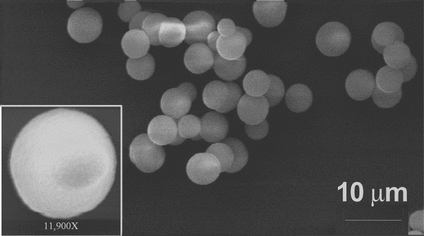
The templates for GOx and polypeptide multilayer film assembly were spherical CaCO3 microparticles produced by a plasma chemical process. Dissolution of the templates at neutral pH by the divalent cation chelator EDTA [Citation[12], Citation[13]] is more favorable than dissolution at strongly acidic pH for preservation of protein functionality and polypeptide film structure. Near-monodispersity of the CaCO3 microparticles used here was verified by scanning electron microscopy (). The diameter was 4.44 ± 0.45 µm. The particles were positively charged and nanoporous, enabling adsorption by electrostatic attraction of a relatively large quantity of a negatively charged chemical species; the net charge of GOx (pI = 5.2) is negative at neutral pH.
Figure 1 Scanning electron micrograph of sacrificial CaCO3 templates used for polypeptide capsule fabrication. Inset, enlarged view of a single particle. The sample was prepared by depositing a drop of aqueous particle suspension on a glass slide. Solvent was evaporated and particles were sputtered with a thin gold film. The Amray 1830 instrument (USA) was operated at an acceleration voltage of 20 keV.
GOx Adsorption on CaCO3 Microparticles
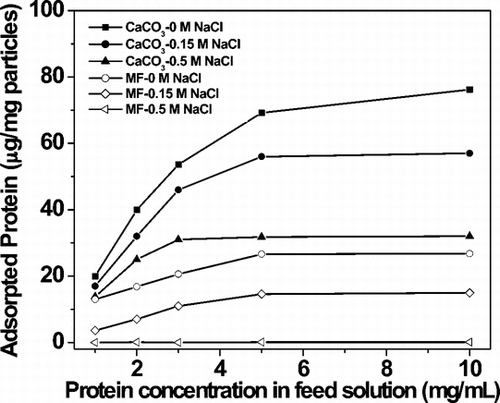
shows the adsorption capacity of GOx onto CaCO3 microparticles and dependence on ionic strength. Evidently, adsorption of this enzyme onto CaCO3 microparticles was much better than non-porous melamine formaldehyde (MF) particles of similar size (Microparticles GmbH, Germany) under otherwise identical conditions. Maximum GOx adsorption achieved was c. 76 µg per mg of CaCO3 microparticles. The mass m of one CaCO3 particle was 4πr3ρ/3 = 1.24 × 10−10 g, where the radius r is 2.2 × 10−4 cm and the density ρ is 2.7 g/cm3. The number of microparticles per mg of material and therefore the number of capsules that could be generated by per mg of material was 8.1 × 106. 76 µg of GOx per mg of microparticles thus translates into approximately 9.4 × 10−12 g of GOx per microparticle. Not all GOx initially adsorbed, however, was present in capsules, as multiple washing steps and sequential adsorption of polypeptide resulted in some loss of enzyme to solution.
Displacement of GOx from CaCO3 Particles During Polypeptide-based Artificial Cell Assembly
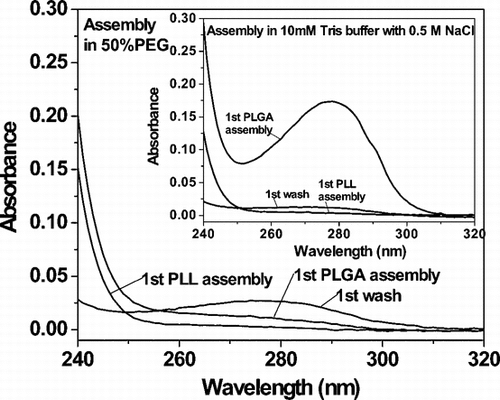
The scheme for artificial cell assembly was GOx:(PLL/PLGA)n. presents UV spectra of supernatants collected during particle rinsing and sequential deposition of PLL/PLGA. The inset shows that an aromatic absorbance peak at λ = 280 nm appeared in the assembly solution on deposition of the first layer of PLGA (third layer overall). This must be due to loss of GOx from microparticles, as none of the chemical groups in PLL or PLGA has a substantial absorption dipole moment near 280 nm. Up to half of adsorbed GOx was dislodged from the CaCO3 particles in this step, despite the presence of one layer of PLL. By contrast, little or no GOx was lost during PLL adsorption steps (see Supplementary for an illustration). Further loss of GOx was detected when PLGA was added as the fifth layer overall, though less than in the previous PLGA adsorption step (not shown). To improve enzyme “loading,” a means was needed of creating physicochemical conditions that reduced enzyme solubility in bulk solvent, limiting undesirable loss of enzyme from the template surface.
Increasing Loading Efficiency by Addition of PEG in Assembly Solutions
PEG is a useful precipitating agent in protein crystallization [Citation[14]]. shows that addition of 50% PEG to the polypeptide assembly solutions promoted retention of absorbed GOx and therefore increased capsule loading. This interpretation was corroborated by quartz crystal microbalance experiments: PEG gave stepwise deposition of polypeptides onto the GOx-coated surface and substantially reduced loss of adsorbed protein relative to control during deposition of PLL and PLGA (see Supplementary ). Complementary experiments involving assay of GOx activity and labeling of GOx with a fluorescent dye were carried out to confirm encapsulation of GOx and to assess enzyme functionality.
Activity Measurement of GOx in Capsules
shows measured activity of GOx after polypeptide encapsulation. CaCO3 particle surfaces in 0.10 mg-aliquots of 2% suspension were saturated with GOx using a 5 mg-mL−1 solution. CaCO3 cores were dissolved by addition of 0.2 M EDTA at pH 7.4, transforming polypeptide-coated, enzyme-bound colloidal microparticles into polypeptide-based artificial cells. A colorimetric assay based on the compound Amplex Red was used to measure enzyme activity in the cells (see Supplementary and associated text). As shown in , when polypeptide assembly was carried out in the absence of PEG, GOx activity associated with microparticles decreased markedly on deposition of PLL/PLGA up to 2 bilayers. Measured activity tended to reach a constant value – about 11% of the value prior to deposition of polypeptide – independent of the number of layers in the 3–6 bilayer range.
Figure 4 Activity of encapsulated enzyme as a function of the number of layers of polypeptide. Measurements are shown for microparticle cores before and after encapsulation, and with and without addition of PEG in the polypeptide assembly solutions. Capsules were not separated from EDTA prior to assay for enzyme activity. The right-most column shows the GOx activity in a sample of capsules following sonication.
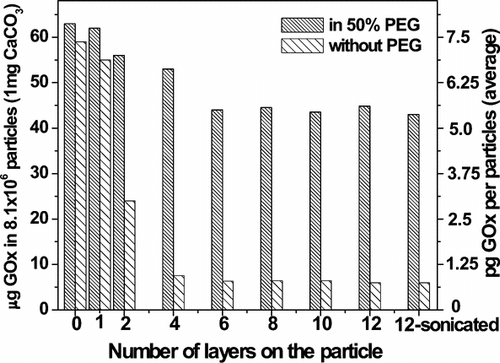
Addition of PEG to the PLL and the PLGA assembly solutions substantially improved the “loading” of polypeptide-based artificial cells with GOx. About 70% of enzyme activity was retained in the cells after deposition of 6 bilayers but prior to phase separation. Sonication for 20 min of 6-bilayer cells assembled using PEG resulted in no decrease in enzyme activity. This suggests that encapsulation of GOx by a polypeptide multilayer film neither inhibited enzyme activity nor prevented diffusion of small molecules—e.g. glucose (enzyme substrate) and reaction products across the barrier. This research did not seek to determine the extent of migration of GOx into the artificial cell wall.
Confirmation that measured enzyme activity was in fact associated with artificial cells was obtained as follows. GOx activity in solution was quantified after separation of the 6-bilayer cells from the fluid phase with a 0.22 µm filter. No GOx activity was detected in the filtrate in the absence of PEG; the enzyme activity was in the artificial cells. Some enzyme (c. 1.5 × 10−12 g-capsule−1) was detected in the filtrate when the PEG protocol was used. Presumably this was due to partial collapse of the coated templates/cells during repeated centrifugation and washing. No further activity was detected in the filtrate after re-suspension of the cells, indicating that loaded GOx did not leach out. The same approach was used to quantify retention of GOx in cells.
Assuming that “membrane” thickness was negligible in comparison with artificial diameter, the volume of one cell was 4πr3/3 = 4.58 × 10−11 cm3. The mass of GOx loaded into a cell when PEG was present in polypeptide assembly solutions was 3.8 × 10−12 g (see above). The GOx concentration within artificial cells, then, was 82 mg-mL−1, about 40% of the amount bound to CaCO3 particles prior to encapsulation. For comparison, when no PEG was present only 7.4 × 10−13 g of GOx was loaded—about 16 mg-mL−1 or 11% of the calculated maximum concentration.
Visualization of GOx-Loaded Polypeptide Microcapsules
GOx was labeled with a fluorescent dye, Cy3 mono-reactive NHS-ester (Amersham Biosciences, UK). Encapsulation of Cy3-GOx within a PLL/PLGA film followed adsorption onto CaCO3 microparticles. Study of influence of layer number on artificial cell stability by fluorescence microscopy revealed that coatings with fewer than 3 bilayers tended to dissociate after core particle dissolution, whereas capsules with 3 or more bilayers usually were stable in aqueous solution on a time scale of weeks, similar to previous work [Citation[8]]. This is consistent with the GOx activity measurements reported here. Film thickness is directly related to layer number: we have found by ellipsometry that it is 1.5–1.6 nm-layer−1 for dry films of 84.0 kDa PLL and 84.6 kDa PLGA [Citation[15]]. Thickness is approximately the same for PLL and PLGA chains of about 14 kDa [Citation[16]]. Further evidence of loading of Cy3-labeled GOx into PLL/PLGA 6-bilayer artificial cells was obtained by confocal laser fluorescence microscopy (a, left panel). The right panel, obtained by brightfield illumination, shows that the microparticle templates were completely dissolved by treatment with EDTA.
Figure 5 Visualization of GOx-Cy3-loaded polypeptide microcapsules. (a) Confocal microscopy image after dissolution of the template. Left, fluorescence; right, brightfield. (b) Fluorescence intensity profile of a capsule. Left, after dissolution of the core; right, coated core prior to dissolution of the template. Variations in fluorescence intensity of capsules reflect differences in distance between capsule and objective lens. The Leica instrument (Germany) was equipped with a 62 × oil immersion lens.
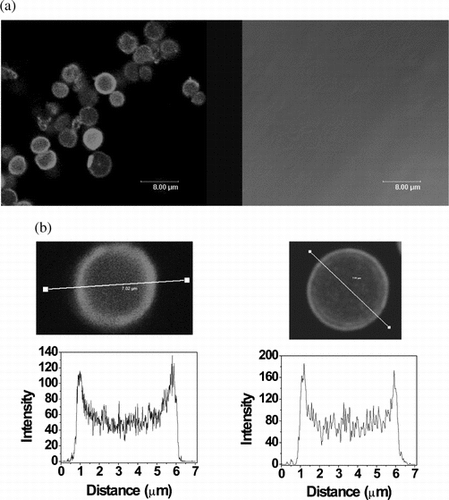
Fluorescence intensity cross-sectional profiles of a 6-bilayer capsule revealed that the artificial cell interior was filled with a substantial quantity of unbound Cy3-labeled GOx (b, left). The diameter of the cell was approximately that of the original template (b, right). The appearance of two peaks in the intensity profile indicates that some GOx was bound to the cell “membranes,” probably due to electrostatic attraction and dissipation. Further evidence of GOx encapsulation in polypeptide cells was obtained by circular dichroism spectroscopy (see Supplementary and related text).
CONCLUSIONS
The foregoing has shown that a straightforward approach has been developed to encapsulate functional protein in a stable polypeptide-based artificial cell. Use of a template soluble under mild solution conditions is advantageous for preserving polypeptide “membrane” integrity and protein function. Addition of PEG to polypeptide adsorption solutions can increase loading of a macromolecule into an artificial cell in an aqueous medium. Presumably this occurs by PEG's decreasing protein solubility in aqueous solution, thereby limiting loss from the template surface during polyelectrolyte self-assembly. In this work about 40% of GOx activity was retained in artificial cells after polypeptide multilayer film formation in the presence of PEG and core dissolution, about a 4-fold increase over no PEG.
The results indicate that a polypeptide thin film can inhibit leakage of macromolecule from a polypeptide-based artificial cell without precluding permeability to small molecules, e.g. substrate and reaction products. The inherent biocompatibility of polypeptide-based artificial cells presents a range of advantages to biomedical applications over more usual synthetic polyelectrolytes, e.g. poly(allylamine hydrochloride) and poly(styrene sulfonate) [Citation[13]]. For example, loaded proteins could be released over time by proteolytic hydrolysis of the capsule shell in vivo. Moreover, artificial cells formed from designed polypeptides containing cysteine can be stabilized by disulfide cross-links [Citation[8], Citation[17]]; the extracellular environment is oxidizing. Polypeptides could be designed on the basis of solvent-exposed surfaces of known proteins to optimize biochemical properties, for instance immunogenicity [Citation[7]]. Finally, there is considerable potential for automating the encapsulation approach outlined here.
We thank Dr. Tatsiana Shutava for assistance with confocal laser scanning microscopy and Dr. Chuanding Cheng for assistance with scanning electron microscopy. This research was supported by a Nano-scale Exploratory Research award from the National Science Foundation (DMI-0403882), an enhancement grant from the Louisiana Space Consortium (Louisiana NASA EPSCoR, project R127172), and the 2002 Capital Outlay Act 23 of the State of Louisiana (Governor's Biotechnology Initiative).
REFERENCES
- Pérez, C., Castellanos, I.J., Costantino, H.R., Al-Azzam, W., Griebenow, K. (2002). Recent trends in stabilizing protein structure upon encapsulation and release from bioerodible polymers. J. Pharm. Pharmacol. 54: 301–313, [CSA]
- Peyratout, C.S., Dähne, L. (2004). Tailor-made polyelectrolyte microcapsules: From multilayers to smart containers. Angew. Chem. Int. Ed. 43: 3762–3783, [CROSSREF], [CSA]
- Sukhorukov, G.B., Antipov, A.A., Voigt, A., Donath, E., Möhwald, H. (2001). pH-controlled macromolecule encapsulation in and release from polyelectrolyte multilayer nanocapsules. Macromol. Rapid Commun. 22: 44–46, [CROSSREF], [CSA]
- Lvov, Y., Antipov, A.A., Mamedov, A., Mohwald, H., Sukhorukov, G.B. (2001). Urease encapsulation in nanoorganized microshells. Nano Lett. 1: 125–128, [CROSSREF], [CSA]
- Ghan, R., Shutava, T., Patel, A., John, V.T., Lvov, Y. (2004). Enzyme-catalyzed polymerization of phenols within polyelectrolyte microcapsules. Macromolecules 37: 4519–4524, [CROSSREF], [CSA]
- Balabushevich, N.G., Tiourina, O.P., Volodkin, D.V., Larionova, N.I., Sukhorukov, G.B. (2003). Loading the multilayer dextran sulfate/protamine microsized capsules with peroxidase. Biomacromolecules 4: 1191–1197, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Zheng, B., Haynie, D.T., Zhong, H., Sabnis, K., Surpuriya, V., Pargaonkar, N., Sharma, G., Vistakula, K. (2005). Design of peptides for thin films, coatings, and microcapsules for applications in biotechnology. J. Biomater. Sci. Polym. Edn. 16: 285–300, [CROSSREF], [CSA]
- Haynie, D.T., Palath, N., Liu, Y., Li, B., Pargaonkar, N. (2005). Biomimetic nanostructured materials: Inherent reversible stabilization of polypeptide microcapsules. Langmuir 21: 1136–1138, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Haynie, D.T., Li, B. (2005). Nanomanufacturing polypeptide multilayer thin films for applications in biomedicine and other areas. NSF Design, Service and Manufacturing Grantees and Research Conference, January, Scottsdale, Arizona.
- Boulmedais, F., Bozonnet, M., Schwinté, P., Voegel, J.-C., Schaaf, P. (2003). Multilayered polypeptide films: Secondary structures and effect of various stresses. Langmuir 19: 9873–9882, [CROSSREF], [CSA]
- Zhang, L., Li, B., Zhi, Z.-I., Haynie, D.T. Perturbation of nano-scale structure of polypeptide multilayer thin films. Langmuir 21: 5439–5445, [CROSSREF], [CSA]
- Petrov, A.I., Antipov, A.A., Sukhorukov, G.B. (2003). Base-acid equilibria in polyelectrolyte systems: From weak polyelectrolytes to interpolyelectrolyte complexes and multilayered polyelectrolyte shells. Macromolecules 36: 10079–10086, [CROSSREF], [CSA]
- Volodkin, D.V., Petrov, A.I., Prevot, M., Sukhorukov, G.B. (2004). Matrix polyelectrolyte microcapsules: New systems for macromolecular encapsulation. Langmuir 20: 3398–3406, [PUBMED], [INFOTRIEVE], [CSA]
- Patel, S., Cudney, B., McPherson, A. (1995). Polymeric precipitants for the crystallization of macromolecules. Biochem. Biophys. Res. Commun. 207: 819–828, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Zhi, Z.-I., Haynie, D.T. (2004). Direct evidence of controlled structure reorganization in a nanoorganized polypeptide multilayer thin film. Macromolecules 37: 8668–8675, [CROSSREF], [CSA]
- Balkundi, S. (2004). Insight into polypeptide layer-by-layer assembly using the model peptides poly-L-lysine and poly-L-glutamic acid. MS thesis, Louisiana Tech University.
- Li, B., Haynie, D.T. (2004). Multilayer biomimetics: Reversible covalent stabilization of a nanostructured biofilm. Biomacromolecules 5: 1667–1670, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
APPENDIX: SUPPORTING INFORMATION
Displacement of GOx From CaCO3 Particles DuringMicrocapsule Assembly
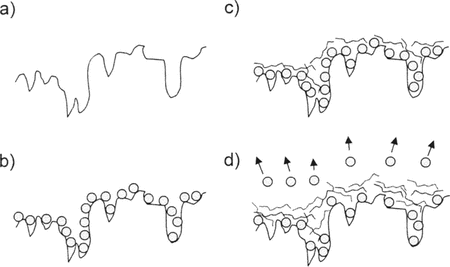
Supplementary illustrates the proposed mechanism for loss of GOx activity in capsules during assembly of polypeptide layers but before dissolution of the colloidal template. Most of the GOx molecules that did not contribute to activity measurements after deposition of polypeptide were presumably displaced from the particles by mass action. This suggestion is plausible because it is known that binary polyelectrolyte films prepared by layer-by-layer adsorption are poorly stratified [1]. For mass action to be effective, however, it is necessary for PLGA to penetrate the adsorbed layer of PLL. This process is aided by thermal fluctuations.
Supplementary Figure 1 Cartoon showing adsorption and desorption of GOx from the surface of a CaCO3 particle during microcapsule fabrication. a) The microparticle surface. b) Adsorbed GOx. c) Adsorbed PLL. d) Adsorption of PLGA results in loss of GOx, the latter being displaced by the former from the microparticle surface. The displacement process is driven by thermal fluctuations and mass action, and it involves the penetration of PLGA beneath the layer of PLL.
Monitoring of Polypeptide Multilayer Formation by QCM
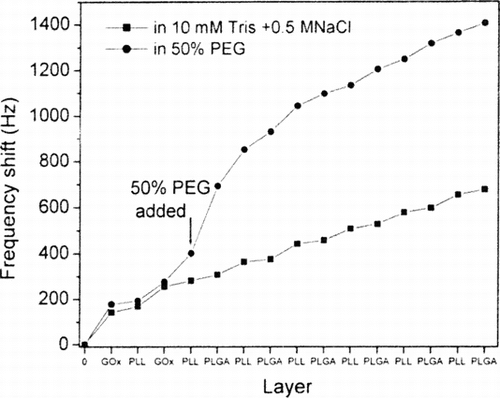
QCM measurements of stepwise growth of GOx/PLL/PLGA multilayers were made using an Agilent 53131 A 225 MHz universal counter (USA) and silver-coated quarts resonators (Sanwa Tsusho Co. Ltd., Japan). GOx was deposited from a solution containing 5 mg/mL of GOx in 10 mM Tris, pH 7.4. PLL/PLGA multilayers were deposited stepwise on resonators from a solution containing 1 mg/mL of PLL or PLGA in 10 mM Tris with 0.5 M NaCl, pH 7.4, with or without added 50% PEG (v/v). Two bilayers of GOx/PLL were formed, followed by some number of bilayers of PLL/PLGA. Frequency shift as a function of numbers of deposited layers was recorded and plotted (Supplementary ). Addition of PEG to solutions of PLL and of PLGA resulted in a substantial increase in mass deposited. In the absence of PEG, there was a much smaller increase of mass during the PLGA deposition step. It seems likely that previously deposited material was displaced during deposition of PLGA and resolubilized.
Supplementary Figure 2 Comparison of the assembly behavior of GOx/PLL/PLGA on a QCM resonator with and without addition of PEG in polypeptide assembly solutions. The experiment described here differs from the procedure used in capsule fabrication in that two bilayers of GOx/PLL were deposited before the first layer of PLL.
Detection of GOx Activity in Polypeptide Microcapsules by Spectrophotometry
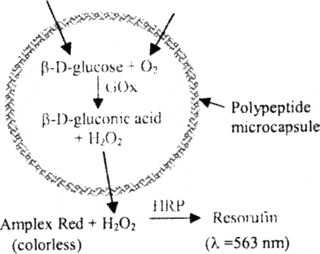
A colorimetric enzyme-coupled assay involving Amplex Red as substrate was used to quantitate GOx activity (see Supplementary ). Glucose and oxygen diffuse inside the capsule; aided by GOx they react to produce H2O2. This compound passes out of the capsule and is detected, indirectly, by Amplex Red. Initially colorless, this reagent is oxidized by H2O2 in the presence of horseradish peroxidase (HRP) (250–330 units/mg solid), forming resorufin, which can be detected by absorbance at λ = 563 nm.
Supplementary Figure 3 Reaction scheme for photometric measurement of GOx activity in a polypeptide microcapsule. The microcapsule shell is shown in blue. Enzymes are shown in green type. β-D-glucose and molecular oxygen enter the capsule from outside, and hydrogen peroxide leaves the capsule. Reactants and products inside the microcapsule are shown in purple.
All chemicals were from Sigma unless indicated otherwise. The following stock solutions were prepared: 10 mM Amplex Red reagent (Molecular Probes, USA) in DMSO, 10 U/mL HRP in 50 mM phosphate buffer and 0.15 M NaCl (PBS), 400 mM glucose in 50 mM PBS, and 100 U/mL GOx in 50 mM PBS. A working solution was prepared by mixing three solutions: 30 µL Amplex Red, 75 µL HRP, and 450 µL glucose. Standard solutions of GOx, each 500 µL and containing 0–10 mU/mL, were prepared by diluting the 100 U/mL GOx stock solution. Capsule samples and control samples without GOx each were diluted to 500 µL with 50 mM PBS buffer. Reactions were initiated by adding 40 µL Amplex Red/HRP/glucose working solution to tubes representing standards, controls, and capsule samples. Reaction mixtures were incubated for 30 min in the dark at ambient temperature with gentle agitation. Absorbance of resorufin was measured at 563 nm.
Analysis of Ordered Structure in Polypeptide Microcapsules by Circular Dichroism Spectrometry (CD)
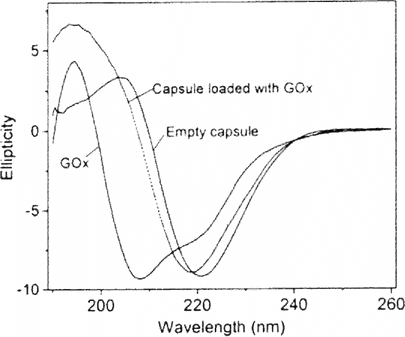
Experiments were done at ambient temperature with a Jasco J-810 spectropolarimeter. The quartz cell had a 2 mm optical path length. Prior to measurement, capsules were separated from EDTA by centrifugation. Pelleted material was rinsed with water and resuspended in 10 mM Tris buffer. Supplementary shows typical CD spectra of PLL/PLGA microcapsules, of GOx, and of PLL/PLGA capsules loaded with GOx. The CD spectrum of unloaded PLL/PLGA capsules closely resembles that of a β sheet, consistent with our previous work on the structure of polypeptide multilayer films formed on a planar quartz substrate [2]. By contrast, the spectrum of GOx indicates a large percentage of α helix. The spectrum of PLL/PLGA capsule with loaded GOx shows a combination of α helix and β sheet, evident from the shifts in magnitude and position of Cotton effects. Spectral deconvolution suggests that the secondary structure content of empty PLL/PLGA capsules was 1.5% α helix and 40% β sheet, while that of GOx was 57% α helix and 11% β sheet and that of capsules with loaded GOx was 3% α helix and 35% β sheet. CD thus provided complementary evidence of GOx encapsultion.
REFERENCES
Glinel, K., Jonas, A.M., Laschewsky, A., Vuillaume, P.Y. (2003). “Internally structured polyelectrolyte multilayers,” in Multilayer Thin Films, G. Decher, J.B. Schlenoff, Eds., Weinheim: Wiley-VCH.
Zhi, Z.-L., Haynie, D.T. (2004). Direct evidence of controlled structure reorganization in a nanoorganized polypeptide multilayer thin film. Macromolecules 37: 8668–8675.