Abstract
Information that can be obtained by magnetic resonance imaging (MRI) of explanted endovascular devices must be validated as this method is non-destructive. Histology of such a device together with its encroached tissues can be elegantly performed after polymethymethacrylate (PMMA) embedding, but this approach requires destruction of the specimen. The issue is therefore to determine if the MRI is sufficient to fully validate an explanted device based upon the characterization of an explanted specimen.
An AneuRx device deployed percutaneously 25 months earlier in a 75-year-old patient was removed en bloc at autopsy together with the surrounding aneurysmal sac and segments of the upstream and downstream arteries. Macroscopic pictures were taken and a slice of the cross-section was processed for histology after polymethylmethacrylate (PMMA) embedding. For the magnetic resonance imaging investigation, the device was inserted in a Biospec 4.7 T MRI system with a 20 mm diameter birdcage resonator used for both emission and reception. A Spin-Echo (SE) was used to acquire both T1 proton density (PD) and T2 weighted images. A gradient-echo (GE) sampling of a free induction decay (GESFID) was used to generate multiple GE images using a single excitation pulse so that four images at different TE were obtained in the same acquisition.
The selected explanted device was outstandingly well-healed compared to most devices harvested from humans. No inflammatory process was observed in contact or at distance of the materials. In MRI T1 images display no specific contrast and were homogeneous in the different tissues. The contrast was improved on proton density weighed images. On the T2 weighed images, the different areas were well indentified. The diffusion images displayed in the surrounding B region had the greatest diffusion coefficient and the greatest anisotropy.
The MRI analysis of the explanted AneuRx device illustrates the possibilities of this technique to characterize the interaction of the endovascular graft with the surrounding tissues. MRI is a breakthrough to investigate explanted medical devices but it also can be advantageously used in vivo to obtain virtual biopsies, because real biopsies to determine the 3 Bs (biocompatibility, biofunctionality and bioresilience) cannot be carried out as they could obviously initiate infection and degradation of the foreign materials.
INTRODUCTION
A flurry of activity in endovascular surgery to recanalyze the blood flow through an aneurysm sac followed the original work of Parodi [Citation[1-7]], as many innovative devices were developed and implanted without extensive validation. This was accompanied by patient competition between surgeons and radiologists [Citation[8]]. As patients were likely to recover faster (shorter duration of anesthesia, length of stay), hospital administrations were very interested in the technological advance that could result in day surgery. In addition, manufacturers identified new market areas in vascular surgery. Regulatory agencies also became rapidly involved [Citation[9]]. Unfortunately, a number of clinical trials appeared but in retrospect may have been premature as evolutive complications were not uncommon. Numerous reports were published, mostly as individual cases, with different proposed modes of failure [Citation[10-15]]. The explanted devices were usually investigated in detail but destructively. Subsequently, the pertinent question was to explore whether it was possible obtain equivalent results or more reliable information without any destructive methodology before the device explantation. This may allow or indicate appropriate intervention before further complications occur. Doppler surveillance, X-ray angiography or spiral CT scan are good indicators of the in vivo biofunctionality of the prostheses, but they may not be able to comment about the biocompatibility and the biodurability of the device [Citation[16-21]]. Magnetic resonance imaging (MRI) appears theoretically as the most attractive approach to perform virtual biopsies in vivo that corroborate the pathological observations. MRI is not only efficient and non-traumatizing [Citation[22-26]], but also environmentally friendly (absence of ionizing radiations).
As was previously demonstrated, it is feasible to perform in vivo follow-up of tissues and/or biomaterials and correlate MRI and histology [Citation[27]]. However, investigations were restricted to polymers, i.e. polyesters, polylactic acid derivatives and hydrogels. The stent-grafts are much more complex devices because they incorporate a metallic structure sewn with a polymer conduit or incorporated in the wall. As metal induced artefacts can be neutralized to permit the observation of the wires or the stents, it is believed that such a follow-up is feasible. Testing viscoelastic properties of soft tissues sandwiching foreign materials are realistic and worth investigation. However, the issue of corrosion may still require destructive testing.
Therefore, we hereby propose to further highlight the capacity of MRI to permit virtual biopsies in an AneuRx explanted device with light microscopy being used as a reference [Citation[28]]. Further to the observations carried out, we recommend new approaches to sequentially monitor implants in situ. Virtual biopsies can be done after scheduled durations of implantation, and they are environmentally friendly. However, destructive analyses still hold key information that MRI may not be able to reproduce.
MATERIALS AND METHODS
1. The Explanted Device
An explanted AneuRx stent-graft manufactured by Medtronic AVE, Santa Rosa, CA, USA, was selected amongst several devices harvested at autopsy or reoperation based upon the apparent completeness of the healing, i.e., the graft was well encapsulated, the content of the aneurysmal sac had solidified, and the wall of the original artery was still delineated.
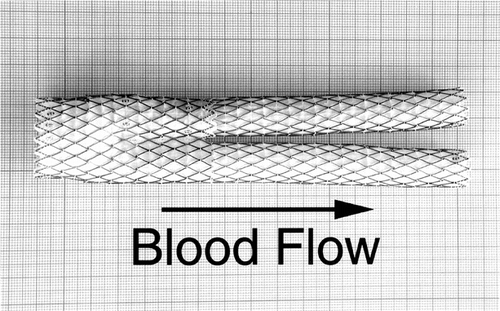
The AneuRx device is made of a thin wall woven polyester graft externally supported by self expanding stents made of Nitinol, a shape memory alloy (). The stent-graft of the specimen investigated had a bifurcated configuration together with an ipsilateral straight graft. This device was developed to recanalyze the blood stream through an aortic aneurysm sac and is sufficiently versatile to fit a broad range of patients whose anatomy and pathology can differ dramatically.
Figure 1 An unimplanted AneuRx device fully deployed and manufactured by Medtronic AVE: it consists of a bifurcation with limbs of different lengths, one long and one short, in which an ipsilateral limb is inserted. The thin wall of the polyester is externally supported by self-expanding stents made of shape memory alloy (Nitinol) and sewn by means of multifilament polyester sutures.
2. Clinical Report
2.1. Patient Summary
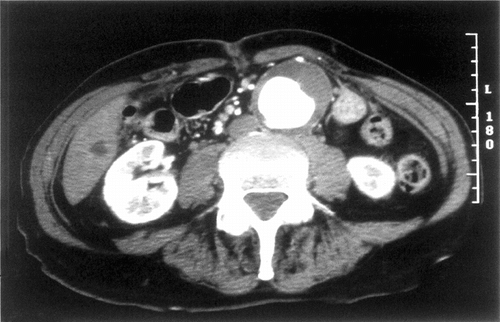
The patient was a 75-year-old man with a symptomatic 5.7 cm abdominal aortic aneurysm (AAA). The aneurysm discovered two years before had become painful and caused the patient discomfort by its prominent pulsation. Severe coronary artery disease and cardiomyopathy required treatment with coronary angioplasty and stenting on five occasions. The patient had hypertension, hyperlipidemia, and a long smoking history. He had COPD with dyspnea on exertion and generalized fatigue with a 30-pound weight loss over the past five years. Pre-operative CT scan demonstrated a 5.7 cm AAA with a 2.0 cm left common iliac aneurysm (). The infrarenal aortic neck was 22 mm in diameter with a length of 30 mm below the main renal arteries. There was an accessory left renal artery supplying the lower half on the left kidney with 10 mm of infrarenal neck below the accessory renal artery.
2.2. Treatment
A 24 × 14 × 16.5 AneuRx bifurcated module (early stiff device with pre-RPM material) placed through the femoral artery and a 14 × 8.5 iliac limb placed through the left femoral artery were deployed. The device was placed below the accessory left renal artery, and both iliac limbs were dilated with 14 mm balloons. Completion angiography revealed a “transgraft leak”
2.3. Post Procedure
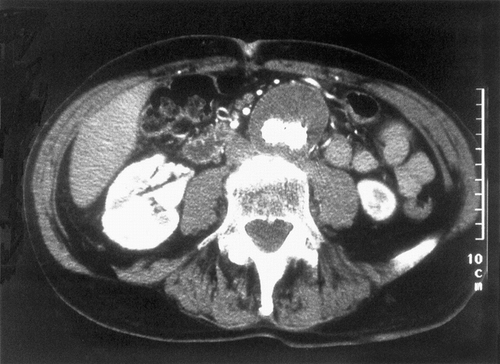
Duplex ultrasound on the first post-operative day revealed an endoleak with two small jets, one from each iliac limb, exiting through the graft with outflow through a patent IMA. This finding prompted an angiogram at 24 hours, which demonstrated good proximal and distal fixation of the stent graft with a local contrast leak from the right limb of the graft and a tiny collection of contrast at the left iliac limb. Duplex ultrasound at 2 weeks revealed no evidence of endoleak, although a small “pinhole” was evident at the left limb of the graft with no flow entering the aneurysm. This “pinhole” was evident by Duplex at 1 month and 3 months, but disappeared at 6 months, with no evidence of endoleak by Duplex at any time other than the first post-op day. CT scan at 1 month, 6 months 12 months and 18 months revealed no endoleak and the aneurysm decreased in size from 5.7 cm pre-op to 4.6 cm at 18 months (). The patient had complete resolution of his abdominal and back pain and no longer felt pulsation in his aneurysm immediately following the stent graft procedure. He gained weight and had no symptoms referable to his aneurysm. However, the patient was quite thin and follow–up clinical examinations revealed pulsation of his aneurysm.
2.4. Terminal Events
At 25 months post procedure, the patient developed back pain and azotemia. A diagnosis of multiple myeloma was made on bone marrow biopsy and the patient was begun on chemotherapy. Abdominal examination was normal. He developed bronchopneumonia and sepsis and died shortly thereafter. An autopsy confirmed the above diagnoses. The aorta was removed intact with the stent graft in place for explant analysis.
3. Histology
A slice of the cross-section less than 1 cm thick was cut from the as-received specimen, i.e., approximatively in the middle of the aneurysm sac. As the evaluation of the tissue reaction to the polyester structure (tube and suture) and to the shape memory alloy is the key issue of the investigation, it was of paramount importance to procede to complete embedding of the encapsulated prostheses together with the surrounding aneurysmal sac in polymethylmethacrylate (PMMA). This technique is well adapted to metallic implants and was successfully employed in research in bones and/or bone substitutes together or not with metallic implants [Citation[29], Citation[30]]. Details are given elsewhere [Citation[31]].
4. MRI Investigations
To prevent any damage or contamination to the section of the explanted device to be investigated in MRI, a gelatin powder (Sigma) of 300 bloom gel strength from porcine skin was dissolved in deionized distilled water at 60°C and poured over the specimen left on its own in a container. Four hundreds ppm of NaN3 were added to prevent microbiological growth.
Magnetic resonance imaging experiments were performed on a Biospec 4.7 T MRI system (Bruker, GmbH, Ettlingen, Germany) with a 27-cm-diameter horizontal bore and fast gradient hardware (maximum amplitude 50 mT/m, rise time 260 µsec). A 20-cm-diameter birdcage resonator was used for both emission and reception.
The sample was positioned so that the main axis of the aorta lays roughly parallel to the main magnetic field direction. The best spatial resolution was obtained perpendicularly to the aorta, as the slices were acquired in the axial plane. For all acquisitions, ten contiguous slices of 1-mm–thickness were simultaneously acquired. Matrix size was 256 × 256 with a square field of view of 102.4 × 102.4 mm2 resulting in an isotropic in-plane resolution of 400 µm. A reduced readout bandwidth of 98 Hz per pixel and (Nex=) 2 average acquisitions were used to reach suitable signal-to-noise ratio.
The linear phase selective pulses used for both excitation and refocalization were generated according to the Shinnar-Le Roux (SLR) algorithm using Matpulse software. RF waveforms were optimized with passband and stopband ripples of 1%, bandwidth of 1 kHz and a duration of 4 ms.
A Spin-Echo (SE) sequence was used to acquire both T1-, proton-density (PD) and T2- weighted images using different values of repetition time TR and echo time TE, respectively equal to 500/20.9 ms, 3000/20.9 ms and 3000/80 ms.
A specific sequence, called gradient-echo (GE) sampling of a free induction decay (GESFID), was used to generate multiple GE images using a single excitation pulse [Citation[32]] so that four images at different TE were obtained in the same acquisition. The readout bandwidth was increased to 156 Hz per pixel to decrease the delay between two successive GE (first TE = 7.3 ms, inter-TE = 8.7 ms). The information dispatched in the different GE images of the same sample were summed up in a parametric map giving the value of apparent spin-spin relaxation rate R*2 in each voxel. For that purpose, the mono-exponential model given by I(TE) = PD · exp(−R*2·TE) was fitted voxel-wise on the magnitude decay curve I(TE) using the Levenberg-Marquardt algorithm, removing the points of S/N under 3.
The effective diffusion tensor was estimated by measuring the SE attenuation produced by different combinations of magnetic field gradient-pulses [Citation[32]]. Since
is symmetric, a simplified protocol requiring only seven images was chosen; it consists of acquiring a SE image without diffusion attenuation and six diffusion-weighted images produced by a pair of symmetric trapezoidal gradient pulses applied in six non-collinear directions.
The diffusion encoding gradients were applied for a duration δ = 20 ms on each side of the refocusing SLR pulse, and a time Δ = 27 ms between the beginning of the rises of the two gradient pulses. The matrix b, which quantifies the influence of imaging and diffusion gradient pulses in the SE attenuation due to diffusion, was calculated analytically according to Mattiello [Citation[33]]. This resulted in a TE of 58.4 ms and a maximum b-factor of ≈ 800 s·mm−2. With a TR of 3000 ms, the total acquisition time was 3 h for the whole diffusion tensor protocol.
In each voxel, the seven unknowns (i.e., the six components of and the T2-weighted echo amplitude) were calculated by solving seven linear equations using a multivariate linear regression routine [Citation[34]]. To characterize water diffusion in the sample, which may be anisotropic, parametric images representing rotationally invariant parameters were calculated from
. The first one is the scalar mean diffusivity 〈D〉 defined by Basser [Citation[35], Citation[36]].
and the second one is a measure of anisotropy named fractional anisotropy (FA).
where Dij are the elements of
measured in the imaging frame of reference, and IÜ are the elements of the identity tensor.
Computations were performed on a IRIX O2 workstation (Silicon Graphics, Inc., Mountain View, CA) using the subroutines of the IMSL C libraries (Visual Numerics, Inc., Houston, TX).
RESULTS
1. Macroscopy
The specimen as received was the bloc incorporating the aneurysmal abdominal aorta together with the prosthesis from the infra-renal site to the iliac arteries included. The aneurysm sac site had an external diameter in the range of 50 mm, slighly flattened on its back.
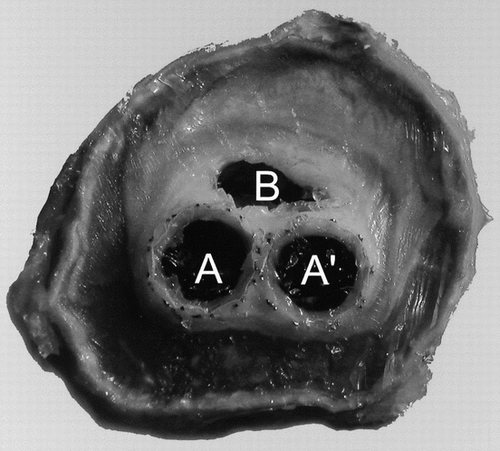
The specimen was cut in two sections perpendicular to the blood flow axis in the middle of the sac. The limbs of the prosthesis were visible and were well encapsulated as the body of the device was limited to the stents that were anchored immediately downstream from the renal arteries. The contents of the sac were mostly solidified as gelatinous masses of different densities with only a void in the vicinity of the limbs of the prosthesis. The wall of the original artery was irregular with some fractures but still well delineated ().
Figure 4 Cross-section of the device together with the reorganized aneurysmal sac where both limbs of the device are still very close, i.e., in the downstream vicinity of the crotch. The lumens of both limbs are clearly delineated (A and A′), whereas the content of the aneurysmal sac has solidified except in a small section (B).
2. Histology
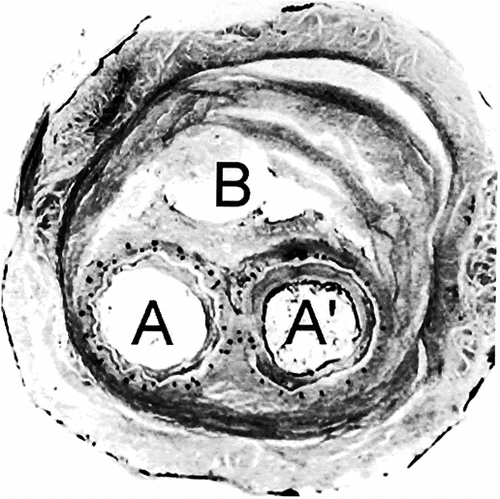
As the specimen was transversely sectioned, the two lumens of the two limbs of the stent graft were well delineated. Both limbs were very well encapsulated with fibrinous layers within a solidified gelatinous plug inside the aneurysmal sac. The aortic wall was not so clearly characterized as anticipated because only circular collagen bundles were observed in polarized light, but disruption were not scarce.
Fibrinous deposits were observed in the lumens of both limbs of the device. They were layered but no cell was observed, neither within this internal capsule, nor on the luminal surface. In places the deposits were retracted to various degrees as the result of fixation and/or processing. Disorganized or poorly organized fibrous deposits were also observed surrounding the prosthesis within the aneurysmal sac. No inflammatory process was observed in contact or at distance of the polyester tube and the Nitinol frame. Lymphocytes macrophages and giant cells were not seen. The content of the aneurysmal sac was essentially acellular ().
Figure 5 Histology of the cross section slightly upstream of . The stents of the bifurcation of the short limb of the device and the one of the ipsilateral limb are superposed, resulting in the compression of the polyester tube, which loses its cylindrical characteristics (A). On the opposite, the polyester tube preserves its cylindrical shape (A′). The content of the aneurysmal sac has solidified; however, a small canal is still visible (B). The fibrous tissues are almost acellular and the wall of the artery is stretched but mostly keeps its continuity. There are, however, some disruptions.
3. MRI
On all the images, the Nickel/Titanium alloy blurred the region of stent graft because of the susceptibility effect. On the different plans, 3 lumens were shown and noted A, A′ & B on the images. The A and A′ area were the lumens from the limits of the stent graft. The B area was a void in the aneurysmal sac that did not solidified.
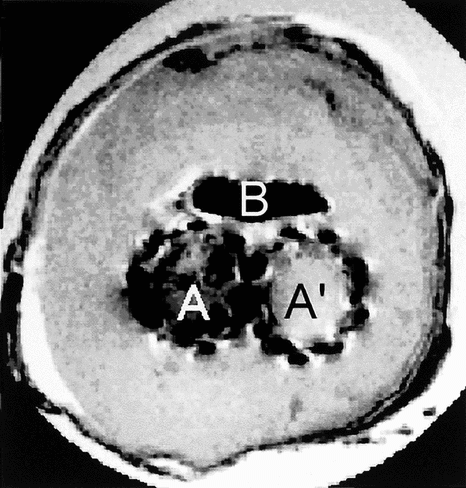
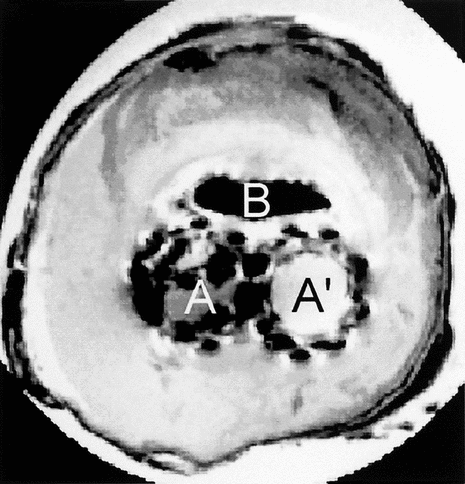
The T1 images displayed no specific contrast and we concluded the T1 should be homogeneous in the different tissues (). The low contrast probably came from the proton density variations. On the proton density weighted images, the contrast was improved (). The area that surrounded the B region showed a hyper signal. Ring–shaped sheets were displayed because of the alternation of hyper and hypo signals. The outer side showed a hypo signal. In the structure from A and A′ an intermediate signal was observed. Some cavities in black were detected.
Figure 6 Proton density weighted image of the cross-section of the explanted device with two lumens, A, and A′, corresponding to both limbs of the device. The content of the aneurysmal sac has solidified whereas there is still an open canal likely to let fluids or blood flow.
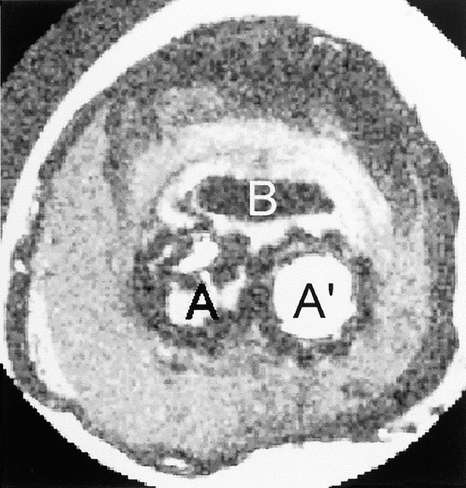
On the T2 weighted images the different areas were recognized (). The hyper signal zone displayed more clearly the sheet structure in B surrounding area. In the bottom of this area the signal was more intense in the 6, 7 and 8 plans.
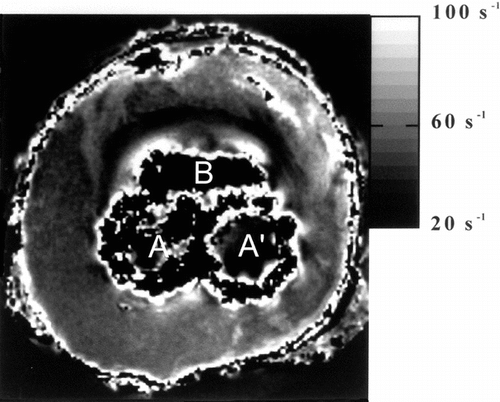
On the R*2 images, the susceptibility effect was easily visible at the tissue interface (). The contrast was similar to this one obtained with proton density images. The signal to noise ratio was increased in this set of R*2 images in comparison with T2 weighted images.
Figure 9 Parametric MR image giving the value of apparent spin-spin relaxations rate R2 in each voxel. The image is windowed between 20 and 100 s−1.
The diffusion images displayed in the surrounding B region had the greatest diffusion coefficient and the greatest anisotropy (, ).
Figure 10 Parametric MR image giving the value of mean diffusivity in each voxel. The image is windowed between 0 and 1.5 × 10−3 mm.s−1.
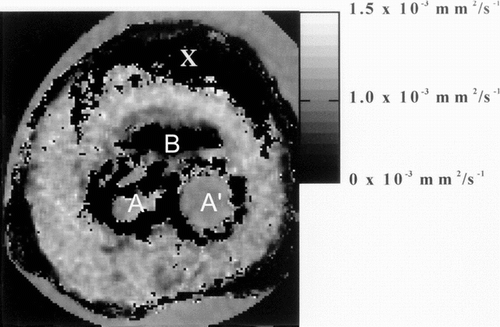
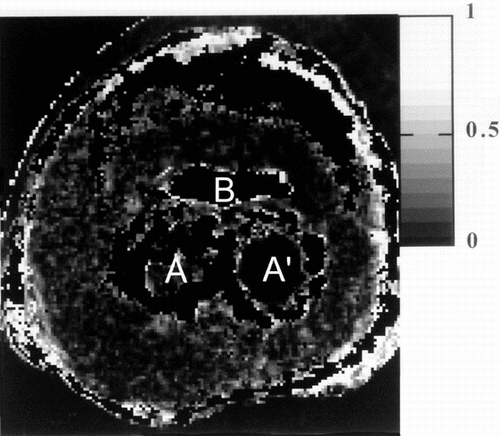
Figure 11 Parametric MR image giving the value of the fractional anisotropy index in each voxel. The image is windowed between 0 and 1.0 corresponding to isotropic diffusion and 1 to infinite anisotropy.
Using diffusion tensor imaging, two different layers in the region surrounding B could be delineated on the images of mean diffusivity, the closest region being characterized by a value lower (≈0.65 × 10−3 mm2/s) than the second layer for which 〈D〉≈ 1.1 × 10−3mm2/s. With Proton density and T2-weighted images there was no difference between these regions. The mean diffusivity was also reduced in the cavities observed near A and A′.
Moreover, fractional anisotropy indexes were homogeneous with a value close to the one measured in gelatine (≈0.1). The diffusion process was isotropic throughout the sample for the diffusion delay used in the protocol.
In the region X, the signal-to-noise ratio was not sufficient to estimate diffusion tensor because of the proton-density and T2 values and no calculation is performed.
DISCUSSION
As the worldwide experience with the AneuRx device now approaches 10,000 patients, this device appears to have been used with great success overall [Citation[37]]. Freedom from aneurysm rupture was 99.7% at 1 year, 99.5% at 2 years and 99.5 at 3 years. The overall patient survival rate was 93% at 1 year and 86% at 3 years.
Endovascular surgery appears to be an alternative treatment option but will not totally replace conventional surgery for patients with AAA. Poor risk patients who were not candidates for medical contraindications may be considered candidates, [Citation[38]]. The percentage of patients who can take advantage of endovascular surgery appears to be higher than anticipated and previously reported [Citation[39-41]].
Unfortunately, individual aneurysm behavior is unpredictable either with or without endovacular prostheses [Citation[42], Citation[43]]. As analytical modeling and computational fluid dynamics (CFD) of aneurysmal aorta fitted with an endovascular graft is still in its infancy [Citation[44]], it is of particular importance to understand the long-term behavior of an aneurysmal sac in terms of biomechanics. The endovascular graft must behave in a predictable way [Citation[45]]. There have been several endovascular devices with early failure performances. However, it is important to report all failures, with the goal to improve forthcoming generations of devices. More than ten years after the pioneering work of Parodi [Citation[1]], the era of premature usage of new medical devices has passed.
MRI presents the advantage allowing both vizualisation and reliable tissue characterization [Citation[46-48]]. These virtual biopsies allow determination of biocompatibility, biofunctionality and biodurability. Examination of the viscoelastic properties of soft tissues is also possible. Metal induced artefacts can be neutralized to permit the observation of the wires or the stents [Citation[49], Citation[50]]. However, it is still premature to comment on corrosion in early stages of development.
Therefore analysis of explanted devices by MRI may represent a time advance providing clinicians with information about the in vivo characteristics of the implant. As the initial concepts of endovascular devices were mostly clinician–driven to address obvious and urgent clinical requirements, engineering contributions were less than optimal. MRI permits complete investigation of the device harvested at reoperation or at autopsy. Failures and/or residual life expectancies can be explained by sharing the information between clinicians, manufacturers and engineers. Destructive testing does not permit a global vision of the device but detailed investigations on the biomaterials are of paramount importance to determine the presence of corrosion of the alloys and/or the degradation of polymers.
In addition, destructive testing is restricted to explanted specimens whereas MRI can also be performed in vivo. According to the policy of precaution related to implantable devices and defined as materiovigilance, patients must be regularly investigated by environmentally friendly methods. As a real biopsy of an implant is totally unrealistic because it can initiate infection and damage, the concept of virtual biopsy must be proposed. MRI is far more attractive than conventional X-rays as there is no emission of ionizing radiations, and it also permits better differentiation of the characteristics of the tissues [Citation[51], Citation[52]].
CONCLUSIONS
The MRI analysis of an explanted AneuRx device illustrates the possibilities of this technique to characterize the interactions of the endovascular graft with the surrounding tissues. However, only destructive testing guarantees the determination of the degradation and/or corrosion state of the device, but it is generally too late. Such observations shall be matched with innovative imaging techniques, particularly MRI, to guarantee a reliable follow-up of the patients [Citation[53-56]].
Special thanks are due to Suzanne Bonnet and Michel Letort for taking care of the retrieval program at Medtronic AVE, Santa Rosa CA, USA.
REFERENCES
- Parodi, J.C., Palmaz, J.C., Barone, H.D. (1991). Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann. Vasc. Surg. 5: 491–499, [PUBMED], [INFOTRIEVE], [CSA]
- Mialhe, C., Amicabile, C., Becquemin, J.P. (1992). Endovascular treatment of infra renal abdominal aneurysms by the Stentor system: Preliminary results of 79 cases. Stentor retrospective study group. J. Vasc. Surg. 26: 199–209, [CROSSREF], [CSA]
- May, J., White, G.H., Yu, W., Waugh, R.C., Stephen, M.S., McGahan, T.J., Harris, J.P. (1995). Early experience with the Sydney and EVT prostheses for endoluminal treatment of abdominal aortic aneurysms. Endovasc. Surg. 2: 240–247, [CROSSREF], [CSA]
- Wilson, G.J., Klement, P., Kato, Y.P., Martin, J.B., Kahn, I.J., Alcine, R., Dereume, J.P., MacGregor, D.C., Pinchuk, L. (1996). A self-expanding bifurcated endovascular graft for abdominal aortic aneurysm repair—An initial study in the canine model. ASAIO 42: M386–M393, [CSA]
- White, R., Kopchok, G., Zalewski, M., Ayres, B., Wilson, W.E., De Virgilio, C., Donayre, C. (1996). Comparison of the deployment and healing of thin-walled expanded PTFE stented grafts and covered stents. Ann. Vasc. Surg. 10: 336–346, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Koskas, F., Cluzel, P., Benhamou, A.C., Kieffer, E. (1999). Endovascular treatment of aortoiliac aneurysms: Made to measure stent-grafts increase feasability. Ann. Vasc. Surg., Moll Fl. 13: 239–246, [CROSSREF], [CSA]
- Zarins, C.K., White, R.A., Hodgson, K.J., Fillinger, M.F., Fogarty, T.H. (2001). The AneuRx stent-graft: four year experience and worldwide experience 2000. J. Vasc. Surg. 33(Suppl. 2): S135–S145, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Veith, F.J., Marin, M.L. (1995). Endovascular surgery and its effect on the relationship between vascular surgery and radiology. J. Endovasc. Surg. 2: 1–7, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- AFSSAPS. (2001). Evaluation des endoprothèses aortiques utilisées pour le traitement endovasculaire des anévrismes de l'aorte abdominale sous-rénale. AFSSAPS, Paris, France, February[CSA]
- Alimi, Y.S., Chakfé, N., Rivoal, E., Slimane, K.K., Valerio, N., Riepe, G., Kretz, J.G., Juhan, C. (1998). Rupture of an abdominal aortic aneurysm after endovascular graft placement and aneurysm size reduction. J. Vasc. Surg. 28: 178–183, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Krohg-Sorensen, K., Brekke, M., Drolsum, A., Kvernebo, K. (1999). Periprosthetic leak and rupture after endovascular repair of abdominal aortic aneurysm: The significance of device design for long-term results. J. Vasc. Surg. 29: 1152–1158, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Riepe, G., Heilberger, P., Umscheid, T., Chakfe, N., Raithel, D., Stelter, W., Morlock, M., Kretz, J.G., Schröder, A., Imig, H. (1999). Franme dislocation of body middle rings in endovascular stent tube grafts. Eur. J. Vasc. Endovasc. Surg. 17: 28–34, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Shin, C.K., Rodino, W., Kirwin, J.D., Ramirez, J.A., Wisselink, W., Papierman, G., Panetta, T.F. (1999). Histology and electron microscopy of explanted bifurcated endovascular aortic grafts: evidence of early incorporation and healing. J. Endovasc. Surg. 6: 246–250, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Guidoin, R., Marois, Y., Douville, Y., King, M.W., Castonguay, M., Traoré, A., Formichi, M., Staxrud, L.E., Norgren, L., Bergeron, P., Becquemin, J.P., Egana, J.M., Harris, P.L. (2000). First generation of aortic endografts analysis of explanted Stentor devices from the Eurostar registry. J. Endovasc. Ther. 7: 105–122, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- McArthur, C., Teodorescu, V., Eisen, L., Morrissey, N., Faries, P., Hollier, L., Marin, M.L. (2001). Histopathologic analysis of endovascular stent graft from patients with aortic aneurysms: Does healing occur? J. Vasc. Surg. 33: 733–738, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Hilfiker, P.R., Quick, H.H., Pfammater, T., Schmidt, M., Debatin, J.F., (1999a). Three-dimensional MR angiography of a nitinol-based abdominal aortic stent-graft: Assessment of heating and imaging characteristics. Eur. Radiol. 9: 1775–1780, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Hilfiker, P.R., Quick, H.H., Pfammater, T., Schmidt, M., Debatin, J.F. (1999b). Plain and covered stent-grafts: In vitro evaluation of characteristics at three-dimensional MR angiography. Radiology 211: 693–697, [PUBMED], [INFOTRIEVE], [CSA]
- Haulon, S., Lions, C., McFadden, E.P., Koussa, M., Gaxotte, V., Halma, P., Beregi, J.P. (2001). Prospective evaluation of magnetic resonance imaging after endovascular treatment of infra-renal aortic anevrysm. Eur. J. Vasc. Endovasc. Surg. 22: 62–69, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Cejna, M., Loewa, C., Schoder, M., Dirisamer, A., Holzenbein, T., Kretschner, G., Lammer, J., Thurnher, C. (2002). MR angiography vs. CT angiography in the follow-up of nitinol stent grafts in endoluminally treater aortic anevryses. Eur. Radiol. 12: 2443–2450, [PUBMED], [INFOTRIEVE], [CSA]
- Gawenda, M., Gossmann, A., Krüger, K., Zaxkringer, M., Hakn, M., Wassner, G., Brunkwall, J. (2004). Comparison of magnetic resonance imaging and computed tomography of 8 aortic stent-grafts models. J. Endlvasc. Ther. 11: 627–634, [CROSSREF], [CSA]
- Van der Laen, M.J., Bartels, L.W., Bakker, C.J.G., Viergever, M.A., Blankensteijn, J.D. (2004). Suitability of 7 aortic stent-grafts models for MRI-based surveillance. J. Endovasc Ther. 11: 366–371, [CROSSREF], [CSA]
- Martin, A.J., Ryan, L.K., Gotlieb, A.I., Henkelman, R.M., Foster, F.S. (1997). Arterial imaging comparison of high resolution US and MR imaging with histologic correlation. Radiographics 17: 189–202, [PUBMED], [INFOTRIEVE], [CSA]
- Debatin, J.F., Hany, T.F. (1998). MR-based assessment of vascular morphology and function. Eur. Radiol. 8: 528–539, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Fattori, R., Nienaber, C.A. (1999). MRI of acute and chronic aortic pathology: Pre-operative and postoperative evaluation. J. Magn. Reson. Imag. 10: 741–750, [CROSSREF], [CSA]
- Cordoliani, Y.S., Hazebroucq, V., Sarrazin, J.L., Lévêque, C., Vincent, B., Jouan, E. (1999). Irradiation et bonnes pratiques en tomodensitométrie hélicoïdale. J. Radiol. 80: 903–911, [PUBMED], [INFOTRIEVE], [CSA]
- Hilfiker, P.R., Quick, H.H., Schmidt, M., Debatin, J.P. (1999c). In vitro image characteristics of an abdominal aortic stent graft: CTA versus 3 D MRA. MAGMA 8: 27–32, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Traoré, A.S., Woerly, S., Doan, V.D., Marois, Y., Guidoin, R. (2000). In vivo magnetic resonance imaging and relaxometry study of a porous hydrogel implanted in the trapezius muscle of rabbits. Tissue Eng. 6: 265–278, [CROSSREF], [CSA]
- Guidoin, R., Langevin, F., Baslé, M.F., Alarcone, C., Legrand, A.P., Zhang, Z., Bassé-Cathalinat, B., Fianconi, J.M., Douville, Y., Baqney, C. (2004). Can magnetic resonance imaging be the key technique to visualise and investigate endovascular biomaterials? Art. Cells. Blood Subst. Biotech. 32: 105–127, [CROSSREF], [CSA]
- Emmanuel, J., Hornbeck, C., Blocbaum, R.D. (1987). A polymethylmethacrylate method for large specimens of mineralized bone with implants. Stain Techn. 62: 401–410, [CSA]
- Chappard, D., Legrand, E., Andran, M., Basle, M.F. (1999). Histomorphometric measurement of the architecture of the trabecular bone in osteoporosis: Comparative study of several methods. Morphologie 83: 17–20, [PUBMED], [INFOTRIEVE], [CSA]
- Guidoin, R., Basló, M.F., Grizon, F., Marinov, G.R., Zarins, C.K., Legrand, A.P., Shang, Z., Guzman, R., Douville, Y. (2005). Polymethylthacrylate (PMMA) as an embedding medium preserving tissues and foreign materials encroaching in endovascular devices. Art. Cells, Blood Subst. Biotech ( submitted for publication) [CSA]
- Basser, P.J., Pierpaoli, C. (1998). A simplified method to measure the diffusion tensor from seven MR images. Magn. Reson. Med. 39: 928–934, [PUBMED], [INFOTRIEVE], [CSA]
- Mattiello, J., Basser, P.J., LeBihan, D. (1994). Analytical expressions for the b matrix in NMR diffusion imaging and spectroscopy. J. Magn. Reson. Series A, 108: 131–141, [CROSSREF], [CSA]
- Basser, P.J., Mattiello, J., LeBihan, D. (1994a). Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Res. Series B, 103: 247–254, [CSA]
- Basser, P.J., Mattiello, J., LeBihan, D. (1994b). MR diffusion tensor spectroscopy and imaging. Biophys. J. 66: 259–267, [PUBMED], [INFOTRIEVE], [CSA]
- Basser, P.J., Pierpaoli, C. (1996). Microstructural and physiological features of tissues elucidated by quantitative diffusion tensor MRI. J. Magn. Res. Séries B, 111: 209–219, [CROSSREF], [CSA]
- Zarins, C.K. AneuRx Clinical Investigators. (2003). The US AneuRx clinical trial: 6 years clinical update 2002. J. Vasc. Surg. 37: 904–908, [PUBMED], [INFOTRIEVE], [CSA]
- Harris, P.L., Vallabhaneni, S.R., Desgranges, P., Becquemin, J.P., Van Marrewijk, C., Laheij, R.F.J. (2000). Incidence and risk factors of late rupture, conversion and death after endovascular repair of infrarenal aortic aneurysms: The Eurostar experience. European Collaborators on Stent/graft techniques for aortic aneurysm repair. J. Vasc. Surg. 32: 739–749, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Abul-Khoudand, O., Criado, F. (2004). A decade of thoracic endografting: Planning the next ten years. J. Endovasc. Ther. 11(Supl. II): II 72–II 81, [CSA]
- Chuter, T.A.M., Parodi, J.C., Lawrence-Brown, M. (2004). Management of abdominal aortic anevrysm: A decade of progress. J. Endovasc. Ther. 11(Supl. II): II 82–II 95, [CSA]
- Fogarty, T.J., Arko, F.R., Zarins, C.K. (2004). Endograft technology: Highlights of the past 10 years. J. Endovasc. Ther. 11(Supl. II): II 191–II 197, [CSA]
- Wolf, Y.G., Hill, B.B., Rubin, G.D., Fogarty, T.J., Zarins, C.K. (2000). Rate of change in abdodminal aortic aneurysm diameter after endovascular repair. J. Vasc. Surg. 32: 108–115, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Hatakeyama, T., Shigematsu, H., Muto, T. (2001). Risk factors for rupture of abdominal aortic aneurysm based on three dimensional study. J. Vasc. Surg. 33: 543–461, [CROSSREF], [CSA]
- Liffman, K., Lawrence-Brown, M., Semmens, J.B., Bui, A., Rudman, M., Hartley, D.E. (2001). Analytical modeling and numerical simulation of forces in an endoluminal graft. J. Endovasc. Ther. 8: 358–371, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Formichi, M., Marois, Y., Roby, P., Marinov, G., Stroman, P., King, M.W., Douville, Y., Guidoin, R. (2000). Endovascular repair of thoracic aortic aneurysm in dogs: Evaluation of a nitinol-polyester self-expanding stent-graft. J. Endovasc. Ther. 7: 47–67, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Lewa, C.J., DeCertaines, J.D. (1996). Viscoelastic property detection by elastic displacement NMR measurements. J. Magn. Reson. Imag. 4: 652–656, [CSA]
- Krinsky, G., Rofsky, N., Flyer, M., Giangola, G., Maya, M., DeCoroto, D., Earls, J., Weinreb, J. (1996). Gadolinium enhanced three dimensional MR angiography of acquired arch vessel disease. Am. J. Roentgenol. 167: 981–987, [CSA]
- Lewa, C.J., Roth, M., Nicol, L., Franconi, J.M., DeCertaines, J.D. (2000). A new fast and unsynchronized method for MRI of viscoelastic properties of soft tissues. J. Magn. Reson. Imag. 12: 784–789, [CROSSREF], [CSA]
- Marshall, M.W., Teitelbaum, G.P., Kim, H.S., Deveikis, J. (1991). Ferromagnetisin and magnetive ressource artifacts of platinium embolization microcoils. Cardiovasc. Intervent. Radiol. 14: 164–166, [CSA]
- Wang, Y., Truong, T.N., Yan, C., Bilecen, D., Watts, R., Trost, D.W., Prince, M.R. (2003). Quantitative evaluation of susceptibility and shielding effects of nitinol, platinium, cobalt-alloy and stainless stell stents. Magn. Reson. Med. 49: 972–976, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Merkle, E.M., Klein, S., Wisianowsky, C., Boll, D.T., Fleiter, T.R., Pamler, R., Gorich, J., Brambs, H.J. (2002 a). Magnetic resonance imaging versus multislice computed tomography of thoracic aortic endografts. J. Endovasc. Ther. 9(suppl. 2): II 2–II 13, [CSA]
- Merkle, E.M., Klein, S., Wisianowsky, C., Boll, D.T., Fleiter, T.R., Pamler, R., Gorich, J., Brambs, H.J. (2002 b). MR angiographic guidings in patients with aortic endoprostheses. Am. J. Roentgenol. 178: 641–648, [CSA]
- Reid, A.M., Reid, D.B., Roditi, G.M. (2004). Vascular imaging: An unparalleled decade. J. Endovasc. Ther. 11(Supl. II): II 163–II 179, [CSA]
- Walter, F., Henrot, P., Blum, A., Hirsch, J.J., Béot, S., Guillemin, F., Boccaccini, H., Regent, D. (1998). Valeur comparative de l'angio-IRM, du scanner hélicoïdal et de l'angiographie numérisée dans le bilan préopératoire des anévrismes de l'aorte abdominale. J. Radiol. 79: 519–539, [CSA]
- Wever, J.J., Blankensteijn, J.D., Van Rijn, J.C., Broeders, I.A., Eikelboom, B.C., Mali, W.P. (2000) Inter and intraobserver variability of CT measurements obtained after endovascular repair of abdominal aortic aneurysms. Am. J. Rad. 175: 1279–1282, [CSA]
- White, R.A., Verbin, C., Kopchok, G., Scoccianti, M., DeVirgilio, C., Donayre, C. (1995). The role of cinefluoroscopy and intrvascular ultrasonography in evaluating the deployment of experimental endovascular prostheses. J. Vasc. Surg. 21: 365–374, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]