Abstract
In this study, chitosan [(1 → 4) linked 2-amino-2-deoxy-β-D-glucopyranose] beads were prepared by interacting this polycation (> 90% deacetylated) with the tripolyphosphate (TPP) polyanion. The resulting chitosan-TPP beads (C) were modified either by coating with sodium alginate (CA) or by cross-linking with glutaraldehyde (CGA). The in vitro degradation of C beads was found to be faster than its CA and CGA counterparts. C beads degraded faster at pH 6.5, compared to pH 7.4 conditions. At pH 7.4, about 41%, 37% and 10% of dry mass loss after 12 months was determined for C, CA and CGA, respectively. At pH 6.5, the dry mass loss of CA and CGA after the same period of time was found to be 73% and 37%, respectively. However, C beads completely degraded at pH 6.5 after 8 months of in vitro incubation. The in vivo biodegradation experiments were performed on Wistar rats (n = 24) for a duration of 6 months. No sign of fibrotic capsule formation was observed around any of the implanted beads at 2 and 6 months post-transplantation. At 2 months, the in vivo-degradation was slow-going and the beads in all groups were intact; CGA beads had more tissue reaction than C and CA beads at this time point. While the C beads had almost completely degraded after 6 months, the biodegradation process in CA and CGA beads was progressing. Histomorphometric analysis revealed that the in vivo biodegradation was in the order of C (∼85%) > CA (∼50%) > CGA (∼25%) after 6 months. Neovascularization was observed at the vicinity of the bead implants close to major blood vessels, both at 2 and 6 months time-points.
INTRODUCTION
Chitosan is an aminopolysaccharide obtained from the alkaline N-deacetylation of the natural biopolymer chitin found in the shells of crabs, lobsters, krills and shrimps. Due to its unique cationic polymer character, its gel and film-forming properties, this biocompatible and biodegradable polymer has been examined extensively in the pharmaceutical industry for its potential in the development of chemoembolization and controlled release systems [Citation[1-3]], in wound healing [Citation[4]], as well as in cell encapsulation and tissue engineering applications [Citation[5], Citation[6]]. Chitosan hydrogel networks are usually stable at the physiological pH, and retain high swelling ability under aqueous conditions. Chitosan spheres (nano-, micro-spheres or beads) can be prepared by various methods including cross-linking with anions [Citation[7]], precipitation [Citation[8]], complex-coacervation [Citation[9]], emulsification and ionotropic gelation [Citation[10]], precipitation-chemical cross-linking [Citation[11], Citation[12]], and thermal cross-linking [Citation[13]]. Interaction of chitosan with the polyanion, tripolyphosphate (TPP), by electrostatic forces is a preferred method [Citation[14]] since it does not involve harsh steps and can also be applied to cell encapsulation applications. The mechanical strength of chitosan-TPP beads is relatively poor, however it can be strengthened by coating with a negatively charged polymer, such as sodium alginate, to form a polyelectrolyte complex film, or by covalent crosslinking.
Although chitosan and chitosan-based biomaterials have been extensively studied for several biomedical applications, studies on its biological properties are limited: in an ultrastructural study by Muzzarelli et al. [Citation[15]], the biological activity of 100–150 µm-thick chitosan membranes as dura mater substitute was investigated; thus the stimulatory effect of chitosan on connective tissue-rebuilding was reported. Jameela and Jayakrishan [Citation[16]] demonstrated that glutaraldehyde-crosslinked chitosan microspheres were well-tolerated by the living tissue. Later, Lu et al. [Citation[17]] reported the mild inflammatory reaction of thin chitosan films in mice. Pratsilp et al. [Citation[18]] demonstrated a relation between the cellular response and deacetylation percentage of chitosan, while higher deacetylated chitosan demonstrated better biocompatility in terms of cell adherence and growth. Kofuji et al. [Citation[19]] reported the biodegradation of chitosan beads produced by precipitation in alkaline amino acid (glycine) solution, however these beads designed for prednisolone release had very limited mechanical stability; thus their biodegradation started within a couple of days following implantation into subcutaneous air pouches of mice. Vande Vord et al. [Citation[20]] evaluated the biocompatibility of a macroporous chitosan scaffold in mice and showed an easy host-tissue invasion.
As can be seen from the related literature, some of the biological properties of chitosan, usually in the form of films or controlled drug release formulations, have been investigated. However, studies using a relatively high amount of chitosan material per subject weight (adaptable to cell encapsulation procedures) and a longer follow-up of the in vivo biodegradation are lacking. In this study, we describe the in vitro degradation of plain, sodium alginate-coated, and glutaraldehyde cross-linked chitosan-TPP beads having a deacetylation percent of >90% for a duration of 1 year. The in vivo biodegradation and tissue biocompatibility of the chitosan-TPP beads transplanted into the epigastric fasciovascular fat pads of Wistar rats are followed up for 6 months.
MATERIALS AND METHODS
A highly purified chitosan (>90% deacetylated, medium MW, Pronova, Norway) was used in the experiments. Sodium alginate (1,4-linked β-D-mannuronic acid and 1,4-linked α-L-guluronic acid heteropolymer, low viscosity) was obtained from Sigma Chemical Company (St. Louis, MO, USA). Other chemicals used were cell culture grade and purchased from Sigma.
Preparation of Chitosan-TPP Beads
Chitosan-TPP beads were prepared by a standard encapsulation method [Citation[14]] under sterile conditions. Briefly, a 2% (w:v) aqueous chitosan solution in a hypodermic syringe (25 G) was added dropwise into 0.6% (w:v) sodiumtripolyphosphate solution containing 0.85% (w:v) NaCl (pH 7.4) at 4°C. For polyanion coating, the beads were incubated in 0.8% (w:v) sodium alginate solution containing 0.85% NaCl for 60 minutes at 4°C. For crosslinking, the beads were reacted with 0.25% (v:v) glutaraldehyde for 15 minutes at 4°C, then incubated in 1.0% glycine solution (w:v) to remove the unreacted glutaraldehyde, and washed excessively with double-distilled water. BSA-containing beads were prepared by using a 5 mg/ml BSA containing chitosan solution during encapsulation. The immobilization of BSA in beads was confirmed by FTIR.
Water Uptake
Water uptake of empty and BSA-loaded beads was determined from the weights of swollen and dry beads. A certain amount (1.000 g) of beads was left in double-distilled water (≥20 MΩ cm at 25°C) for 48 hours. Then, the excess water was removed by using filter paper, and weighed (wet weight, mwet). Later, the beads were dried in a vacuum oven at 50°C for 48 hours, and weighed again (dry weight, mdry). Water uptake was calculated from the following formula.
In Vitro Studies
In vitro degradation of the beads was investigated in 15 mL-sterile dissolution containers with screw caps, under static conditions at 37°C up to 12 months. Five hundred micrograms of beads (about 30 wet beads) were placed in 10 mL of 0.1 M phosphate-buffered saline (at pH 6.5 and 7.4), in triplicate parallel experiments. The medium was replaced with fresh buffer every week. The mass loss of the beads was calculated from the dry weights measured at the beginning and predetermined time points.
In Vivo Studies
In vivo experiments were performed with 250–300 g weighing male Wistar rats at the time of implantation (n = 24). The subjects were housed in duplicates in cages with free access to food pellets and drinking water. All protocols involving animals were conducted according to the standards of international regulations and local ethics committee.
At implantation, the rats were anaesthetized by avertin, the abdominal region was shaved, swabbed with alcohol and then Wescodyne®. Using a sterile surgical set-up, the animals were draped and an incision of ca. 3 cm in length was made through the skin and the microspheres (ca. 0.5 g; 30 each) were placed at the epigastric fasciovascular flaps (right and left). The skin was sutured after the addition of 0.1 mL sodium cefazolin antibiotic, and closed using 9 mm wound clips (Becton Dickinson, Sparks, MD). The subjects were numbered and held for 2 and 6 months. Experimental series consisted of three types of bead implants: (i) chitosan-TPP (n = 8), (ii) alginate-coated chitosan-TPP (n = 8), and (iii) glutaraldehyde-crosslinked chitosan-TPP (n = 8), either empty or BSA-loaded. At set time points, the implants together with some of the surrounding tissue were carefully removed and transferred into PBS for washing and then prepared for histology.
Histology
The implant biocompatibility and the level of biodegradation was evaluated by light microscopy. The angiogenesis and wound healing responses at the implantation interfaces were also searched. The explants were fixed in 2.5% formalin (in PBS at pH 7.4), embedded in paraffin, and sectioned. The samples then were mounted in 1% glycerol and examined under a Leica 4000B model (Germany) light microscope, after staining with hematoxyline and eosin.
Scanning Electron Microscopy
SEM was used to investigate the surface morphology of the beads. The samples were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) at 4°C. Then, they were dehydrated in graded ethanol series, mounted on aluminum supports and were sputter-coated with gold using a Sputter Coater (Desk II, Denton Vacuum, Cherry Hill, NJ, USA). A JEOL JSM 5600 model (Tokyo, Japan) electron microscope was utilized to image samples at a voltage of 20 kV.
Statistical Analysis
The in vitro quantitative results were obtained from at least triplicate samples. Morphometric analysis was performed on histology sections to determine the in vivo biodegradation of C, CA and CGA beads at 2 and 6 months after implantation. Leica QWin Plus (Germany) software was used to process and analyze the images. Twelve counts from 6 different histology sections were performed for each experimental group. Data were expressed as the mean±SD. Statistical analysis was carried out using the unpaired Student's t test for the histomorphometric data. A value of P < 0.05 was considered to be statistically significant.
RESULTS
Physical Properties of Chitosan Beads
The water uptake and size distribution of chitosan-TPP beads are presented in . Depending on the type of modification, the water uptake of the beads was found to be 3.7 to 8.2 times of their dry weight. The water uptake was in the order of C > CA > CGA (). The size distributions of empty and BSA-loaded chitosan beads were found to be 900–1500 µm and 1000–1600 µm, respectively. On the other hand, both coating with alginate and crosslinking with glutaraldehyde decreased the water uptake and size of the beads ().
Table 1 The water uptake and size distribution of chitosan beads
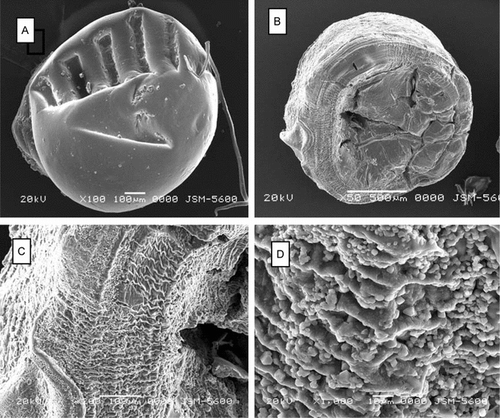
Scanning electron microscopy images of the chitosan beads are presented in . While the new prepared chitosan-TPP (C) bead had a relatively smooth surface (A), its BSA-loaded counterpart (BSA-loaded C) had porous surface topography. Observations at higher magnification clearly revealed the surface roughness of the BSA-loaded chitosan-TPP beads (C and D).
In Vitro Studies
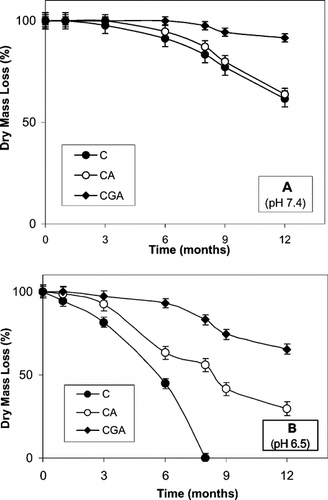
In vitro degradation of chitosan beads is presented in . The in vitro degradation of the chitosan-TPP beads was found to be faster than its alginate-coated and glutaraldehyde-crosslinked counterparts. Secondly, chitosan-TPP beads degraded faster in the buffer at pH 6.5, compared to the pH 7.4 buffer condition (compare A and 2B). At pH 7.4, about 41%, 37% and 10% of dry mass loss after 12 months was determined for C, CA and CGA beads, respectively ( A). At pH 6.5, the dry mass loss of CA and CGA beads after the same period of time was found to be 73% and 37%, respectively (B). During the one-year follow-up of the in vitro experiments, the complete degradation was observed for only the chitosan-TPP beads at pH 6.5 after 8 months (B). Additionally, BSA-loaded beads degraded faster than the empty ones (data not given).
Figure 2 In vitro degradation of chitosan beads in dissolution solutions of PBS at (A) pH 7.4 and (B) pH 6.5 (37°C; static conditions, weekly fresh medium changes). C: chitosan-TPP; CA: alginate-coated chitosan-TPP; CGA: glutaraldehyde-crosslinked chitosan-TPP. All values presented are given as mean ± SD.
In Vivo Studies
None of the subjects in the experimental groups showed any sign of distress or reaction starting from the second day of implantations. The wounds made at the implantation interfaces healed very quickly. The results of the in vivo experiments are presented in and . Generally speaking, fibrotic capsule formation was not observed around the implants, demonstrating an acceptable foreign-body reaction of the implants, both at 2 and 6 months post-transplantation.
Figure 3 Histological appearance of chitosan beads retrieved at 2 months post-implantation from the epigastric groin fascia of Wistar rats (H&E staining). A and B: chitosan-TPP (C) beads; C and D: alginate-coated chitosan-TPP (CA) beads; E and F: glutaraldehyde-crosslinked chitosan-TPP (CGA) beads. F is the detail of E. FP: fat pad; arrows: capillaries. Scale bars: 200 µm.
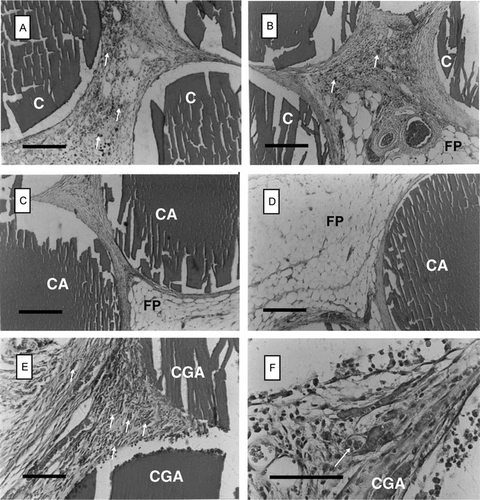
Figure 4 Histological appearance of chitosan beads retrieved at 6 months post-implantation from the epigastric groin fascia of Wistar rats (H&E staining). A and B: chitosan-TPP (C) beads; C and D: alginate-coated chitosan-TPP (CA) beads; E and F: glutaraldehyde-crosslinked chitosan-TPP (CGA) beads. F is the detail of E. FP: fat pad; arrows: capillaries. Scale bars: 200 µm.
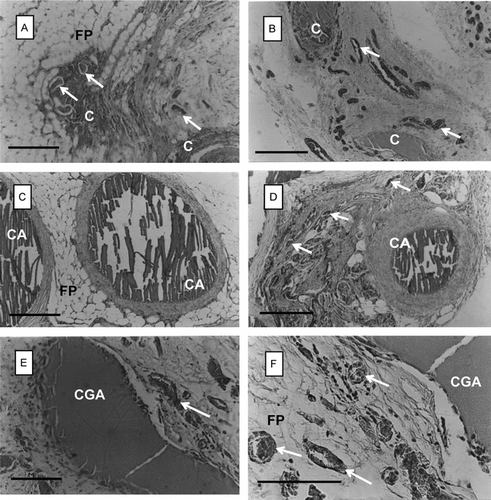
At 2 months post-transplantation, it was found that in vivo-degradation was low, and the chitosan beads in all experimental groups were intact (A–F). Glutaraldehyde cross-linked beads (E and F) had slightly more tissue reaction than C or CA beads (A–D). Neovascularization (new capillary formation) was observed at or around the implanted beads.
Histology of chitosan beads retrieved at 6 months post-transplantation is presented in . It is clear from A and B that the chitosan-TPP (C) beads had almost completely degraded at this time-point. On the other hand, the biodegradation process in CA and CGA beads was much slower compared to that observed for the C beads (C–E). The tissue reaction of alginate-coated chitosan-TPP (CA) beads at 6 months post-transplantation seemed to be higher than that observed after 2 months (compare C, D with C, D). Degradation of CGA was found to be the slowest among all experimental groups after 6 months (E, F). The process of neovascularization was ongoing at the vicinity of bead implants (arrows in ).
Histomorphometric data from the in vivo biodegradation of chitosan beads are presented in . At 2 months post-transplantation, the degradation of chitosan beads was found to be in the order of C (7.7 ± 3.4%) > CA (4.9 ± 2.3%) > CGA (2.7 ± 1.2%) (). At 6 months, the degradation level of chitosan beads was quantified as: C (85.4 ± 4.7%) > CA (50.2 ± 4.3%) > CGA (24.8 ± 2.6%) (). It is clear from the data that statistically significant differences in the in vivo biodegradation of all three chitosan bead types were observed between 2 and 6 months of implantation (p < 0.05). The morphometric analysis also revealed that the biodegradation level was especially different for C and CGA (85.4 ± 4.7 vs. 24.8 ± 2.6), as well as for CA and CGA (50.2 ± 4.3 vs. 24.8 ± 2.6.) at 6 months post-transplantation ().
Table 2 Histomorphometric analysis on the in vivo biodegradation of chitosan beadsFootnote[1]
DISCUSSION
Chitosan has structural similarity to glycosaminoglycans and has been considered as a substratum or carrier material for several biomedical applications. Among these, chitosan receives increasing attention for use in encapsulated cell therapy and tissue engineering. For these applications, a relatively larger volume of chitosan should practically be transplanted when compared to that of controlled drug release applications.
Initially, we tested the water uptake of the chitosan-TPP beads and found that sodium alginate-coating (CA) or glutaraldehyde cross-linking (CGA) decreased the water uptake of the hydrogel beads (). The decrease in water uptake following alginate-coating can be attributed to diffusion limitations caused by the coating that acts as a barrier. For glutaraldehyde cross-linked chitosan beads, the decrease in water uptake can be explained by the crosslinking of a portion of the hydrophilic groups within the hydrogel network, thus resulting in more restricted diffusion.
Chitosan beads or microspheres have also been used to improve the bioavailability of degradable macromolecules such as proteins and growth factors in vivo [Citation[2], Citation[21]]. In this study, bovine serum albumin was used as the model protein to load chitosan-TPP beads. Loading with BSA increased the water uptake of the beads (). This increase may be explained by the inclusion of new hydrophilic groups of the protein molecule into the network.
In vitro degradation experiments performed at a slightly acidic condition (pH 6.5) caused the swelling of the beads, thus a faster rate of degradation was observed compared to that of the condition at pH 7.4. The pH sensitive swelling is due to the transition of bead network between the collapsed and the expanded rates, which is related to ionization degree of amino groups on chitosan in different pH solutions [Citation[22]]. This slightly acidic buffer condition may partially simulate an environment induced by the macrophages accumulating towards the polymer beads following implantation. However, it is very hard to make a direct comparison between the in vitro and in vivo data related to the extremely dynamic and complex nature of the in vivo conditions.
All implants may cause some degree of irritation whether they are permanent or temporary. The success of an implant depends on a large extent on minimizing the inflammatory response and preventing infection. In our study, we have observed quite low inflammatory response to different types of chitosan-TPP beads. Related to the relatively faster biodegradation, subjects that received unmodified chitosan-TPP beads demonstrated a milder inflammation at 2 months, which slowed down after 6 months post-transplantation. Alginate-coated chitosan-TPP beads showed a moderate level of inflammation at 6 months post-transplantation, in relation to the sustained biodegradation process. The erosion observed in this group was typically at the surface of the beads ( C and D). Glutaraldehyde cross-linked chitosan beads were found to be quite stable for the duration of the in vivo experiments (6 months), and demonstrated a considerably low tissue reaction, in similarity with a previous 3-months follow-up study performed with a 74% deacetylated chitosan [Citation[16]]. The cross-linking procedure affects the mucoadhesive strength; however, there is the possibility that the tissue reaction could have increased at longer periods. Thus, the use of glutaraldehyde is usually not recommended in cell-based procedures.
CONCLUSION
In this study, we have describsed the biodegradation of plain, alginate-coated, and glutaraldehyde cross-linked chitosan-TPP beads having a deacetylation percent of >90%, both in vitro and in vivo. We have used a relatively large volume of chitosan in animal studies; i.e. about one gram of swollen beads per rat (ca.0.5 g, 30 beads per fascia). This corresponds to about 0.05 to 0.1 g dry chitosan/100 g body weight (chitosan beads can swell about 3.7 to 8.2 times of their dry weight). The subjects in the experimental groups did not show any sign of distress or reaction for the duration of 6 months. This is particularly important since a large amount of chitosan may usually be needed in encapsulated cell therapy and tissue engineering applications.
The support of TÜBA-GEBIP (2002-1-10), Ankara University-Biotechnology Institute and BAP, is acknowledged.
REFERENCES
- Pişkin, E., Chang, T.M.S. (1981). 5-Fluorouracil loaded chitosan microspheres for chemoembolization. J. Microencapsul. 1: 343–350, [CSA]
- Elçin, Y.M., Dixit, V., Gitnick, G. (1996). Controlled release of endothelial cell growth factor from chitosan-albumin microspheres for localized angiogenesis: In vitro and in vivo studies. Artif. Cells Blood Substit. Immobil. Biotechnol. 24(3): 257–271, [CSA]
- Gupta, K.C., Kumar, M.N.V. (2000). An overview on chitin and chitosan applications with an emphasis on controlled drug release formulations. Review. J.M.S.-Rev. Macromol. Chem. Phys. C 40(4): 273–308, [CSA]
- Muzzarelli, R.A., Mattioli-Belmonte, M., Pugnaloni, A., Biagini, G. (1999). Biochemistry, histology and clinical uses of chitins and chitosans in wound healing. Review. EXS 87: 251–264, [PUBMED], [INFOTRIEVE], [CSA]
- Elçin, A.E., Elçin, Y.M., Pappas, G.D. (1998). Neural tissue engineering: Adrenal chromaffin cell attachment and viability on chitosan scaffolds. Neurol. Res. 20: 648–654, [CSA]
- Elçin, Y.M., Dixit, V., Lewin, K., Gitnick, G. (1999). Xenotransplantation of fetal porcine hepatocytes in rats using a tissue engineering approach. Artif. Organs 23: 146–152, [CROSSREF], [CSA]
- Bodmeier, R., Paeratakul, O. (1989). Spherical agglomerates of water-insoluble drugs. J. Pharm. Sci. 78: 964–967, [PUBMED], [INFOTRIEVE], [CSA]
- Berthold, A., Cremer, K., Kreuter, J. (1996). Preparation and characterization of chitosan microspheres as drug carrier for prednisolone sodium phosphate as model for anti-inflammatory drugs. J. Control. Rel. 39: 17–25, [CROSSREF], [CSA]
- Bodmeier, R., Oh, K.H., Pramar, Y. (1989). Preparation and evaluation of drug-containing chitosan beads. Drug Dev. Ind. Pharm. 15: 1475–1494, [CSA]
- Singla, A.K., Dhawan, S. (2003). Nifedipine loaded chitosan microspheres prepared by emulsification phase separation. Biotech. Histochem. 78: 243–254, [PUBMED], [INFOTRIEVE], [CSA]
- Thanoo, B.C., Sunny, M.C., Jayakrishnan, A. (1992). Cross-linked chitosan microspheres: Preparation and evaluation as a matrix for the controlled release of pharmaceuticals. J. Pharm. Pharmacol. 44: 283–286, [PUBMED], [INFOTRIEVE], [CSA]
- Berthold, A., Cremer, K., Kreuter, J. (1996). Influence of crosslinking on the acid stability and physicochemical properties of chitosan microspheres. STP Pharm. Sci. 6: 358–364, [CSA]
- Orienti, I., Aiedeh, K., Gianasi, E., Bertasi, V., Zecchi, V. (1996). Indomethacin loaded chitosan microspheres: Correlation between the erosion process and release kinetics. J. Microencapsul. 13: 463–472, [PUBMED], [INFOTRIEVE], [CSA]
- Shu, X.Z., Zhu, K.J. (2000). A novel approach to prepare tripolyphosphate/chitosan complex beads for controlled release drug delivery. Int. J. Pharm. 201: 51–58, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Muzzarelli, R., Baldassare, V., Conti, F., Ferrara, P., Biagini, G., Gazzanelli, G., Vasi, V. (1988). Biological activity of chitosan: Ultrastructural study. Biomaterials 9: 247–252, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Jameela, S.R., Jayakrishan, A. (1995). Glutaraldehyde cross-linked chitosan microspheres as a long acting biodegradable drug delivery vehicle. Studies on the in vitro release of mitoxantrone and in vivo degradation of microspheres in rat muscle. Biomaterials 16(10): 769–775, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Lu, F., Cao, Z., Zhuang, Z., Mou, Z.X., Feng, X. (1998). Biodegradation and biocompatibility of a chitosan film. Sheng Wu Yi Xue 15(2): 183–185, [CSA]
- Pratsilp, M., Jenwithisuk, R., Kongsuwan, K., Damrongchai, N., Watts, P. (2000). Cellular responses to chitosan in vitro: The importance of deacetylation. J. Mater. Sci. Mater. Med. 11(12): 773–778, [CROSSREF], [CSA]
- Kofuji, K., Ito, T., Murata, Y., Kawashima, S. (2001). Biodegradation and drug release of chitosan gel beads in subcutaneous air pouches of mice. Biol. Pharm. Bull. 24(2): 205–208, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Vande Vord, P.J., Matthew, H.W., DeSilva, S.P., Mayton, L., Wu, B., Wooley, P.H. (2002). Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 59(3): 585–590, [CROSSREF], [CSA]
- Elçin, A.E., Elçin, Y.M. (2000). Polycation-coated polyanion microspheres of urease for urea hydrolysis. Artif. Cells Blood Substit. Immobil. Biotechnol. 28(1): 95–111, [CSA]
- Kumar, M.N.V.R. (2000). Nano and microparticles as controlled drug delivery devices. J. Pharm. Pharmaceut. Sci. 3(2): 234–258, [CSA]