Abstract
Covalent attachment of enzymes and other proteins to the smart polymer, poly(N-isopropylacrylamide) [poly (NIPAAm)], has been widely used as a method for the preparation of thermosensitive protein conjugates. In the present study, reversible soluble-insoluble polymer-enzyme conjugates were prepared by conjugating a copolymer of NIPAAm with 5-mol % of 6-acrylaminohexanoic acid to trypsin by the carbodiimide-NHS (N-hydroxysuccinimide) coupling method. Four bioconjugates with different units of enzyme coupled to the matrix were prepared. Increased enzymatic activity in terms of high effectiveness factor (in the range of 3–5) was found in the conjugates. Kinetic parameters for the immobilized and free enzyme were determined. The Vmax/Km value of the enzyme significantly increased on immobilization by the factors in the range of 12–28. The immobilized enzyme also showed stability to autolysis at 50°C.
INTRODUCTION
Enzymes are extensively used for industrial, analytical and biomedical applications. Immobilization of enzymes has become an established approach for enhancing their usefulness in biotechnology [Citation[1-3]]. Enzymes immobilized on soluble supports catalyze the reaction in homogeneous phase rather than heterogeneous phase. This is of great importance as it leads to reduced mass transfer constraints, a major drawback with enzymes immobilized on solid matrices. This factor becomes very significant if the substrate is insoluble/poorly soluble or the substrate is macromolecular like proteins, polysaccharides or nucleic acids. Poor solubility of substrates also results in fouling of carriers. Though these soluble supports enhance the enzyme stability, they do not possess the advantage of reusability. It is in this respect that smart polymers, which are reversibly soluble-insoluble, form ideal support material for design of smart biocatalysts [Citation[4], Citation[5]]. These biocatalysts catalyze an enzymatic reaction in their soluble state and as soon as the product is formed, the conditions are changed to cause the biocatalyst to precipitate so that it can be separated and used in the next cycle after dissolution.
Thermosensitive polymers are smart polymers that de-swell as the temperature is raised beyond lower critical solution temperature (LCST). Conjugation of these polymers with proteins results in thermoresponsive biocatalysts and allows their separation from aqueous solutions induced by a small shift of temperature [Citation[6-9]]. The thermosensitive polymer poly (N-isopropylacrylamide) [NIPAAm] is one of the most commonly studied smart polymers. The present work describes covalent coupling of trypsin to a thermosensitive copolymer of NIPAAm with 5-mol % 6-acrylaminohexanoic acid. This water-soluble copolymer undergoes a phase transition when heated above 30°C [Citation[10]]. In this article, we report the catalytic properties and resistance to autolysis of the conjugates. The results on characterization of the bioconjugates using fluorescence are also described.
MATERIALS AND METHODS
Materials
Trypsin (Type I, from bovine pancreas), N-α-benzoyl-DL-arginine-p-nitroanilide hydrochloride (BAPNA), trypsin inhibitor (Type1-S, from soybean) were bought from Sigma Chemical Co. (St. Louis, MO, USA). 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were procured from Spectrochem Pvt. Ltd., Mumbai, India. N,N-bis (2-hydroxyethyl)-2-aminoethane sulphonic acid (BES buffer) and Casein were obtained from Sisco Research Laboratories, Mumbai, India. All other chemicals were of analytical grade.
Methods
Enzyme Assay and Protein Estimation
Amidase activities of free trypsin and its conjugates with the thermosensitive copolymer were determined using DL-BAPNA as a substrate at 25°C. The enzymatic reaction was monitored by measuring the production of p-nitroanilide spectrophotometrically at 410 nm [Citation[11]]. One enzyme unit liberates 1 nmol of p-nitroaniline/min at 25°C. The proteolytic activity of trypsin was measured using casein as the substrate. The activity with casein was estimated by following the increase in the amount of TCA-soluble peptides [Citation[12]]. The amount of TCA-soluble peptides was measured by Lowry's method [Citation[13]]. One enzyme unit liberates 1 µmol of tyrosine/min at 37°C. The protein content was determined by UV absorption at 280 nm (E1% = 14.3, at 280 nm) [Citation[14]].
Synthesis of Copolymer
The thermosensitive copolymer of NIPAAm with 5-mol% 6-acrylaminohexanoic acid (Number average molecular weight (Mn) of polymer is 1.87 × 105; Polydispersity index (Mw/Mn) of polymer is 1.36) was synthesized by an earlier described protocol [Citation[10]].
Immobilization of Trypsin to Thermosensitive Copolymer
Trypsin was covalently coupled to copolymer of NIPAAm with 5-mol% 6-acrylaminohexanoic acid by carbodiimide-N-Hydroxysuccinimide coupling by following a protocol described by Hermanson et al. [Citation[15]]. A solution of copolymer (0.5% wv−1; 5.0 mL) was prepared in 0.01 M BES buffer (pH 7.5, containing 0.01 M Ca2+), followed by addition of EDC (0.06 g) and NHS (0.01 g). Varying amounts of trypsin (115 U, 229 U, 344 U and 459 U) were added to the polymer solutions. After vortexing, the solutions were incubated in an incubator-shaker at 25°C (100 rpm) for 3 h. Ethanolamine solution (0.14 mL) was added to block the remaining activated sites on the polymer and again incubated for 1 h in the incubator-shaker at 25°C (100 rpm). The solutions were removed and pH was adjusted to 5 with 1 M acetic acid. Then 10% (wv−1) ammonium sulphate was added and the solutions were incubated at 32°C for 15 min to ensure complete precipitation of the bioconjugate. The solutions were then centrifuged at 10,000 g for 10 min. The conjugates were washed twice with 0.01 M BES buffer (pH 7.5, containing 0.01 M Ca2+). After the washings, the precipitates were dissolved in 2 ml of 1 M NaCl solution and incubated at 20°C (100 rpm) for 1 h. The pH was adjusted to 5 and then solutions were incubated at 32°C for about 15 min, followed by centrifugation at 10,000 g for 10 min. After the NaCl wash, the conjugates were dissolved in 2 ml of 20% (vv−1) ethylene glycol and incubated at 20°C (100 rpm) for 1 h. After 1 h, the copolymer-enzyme conjugate was precipitated as explained above. These washes were given to remove any loosely bound protein. The conjugates were dissolved in 5 ml of 0.05 M Tris-HCl buffer (pH 8.2, containing 0.02 M Ca2+). Enzyme activities were measured in the conjugates, supernatants and washings. Effectiveness factor, η = BA−1 (defined as the rate of the reaction catalyzed by the immobilized enzyme to the rate of the reaction catalyzed by the same enzyme concentration in free form), was calculated for each of the conjugates prepared by dividing enzyme activity expressed in units (B) by the theoretical enzyme activity bound in units (A). Following this procedure, four conjugates were obtained by starting with different amounts of trypsin activity taken initially (Conjugate A, with 115 U of trypsin; Conjugate B, with 344 U of trypsin; Conjugate C, with 229 U of trypsin; Conjugate D, with 459 U of trypsin).
Measurement of pH Optima
The pH optima of free and immobilized trypsin were determined by preparing the substrate solutions in buffers of different pH, ranging from pH 5.5–10.0 (0.05 M acetate buffer, pH 5.5; 0.01 M BES buffer, pH 6.5–7.5; 0.05 M Tris-HCl buffer, pH 8.0–9.0; carbonate-bicarbonate buffer, pH 10.0; all buffers contained 0.02 M Ca2+). The enzyme assays for the free and immobilized enzyme were carried out by the protocol given for trypsin assay with BAPNA as the substrate except that the pH of the assay was varied in the above range. The experiment was carried out with matched enzymatic activities of free and immobilized trypsin. The maximum activity at optimum pH was taken as 100%.
Measurement of Temperature Optima
The temperature optima of free and immobilized trypsin were determined by carrying out the enzyme assay at various temperatures ranging from 20–70°C. The experiment was carried out with matched enzymatic activities of free and immobilized trypsin. The maximum activity at optimum temperature was taken as 100%.
Determination of Kinetic Parameters
The kinetic parameters, Km, Vmax, and Vmax/Km of free and immobilized trypsin, were determined by measurement of enzyme activity at various concentrations of BAPNA and fitting the data in Hanes-Woolf equation using the Leonora software program [Citation[16]] to calculate the kinetic parameters. Matched amidase activities of free and immobilized enzyme were used in these experiments.
Autolysis of Free and Immobilized Trypsin
Free and immobilized trypsin were dissolved in 0.05 M Tris-HCl buffer, pH 8.0 (without the addition of Ca2+). The substrate solution was also made in the same buffer (without Ca2+). The enzyme solutions were incubated at 50°C. Appropriate aliquots of free and immobilized enzyme with matched enzyme activities were drawn at various time intervals and the amidase activities were determined. Starting activity of the enzyme preparation (before incubating at 50°C) was taken as 100%.
STI Accessibility
Free and immobilized trypsin with matched enzyme activities were incubated with different concentrations of soybean trypsin inhibitor (STI) for 15 min at 25°C. Thereafter, activities of free and immobilized trypsin against BAPNA and casein were measured spectrophotometrically at 410 nm and 578 nm, respectively.
Fluorescence Spectroscopy
The fluorescence emission spectra of free trypsin and copolymer-trypsin conjugates were recorded on a Shimadzu RF-5000 spectrofluorophotometer with a band width of 5 nm and 1 cm path length. The emission spectra were recorded using an excitation wavelength of 284 nm. The amount of protein was 12.6 µg in all the cases.
RESULTS AND DISCUSSION
Immobilization of Trypsin to Thermosensitive Copolymer
shows the coupling of various trypsin activity units to the thermosensitive copolymer as assayed with BAPNA as the substrate. It was observed that phase transition temperature of the bioconjugates remained unchanged at 30°C (data not shown). An increase in amidase activity was observed with all the conjugates (η > 1) as compared to free trypsin. This increase was maximum for conjugate A (η of 5.1) wherein 115 U of trypsin activity were coupled to the copolymer. The increase in activity of the immobilized preparation may be due to changes in the microenvironment of the enzyme leading to enhanced partitioning of the substrate in the polymer phase [Citation[8]].
Table 1. Immobilization of trypsin to thermosensitive copolymerFootnote‡
Kinetic parameters of immobilized and free trypsin were calculated using Hanes-Woolf equation and calculated values of Km and Vmax are given in . Immobilization of trypsin resulted in decrease in Km values, and increase in Vmax values for all conjugates as compared to free trypsin. Vmax/Km values for the conjugates were higher than those for the free trypsin. This data suggests that trypsin bound to thermosensitive polymer has higher affinity for the low molecular weight substrate, BAPNA [Citation[8], Citation[17]]. The highest Vmax/Km of 2.24 was obtained for conjugate B. Hence, further work was carried out with conjugate A (highest η) and conjugate B (highest Vmax/Km).
Table 2. Kinetic parameters of free trypsin and conjugates
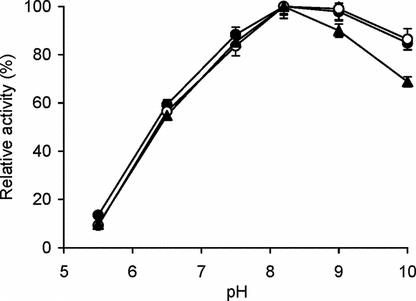
The effect of pH on the enzyme activity of free and immobilized trypsin is shown in . The optimum pH for the two conjugates was found to be same as that of free trypsin, i.e., pH 8.2. Also, the immobilized preparations showed broader pH optima with large activity surviving at alkaline pH [pH 9–10 region] (). These kinds of effects are not uncommon and have been often reported in case of conventional immobilization (on solid supports) [Citation[18], Citation[19]].
Figure 1 Effect of pH on enzyme activity. The enzyme assay for free (▴) and immobilized enzyme (conjugate A, •; conjugate B, ○) with matched enzyme activities was carried out at pH values ranging from pH 5.5–10.0 at 25°C with BAPNA as the substrate as given in materials and methods. The relative activity (%) was calculated by taking the maximum activity at optimum pH to be 100% and calculating activities at other pH values relative to it. The experiment was carried out in duplicate and the error bars represent the variation in the readings. The observed standard deviation in each set of readings was less than 0.1%.
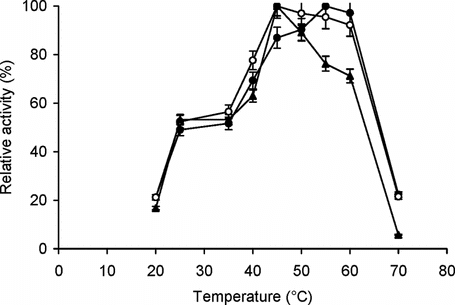
The effect of temperature on enzyme activity of free and immobilized trypsin is shown in . While the optimum temperature of free trypsin and conjugate B was 45°C, for conjugate A, the temperature optimum shifted to 55°C.
Figure 2 Effect of temperature on enzyme activity. The enzyme assay for free (▴) and immobilized enzyme (conjugate A, •; conjugate B, ○) with matched enzyme activities was carried out at temperatures ranging from 20–70°C with BAPNA as the substrate. The relative activity (%) was calculated by taking the maximum activity at optimum temperature to be 100% and calculating activities at other temperatures relative to it. The experiment was carried out in duplicate and the error bars represent the variation in the readings.
The immobilization of trypsin also resulted in its stabilization (). In the absence of Ca2+, at 50°C, while free trypsin lost its activity completely within 20 min owing to thermal denaturation and autodigestion, conjugates A and B retained above 80% activity even after 90 min. This is because at 50°C the copolymer is in the collapsed hydrophobic state and in this precipitated form of the conjugate, enzyme-enzyme interactions may be reduced resulting in reduced autodigestion [Citation[8]].
Figure 3 Autolysis of free and immobilized trypsin. Free (▴) and immobilized trypsin (conjugate A, •; conjugate B, ○) [in 0.05 M Tris-HCl, pH 8.0] with matched enzyme activities were incubated at 50°C. Aliquots were taken out at various intervals and assay was carried out with BAPNA (made in 0.05 M Tris-HCl, pH 8.0) after cooling to 25°C. The relative activity (%) was calculated by taking the activity at 0 min to be 100% and calculating activities at other intervals relative to it. The experiment was carried out in duplicate and the error bars represent the variation in the readings. The observed standard deviation in each set of readings was less than 0.1%.
![Figure 3 Autolysis of free and immobilized trypsin. Free (▴) and immobilized trypsin (conjugate A, •; conjugate B, ○) [in 0.05 M Tris-HCl, pH 8.0] with matched enzyme activities were incubated at 50°C. Aliquots were taken out at various intervals and assay was carried out with BAPNA (made in 0.05 M Tris-HCl, pH 8.0) after cooling to 25°C. The relative activity (%) was calculated by taking the activity at 0 min to be 100% and calculating activities at other intervals relative to it. The experiment was carried out in duplicate and the error bars represent the variation in the readings. The observed standard deviation in each set of readings was less than 0.1%.](/cms/asset/f3d56706-4b26-4bda-84c3-d934105381c6/ianb19_a_168360_f0003_b.gif)
STI Accessibility
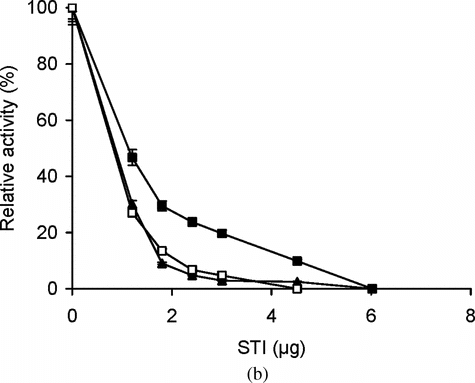
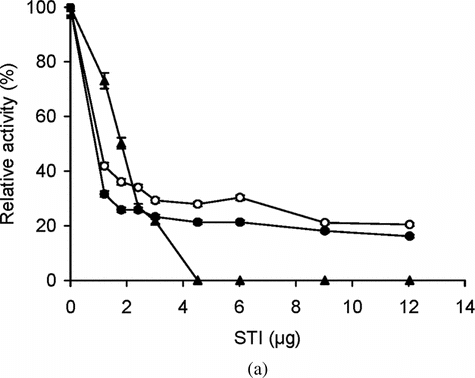
To investigate the effect of conjugation on accessibility to the active sites of trypsin, soybean trypsin inhibitor (STI, molecular weight of 20,000) was employed. STI is a competitive inhibitor of trypsin and, thus, at sufficient concentration totally blocks the enzyme activity. shows the enzyme activity of trypsin and conjugates as assessed by BAPNA (low molecular weight substrate) in the presence of different amounts of STI. This accessibility assay was carried out at 25°C when the bioconjugates are in the solution phase. The data shows that STI was unable to totally block the access of BAPNA to the enzyme active site in conjugates (). This is in agreement with the results reported by Arasaratnam et al. [Citation[17]]. In these cases STI is unable to completely block the active site and there is a limited access for low molecular weight amide substrate, BAPNA. However, with high molecular weight substrate, casein, both trypsin and the conjugates were completely inhibited by STI (), indicating that all enzyme molecules accessible to casein were accessible to STI as well. These accessibility experiments were carried out at 37°C when the bioconjugates are in precipitate (“collapsed state”) phase. Surprisingly, here bioconjugates behave more like the free enzyme.
Figure 4 (a) Effect of different concentrations of STI on the activity of free and immobilized trypsin with BAPNA. Free (▴) and immobilized enzyme (conjugate A, •; conjugate B, ○) with matched enzyme activities were incubated with different concentrations of STI for 15 min at 25°C, then trypsin activity was assayed with BAPNA under standard assay conditions. The relative activity was defined as the ratio of activities after incubating with and without STI. The experiment was carried out in duplicate and the error bars represent the variation in the readings. The observed standard deviation in each set of readings was less than 0.1%; (b) Effect of different concentrations of STI on the activity of free and immobilized trypsin with casein. Free (▴) and immobilized enzyme (conjugate A, ▪; conjugate B, □) with matched enzyme activities were incubated with different concentrations of STI for 15 min at 25°C, then trypsin activity was assayed with casein under standard assay conditions at 37°C. The relative activity was defined as the ratio of activities after incubating with and without STI. The experiment was carried out in duplicate and the error bars represent the variation in the readings. The observed standard deviation in each set of readings was less than 0.1%.
Fluorescence Spectra
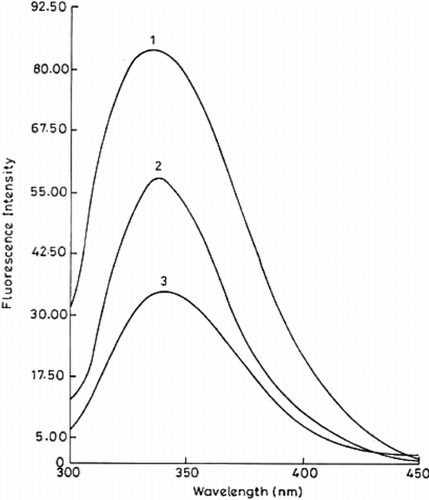
shows the fluorescence spectra of free and immobilized trypsin preparations, obtained using an excitation wavelength of 284 nm. The emission maximum for both free and immobilized systems was 340 nm, which agrees with earlier published work [Citation[20]]. Immobilized enzymes showed a considerable increase (2.4 times for conjugate A and 1.6 times for conjugate B) in quantum yield of fluorescence. This increase in quantum yield may be due to greater exposure of tryptophan residues, further confirming the changes in the microenvironment of the enzyme.
Figure 5 Fluorescence spectra of free and immobilized trypsin. The fluorescence emission spectra of immobilized (conjugate A, curve 1; conjugate B, curve 2) and free trypsin (curve 3) were recorded on a Shimadzu RF-5000 spectrofluorophotometer with a band width of 5 nm and 1 cm path length (at an excitation wavelength of 284 nm). The amount of protein was 12.6 µg in all the cases.
It may be relevant to compare the properties of these bioconjugates to the one described earlier [Citation[20]]. While Ca2+-responsive alginate–trypsin conjugate had the same Vmax/Km as free trypsin, the bioconjugates described in the present work represent more efficient designs in catalysis. Also, alginate-trypsin conjugate showed fluorescence emission spectra, which had contributions from tyrosine residues as well (presumably due to inhibition of energy transfer from tyrosine to tryptophan residues), the thermoresponsive bioconjugates described here show fluorescence emission spectra identical to the free enzyme (as far as λmax of emission is concerned).
CONCLUSION
While immobilization of enzymes on solid supports has been extensively carried out over the last few decades, much less work has been done using soluble supports as carriers/matrices. Use of smart or stimuli-sensitive polymers is a further improvement in this approach since it allows the advantages of homogeneous catalysis as well as easy recovery of a heterogeneous catalyst.
The conjugates described in this work show enhanced efficiency as amidase activity. These smart biocatalysts represent the evolving designs in applied biocatalysis.
The financial support provided by Department of Science and Technology, Government of India, is gratefully acknowledged. Partial financial support was also provided by Council for Scientific and Industrial Research (Technology Mission on Oilseeds, Pulses and Maize and Extramural Division). The financial support provided by IIT to KM, in the form of a senior research fellowship, and ICMR to SR, in the form of a junior research fellowship, is also acknowledged.
REFERENCES
- Guisán, J.M., Bastida, A., Cuesta, A.C., Fernández-Lafuente, R., Rosell, C.M. (1991). Immobilization-stabilization of chymotrypsin by covalent attachment to aldehyde agarose gels. Biotechnol. Bioeng. 39: 75–84, [CSA]
- Adlercreutz, P. (1993). Immobilized enzymes, in Enzymes in Food Processing, G. Reed, Ed., Academic Press: New York, pp. 103–119.
- Saleemuddin, M. (1999). Bioaffinity based immobilization of enzymes. Adv. Biochem. Eng. Biotechnol. 64: 203–226, [INFOTRIEVE], [CSA]
- Roy, I., Sharma, S., Gupta, M.N. (2003). Smart biocatalysts: Design and applications. Adv. Biochem. Eng/Biotechnol. 86: 159–189, [CSA]
- Fujii, M., Taniguchi, M. (1991). Application of reversibly soluble-insoluble polymers in bioprocessing. Trends. Biotechnol. 9: 191–196, [INFOTRIEVE], [CROSSREF], [CSA]
- Shiroya, T., Yasui, M., Fujimoto, K., Kawaguchi, H. (1995). Control of enzymatic activity using thermoresponsive polymers. Colloids Surf. B: Biointerfaces. 4: 275–285, [CROSSREF], [CSA]
- Chen, J.-P., Hsu, M.-S. (1997). Preparations and properties of temperature-sensitive poly(N-isopropylacrylamide)-chymotrypsin conjugates. J. Mol. Catal. B Enzymatic. 2: 233–241, [CROSSREF], [CSA]
- Ding, Z., Chen, G., Hoffman, A.S. (1998). Unusual properties of thermally sensitive oligomer-enzyme conjugates of poly (N-isopropylacrylamide)-trypsin. J. Biomed. Mater. Res. 39: 498–505, [INFOTRIEVE], [CROSSREF], [CSA]
- Ivanov, A.E., Edink, E., Kumar, A., Galaev, I.Y., Arendsen, A.F., Bruggink, A., Mattiasson, B. (2003). Conjugation of penicillin acylase with the reactive copolymer of N-Isopropylacrylamide: A step toward a thermoresponsive industrial biocatalyst. Biotechnol. Prog. 19: 1167–1175, [INFOTRIEVE], [CROSSREF], [CSA]
- Kuckling, D., Adler, H.-J.P., Arndt, K.-F., Ling, L., Habicher, W.D. (2000). Temperature and pH dependent solubility of novel poly(N-isopropylacrylamide) copolymers. Macromol. Chem. Phys. 201: 273–280, [CROSSREF], [CSA]
- Erlanger, B.F., Kokowsky, N., Cohen, W. (1961). The preparation and properties of two new chromogenic substrates of trypsin. Arch. Biochem. Biophys. 95: 271–278, [INFOTRIEVE], [CROSSREF], [CSA]
- Fujimora, M., Mori, T., Tosa, T. (1987). Preparation and properties of soluble–insoluble immobilized proteases. Biotechnol. Bioeng. 29: 747–752, [CROSSREF], [CSA]
- Lowry, O.H., Rosebrough, N.J., Fall, A.C., Randall, R.J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275, [INFOTRIEVE], [CSA]
- Decker, L.A., Ed. (1977). Worthington Enzyme Manual, Worthington Biochemical Corporation: Freehold, New Jersey, pp. 221–224.
- Hermanson, G.T., Mallia, A.K., Smith, P.K., Ed. (1992). Activation methods, in Immobilized Affinity Ligand Techniques, Academic Press: San Diego, pp. 53–95.
- Cornish-Bowden, A., Ed. (1995). Analysis of Enzyme Kinetic Data, Oxford University Press: Oxford, pp. 27–36.
- Arasaratnam, V., Galaev I.Y., Mattiasson, B. (2000). Reversibly soluble biocatalyst: Optimisation of trypsin coupling to Eudragit S-100 and biocatalyst activity in soluble and precipitated forms. Enzyme Microb. Technol. 27: 254–263, [INFOTRIEVE], [CROSSREF], [CSA]
- Palmieri, G., Giardina, P., Desiderio, B., Marzullo, L., Giamberini, M., Sannia, G. (1994). A new enzyme immobilization procedure using copper alginate gel: application to a fungal phenol oxidase. Enzyme Microb. Technol. 16: 151–158, [INFOTRIEVE], [CROSSREF], [CSA]
- Markvicheva, E.A., Tkachuk, N.E., Kuptsova, S.V., Dugina, T.N., Strukova, S.M., Kirsh, Yu.E., Zubov, V.P., Rumsh, L.D. (1996). Stabilization of proteases by entrapment in a new composite hydrogel. Appl. Biochem. Biotechnol. 61: 75–84, [INFOTRIEVE], [CSA]
- Jain, S., Roy, I., Gupta, M.N. (2004). A smart bioconjugate of trypsin with alginate. Artif. Cells Blood Substit. Biotechnol. 32: 325–337, [CROSSREF], [CSA]