Abstract
The hemoglobin based oxygen carrier (HBOC) Diaspirin Crosslinked Hemoglobin (DCLHb) has been developed to substitute not only the blood volume, but also to restore the oxygen–carrying properties of blood during hemorrhagic shock. However, it has been suggested that HBOCs may enhance the formation of free oxygen radicals through the release of free iron ions via the Haber-Weiss reaction.
The aim of this study was to investigate the effects of DCLHb on the microcirculation, leukocyte-endothelial cell interaction and local tissue oxygenation in striated skin muscle of Syrian golden hamsters during and after resuscitation from hemorrhagic shock. In particular we focused on the local tissue oxygenation after resuscitation with DCLHb (hemoglobin content 10 g%) compared to resuscitation using autologous blood diluted to a hemoglobin content of 10 g%.
Hemorrhagic shock was induced for 45 minutes by bleeding the animals at a rate of 33 ml/kg BW maintaining a mean arterial pressure of 30 ± 5 mmHg. Animals were resuscitated either with 33 ml/kg BW 6% Dextran-60.000 or with 10 g% DCLHb. The control group received shed blood diluted with Ringers to a hemoglobin content of 10 g%. Intravital microscopy was used for investigation of the microcirculatory parameters and a multiwire platinum surface electrode for measurement of local tissue pO2 in striated skin muscle in the dorsal skinfold chamber of Syrian golden hamsters.
Resuscitation from hemorrhagic shock with 10 g% AUB revealed significant increase of leukocytes rolling in postcapillary venules at 30 to 120 minutes after resuscitation compared to baseline values. DCLHb turned out to reduce the number of firmly adherent leukocytes after resuscitation compared to 10 g% AUB. Microvascular permeability as an indicator for functional endothelial integrity revealed no significant differences between the groups. DCLHb and 10 g% AUB led to a significant increase in local tissue oxygenation after resuscitation from hemorrhagic shock. However, 10 g% AUB turned out to be most effective to restore the local tissue pO2 compared to Dx-60.
Our findings indicate that DCLHb restores microvascular perfusion after critical hemorrhagic shock as efficient as Dx-60 and 10 g% AUB. The absence of enhanced leukocyte-endothelium interaction after resuscitation with DCLHb implies that this HBOC does not exacerbate formation of oxygen free radicals during reperfusion. DCLHb effectively increases local tissue pO2 after resuscitation from hemorrhagic shock; however, not as effectively as 10 g% AUB.
INTRODUCTION
Hemoglobin based oxygen carriers (HBOCs [Citation[1]]) are red blood cell (RBC) substitutes, which demonstrate therapeutic efficiency when used for resuscitation from hemorrhagic shock [Citation[2], Citation[3]]. However, to date, large scale clinical application has been impeded due to unwanted side effects of these solutions consisting of nephrotoxicity [Citation[4]], complement activation [Citation[5]] and risk of transmission of infectious diseases, e.g. hepatitis, HIV or prion induced diseases [Citation[6]]. Next to the refinement of the purification procedure and the technical production process of these solutions [Citation[7]], the chemical modification of the hemoglobin molecule was crucial to abolish these adverse effects.
Diaspirin Crosslinked Hemoglobin (DCLHb, HemAssistTM) is a stromafree hemoglobin solution. The hemoglobin molecule is covalently crosslinked between the 99lysin of the αα-chains with a fumaryl bridge [Citation[8]]. The oxygen-binding properties of DCLHb are similar to whole blood and the half-life is approximately 10 hours in humans [Citation[9]]. Various studies revealed that DCLHb is suitable as a resuscitation fluid in hemorrhagic shock, which restores macrohemodynamic parameters such as the mean arterial pressure [Citation[10]] and reverses a potential base deficit [Citation[11]]. However, very limited data are available on the effects of DCLHb on microvascular parameters following resuscitation from hemorrhagic shock [Citation[12], Citation[13]].
Preclinical investigations as well as clinical trials using DCLHb for resuscitation in hemorrhagic shock demonstrated promising results [Citation[2], Citation[14]]. However, the recently published mortality analysis of the efficacy trial of DCLHb raises concerns about safety of these solutions [Citation[15]]. HBOCs are thought to enhance formation of free oxygen radicals, especially during reperfusion of postischemic tissues [Citation[16]], e.g. after resuscitation from hemorrhagic shock. One possible mechanism may be the release of free iron ions from the prosthetic heme group, thus promoting the formation of hydroxyl radicals via the Haber-Weiss-reaction by acting as a Fenton reagent [Citation[17]]. This mechanism might lead to an enhancement of lipid peroxidation and leukocyte/endothelial cell interactions with activation of the inflammation cascade, release of cytosolic enzymes and, ultimately destruction of postischemic tissues [Citation[18]].
DCLHb has proven its efficacy in restoring microvascular parameters in the microcirculation after ischemia and reperfusion [Citation[19]] and hemorrhagic shock [Citation[20]]. Aim of the current study was the investigation of the effects of 10g% DCLHb on the microcirculation, leukocyte-endothelial cell/cell interactions and local tissue oxygenation after resuscitation from severe hemorrhagic shock. Special focus addressed resuscitation efficiency by comparing DCLHb (hemoglobin content 10 g/dl) with autologous blood of identical hemoglobin concentration ie equal oxygen carrying capacity.
MATERIALS AND METHODS
Hemoglobin Solution
Diaspirin Crosslinked Hemoglobin [DCLHb, 99αα-3,5-bis(dibromosalicyl)fumararte hemoglobin, lot-no. HBXR-92-268-42793] was provided by Baxter, Healthcare Corp. (Deerfield, Illinois, USA). The characteristics of the hemoglobin solution have been described previously [Citation[21]]. Purification procedures include virus inactivation by heat pasteurization [Citation[22]].
Animal Model
Syrian golden hamsters (6–8 weeks old, 50–70 g, Charles River, Sulzfeld, Germany) were kept with free access to tap water and pellet food ad libitum. All experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1985) and with the permission of the local ethic committee. The dorsal skin fold chamber in awake Syrian golden hamsters [Citation[23]] was used for investigation of microcirculatory parameters and local tissue oxygen tension. The chamber was implanted 24–48 h prior to the experiment under ketamine/xylazine anesthesia (130/20 mg/kg BW i.p.). Polyethylene catheters (Portex, Hythe, UK) were implanted in the jugular vein for infusion of resuscitation fluids and carotid artery for monitoring of mean arterial pressure, heart rate, arterial blood gases and hematocrit.
Experimental Protocol
Animals were randomly assigned to three different treatment groups as described later. After implantation of the dorsal skin fold chamber a recovery period of at least 48 hrs was followed before assessment of baseline values of microcirculatory parameters and tpO2. Shock was induced by bleeding the animals at a rate of 33 ml/kg BW maintaining a mean arterial pressure of 30 ± 2 mmHg over a period of 45 minutes by bleeding or reperfusion of the shed blood. Intravital microscopy and tissue oxygen measurements were performed at 30 min after onset of hemorrhagic shock and at 10, 30, 60 and 120 minutes following resuscitation.
Animals were resuscitated with either 33 ml/kg BW 6% Dextran 60.000 (Dx-60; Mr 60.000; Schiwa, Germany) as an isooncotic control solution, 10 g% DCLHb or with shed blood diluted to a hemoglobin content of 10.0 ± 0.5 g% from 14.2 ± 0.4 g% with lactated ringer.
Intravital Microscopy
The microcirculation of the striated skin muscle was assessed using a computer controlled microscope (Zeiss, Jena, Germany), connected to a stepping motor [Citation[20]] which allowed for exact repeated measurements of the same vessel segments. In vivo staining of leukocytes was accomplished by intravenous injection of 0.05% rhodamine 6G (0.15 mg/kg BW; Sigma Chemicals, Deisenhofen, Germany). This method enables the quantitative assessment of leukocyte/endothelial interactions [Citation[24]]. Macromolecular leakage was assessed by calculation of the ratio extravascular versus intravascular leakage of the fluorescence marker Fluorescein Isothiocyanate Dextran after intravenous injection of 15 mg/kg BW (FITC-Dx Mr 150.000, Pharmacia, Uppsala, Sweden). Video images were recorded on videotapes and evaluated off-line by a computer assisted microcirculation analysis system [Citation[25]] (CapImage®, Zeintl, Heidelberg, Germany). The following parameters were assessed:
Functional capillary density (FCD) defined as red cell perfused capillaries of the striated skin muscle per area (cm/cm2) [Citation[26]].
Leukocyte/endothelial cell interaction, determined as the number of non-adherent leukocytes (NAL/min), number of rolling leukocytes (cells/min) and firmly sticking leukocytes (cells/mm2). Rolling leukocytes are defined as slowly passing leukocytes along the endothelial lining, whereas sticking leukocytes did not detach from the endothelial lining during 30 seconds.
RBC velocity in capillaries and postcapillary venules (mm/s) and venular shear rate using the following formula:
Tissue Oxygen Measurement
A platinum multi-wire surface electrode (Clark-type MDO-electrode, Eschweiler, Kiel, Germany) connected to a computer assisted amplifier (MID, Steindorf, Germany) was used for measurement of local tissue oxygen tension in the striated skin muscle [Citation[27], Citation[28]]. At the preset time points of investigation, the cover slips of the chamber window was gently removed and the tissue superfused with isotonic saline solution (B. Braun, Melsungen, Germany) at room temperature. The MDO-electrode, containing 8 platinum electrodes with each channel measuring the tpO2 of approximately a spherical area of 25 µm2, was placed on the exposed tissue. An integrated temperature probe allowed for continuous measurement of the local tissue temperature for online correction of the tpO2-values. For the collection of 100–150 individual measurements the electrode was moved via a step-motor every 10 sec as described earlier [Citation[29]].
Statistics
Because of the limited number of animals per treatment group non-parametric tests were used. For analysis between the groups, data were tested using the Kruskal-Wallis-test followed by the Mann Whitney U-Test for analysis between groups or the Friedmann- and Wilcoxon-Test for comparison within the groups. All significant values were corrected using the Bonferroni-Holm procedure. Differences were considered statistically significant at a probability level of 0.05. Despite non-parametric distribution, data are presented as arithmetic means with the standard error of mean values for more rapid interpretation and comparability of the data with data from other studies.
RESULTS
Systemic Hemodynamic Parameters
Prior to induction of hemorrhagic shock, mean arterial pressure and heart rate were in the range of physiological values of hamsters [Citation[30]]. The mean arterial pressure during the shock period of 45 minutes was found to not be significantly different between the experimental groups. The MAP remained between 30 ± 6 and 32 ± 4 mmHg (). A previous study from our laboratory [Citation[20]] had shown that, without resuscitation, animals did not survive longer than 60 minutes when the MAP remained at 30 mmHg. In the present study, resuscitation with Dx-60 failed to restore the MAP to pre-shock values, whereas DCLHb and 10 g% AUB administration resulted in MAP comparable to those observed prior to hemorrhagic shock.
Table 1 Heart rate (HR) and mean arterial pressure (MAP) before, shock, during shock and after resuscitation (mean ± SD, n = 6–8 animals per treatment group; *p < 0.05 vs. NaCl 0.9% and Dx-60, Mann-Whitney U-Test)
The heart rate was monitored using arterial pressure curve and revealed no significant differences between the groups. In animals resuscitated with DCLHb the heart rate increased at 10 minutes after resuscitation to 412 ± 48 beats per minute compared to baseline values of 374 ± 18 min−1, whereas animals resuscitated with Dx-60 or 10 g% AUB did not experience heart rates above the pre-shock values ().
During shock period in almost 50% of the animals it was not possible to draw blood from the intraarterial catheter for arterial blood gas and hematocrit analysis. Therefore these data were not considered for statistical analysis. The arterial blood gas analysis showed a decrease of systemic oxygen tension during shock period in the animals. 120 minutes after resuscitation oxygen tension reached baseline values in animals resuscitated with DCLHb and AUB 10%.
Hematocrit was decreased after resuscitation with Dx-60 and DCLHb whereas 10% AUB caused no changes of systemic hematocrit from baseline values of 39.0 ± 1.4 to 38.2 ± 2.4% after 120 min after resuscitation.
Microcirculatory Parameters
The diameters of arterioles or venules in the striated skin muscle of the dorsal skin fold chamber did not differ significantly between the three groups. A vasoconstriction of the arteriolar vessels, as described in the literature [Citation[31]], was not found in animals resuscitated with DCLHb ().
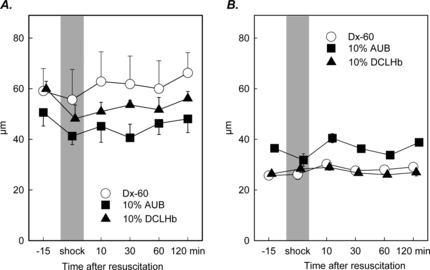
Figure 1 Diameter of arterioles (A) and postcapillary venules (B) in striated skin muscle after hemorrhagic shock and resuscitation (mean ± SEM, n = 6–8 per experimental). No significant changes were observed over the entire observation period.
However, the leukocyte/endothelial cell interactions represented by the number of rolling and sticking leukocytes revealed significant changes. In animals resuscitated with 10 g% AUB, a significant increase in the number of rolling leukocytes was detected at 30, 60 and 120 minutes after resuscitation (). The baseline number of rolling leukocytes prior to induction of shock was 16.2 ± 2.1 cells/minutes. At 30 minutes a value of 33.4 ± 5.0 was observed. The maximum was reached at 120 minutes after resuscitation with 37.6 ± 6.2 cells/minutes (p < 0.05 Wilcoxon Test). No significant differences were found for the number of rolling leukocytes in animals treated either with Dx-60 or DCLHb.
Figure 2 Rolling leukocytes (A) and sticking leukocytes (B) in postcapillary venules after resuscitation from hemorrhagic shock (mean ± SEM, n = 6–8 animals per exp. group; § p < 0.05 vs. Baseline, Wilcoxon test; # p < 0.05 10 g% AUB vs. 10 g% DCLHb, Mann Whitney U-Test).
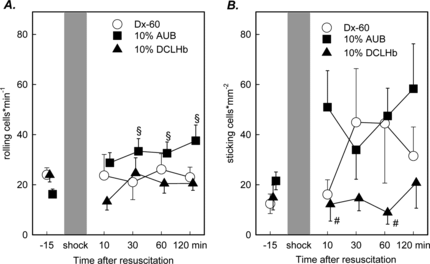
The number of leukocytes sticking to the endothelium of postcapillary venules turned out to be increased in animals receiving Dx-60 and autologous blood, respectively, when compared to the group treated with DCLHb. In the early time course after reperfusion (10 and 60 minutes), the number of sticking leukocytes was significantly reduced in animals resuscitated with DCLHb compared to 10 g% AUB (). No significant changes were noted in animals receiving Dx-60.
The macromolecular leakage of FITC-Dextran (MW 150.000 Dalton), as an indicator for functional endothelial integrity, revealed no significant changes. In animals treated with Dx-60 and DCLHb, an increase of macromolecular leakage during the shock period was notable, although these changes were not seen in the group resuscitated with autologous blood ().
Figure 3 Macromolecular leakage (A) and functional capillary density (B) in the striated skin muscle following hemorrhagic shock and resuscitation. Animals were treated either with Dx-60, 10 g% AUB or 10 g% DCLHb (mean ± SEM, n = 6–8 per exp. group; *p < 0.05 vs. Dx-60 Mann Whitney U-test; § p < 0.05 vs. baseline Wilcoxon test).
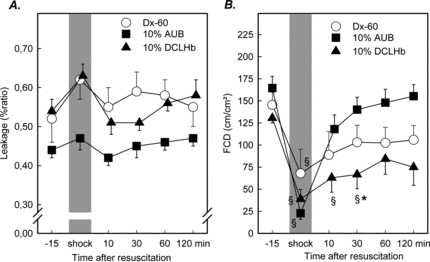
Functional capillary density, i.e. the length of capillaries perfused by red blood cells, was significantly reduced in all experimental groups from baseline values of 148 ± 33.1 cm/cm2 to 41.1 ± 43.4 cm/cm2 during the shock period. Resuscitation with 10 g% AUB almost immediately led to an increase of FCD and almost restored FCD after 120 minutes. Less prominent was this effect after resuscitation with Dx-60, although no significant differences were measurable after 30 minutes. DCLHb was less effective in restoring the FCD. Baseline levels in this group were 130.6 ± 27.0 cm/cm2, 38.8 ± 27.0 during shock, 62.9 ± 39.7 at 10 minutes and 66.6 ± 39.5 cm/cm2 at 30 min after resuscitation. These changes were significantly different when compared to respective data in the DCLHb and 10 g% AUB group during the time course of resuscitation at 30 minutes after resuscitation ().
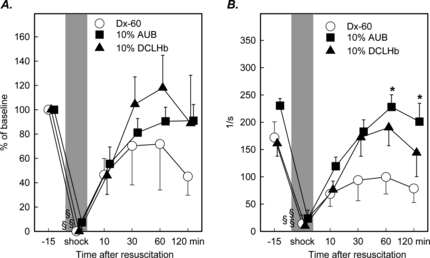
A significant reduction of velocity of red blood cells was detectable in all experimental groups during the shock period with levels of reduced to no flow and pendular flow in postcapillary venules, respectively (). Resuscitation restored the RBCV in all experimental groups, although Dx-60 seemed to be less effective as compared to DCLHb or AUB. The same trend could be observed for shear rates in postcapillary venules (). The mean shear rate at baseline averaged at 193 ± 14/s. The decrease of the shear rates reached significance in all groups during the shock period, when compared to baseline values, and was restored to baseline levels in animals treated with 10% DCLHb or 10% AUB at 60 minutes after resuscitation, but not with Dx-60. At 60 and 120 minutes after resuscitation this rebound was still significantly greater in animals treated with 10% AUB when compared to treatment with Dx-60.
Figure 4 Red blood cell velocity in percentage of baseline values (A) and shear rates (B) in postcapillary venules of animals resuscitated either with Dx-60, 10 g% AUB or 10 g% DCLHb (mean ± SEM, n = 6–8 per exp. group; *p < 0.05 vs. Dx-60 Mann Whitney U-test; § p < 0.05 vs. baseline Wilcoxon test).
Local Tissue pO2
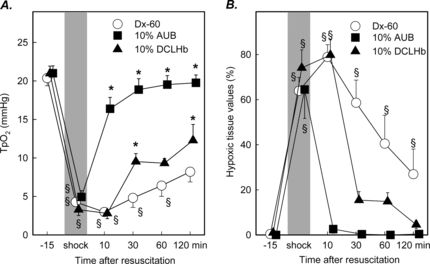
The local tissue pO2 ranged from 20.3 ± 0.3 to 21.0 ± 0.9 mmHg prior to shock in investigated treatment groups. During the shock period a significant decrease of the local tissue oxygenation was measured in all groups ranging from 3.3 ± 0.8 to 4.9 ± 0.8 mmHg. In animals treated with 10 g% AUB tissue pO2 recovered more rapidly after resuscitation than in animals treated with Dx-60 or DCLHb and reached values of 16.4 ± 1.5 mmHg and was found in normal range 60 minutes after resuscitation. The groups treated with Dx-60 demonstrated a prolonged reduction of local tissue pO2 at 120 minutes after resuscitation with a mean of 8.2 ± 1.3 mmHg (). The distribution of local pO2 values indicated a marked shift to hypoxic tissue values. This shift is represented by the marked increase in the hypoxic values between 0 and 5 mmHg (). During shock approximately 70% of all measured tissue pO2 values had to be classified as hypoxic. At 30 minutes following resuscitation the number of hypoxic tissue values initially increased in animals treated with 10 g% DCLHb and Dx-60. In contrast, 60 minutes after resuscitation no significant hypoxic tissue values were observed in animals treated with DCLHb and only 17% of tissue pO2 levels had to be classified as hypoxic 60 minutes after resuscitation. In Dx-60 treated animals tissue hypoxia persisted during the complete time course after resuscitation ().
Figure 5 Local tissue oxygenation (A) and frequency of tissue hypoxia (B) with values ranging from 0–5 mmHg in striated muscle prior to shock and following resuscitation with Dextran, 10 g% AUB or DCLHb (mean ± SEM, n = 6 per exp. group; *p < 0.05 vs. Dx-60 Mann Whitney U-test; § p < 0.05 vs. baseline Wilcoxon test).
DISCUSSION
The current study focused on the effects of DCLHb on the microcirculation and local tissue oxygenation in striated skin muscle after resuscitation from severe hemorrhagic shock. Special focus addressed resuscitation efficiency by comparing DCLHb (hemoglobin content 10 g/dl) with autologous blood of identical hemoglobin concentration, i.e., equal oxygen carrying capacity.
The main findings of this study on resuscitation with DCLHb following severe hemorrhagic shock are: DCLHb (1) reduces leukocyte-endothelial cell/cell interactions in postcapillary venules, (2) effectively increases the local tissue oxygenation, (3) attenuates the loss of functional capillary density after resuscitation, (4) DCLHb was more effective to restore local tissue oxygenation than Dx-60, however not superior to AUB.
The dorsal skin fold chamber is an animal model suitable for the investigation of microvascular disturbances following ischemia/reperfusion as represented by systemic hemorrhagic shock [Citation[32]]. Potential advantages of this model are the possibility to investigate changes in the microcirculation independently of anesthesia during the observation period. The changes due to manipulation of the local tissue are minimized by implantation the chamber several days before investigation. The validity of local tissue oxygenation measurements in this experimental setup has been demonstrated previously [Citation[33]] and the experimental protocol of severe hemorrhagic shock has been validated before. Without resuscitation the animals would die in hemorrhagic shock after a period of approximately 60 minutes [Citation[20]].
The above mentioned studies performed in our laboratory suggested a benefit of DCLHb for ischemia/reperfusion injury and after resuscitation from hemorrhagic shock on the striated skin muscle, when compared to treatment with the colloidal solution Dx-60. These effects were attributed to a reduction of leukocyte/endothelial cell interactions and improvement of local tissue oxygenation. In the present experimental setup, isovolemic resuscitation with autologous blood led to an increase of leukocyte-endothelial cell/cell interactions, i.e., the number of rolling and sticking leukocytes. Since whole blood was reinfused, which was stored for 45 minutes and diluted with lactated ringer solution, the contact of shed blood with artificial surfaces might have induced an inflammatory activation of white blood cells as described by experimental models of extracorporeal circulation [Citation[34]]. As demonstrated in previous studies DCLHb led to a decrease of leukocyte-endothelial cell/cell interactions in animals resuscitated from hemorrhagic shock [Citation[20]]. The exact pathophysiological mechanisms that are responsible for the reduction of leukocyte-endothelial cell/cell interactions remain to be elucidated. DCLHb has been described not to increase free radical formation under conditions of ischemia/reperfusion [Citation[35]]. Data from studies using experimental models of hemorrhagic shock indicate the potential role of DCLHb as a nitric oxide scavenger [Citation[36]]. However, this would imply an activation of leukocyte-endothelial cell/cell interactions [Citation[18]]. The complexity of these interactions of modified stroma free hemoglobin such as DCLHb and leukocyte-endothelial interactions were enhanced by the findings that hemoglobin delivers nitric oxide [Citation[37], Citation[38]]. The paracrine and endocrine functions of nitric oxide bound to hemoglobin are not fully understood and to a lesser extent for modified hemoglobin molecules. Therefore, it can only be speculated why leukocyte-endothelial cell/cell interactions are reduced by DCLHb. Though autologous blood was most effective in restoring the local tissue oxygenation and functional capillary density, the activation of leukocytes was most prominent in this experimental group. The recovery of local tissue oxygenation and the tissue perfusion with red blood cells in the striated muscle is not the single pre-requisite for the reduction of leukocyte-endothelial cell/cell interactions in postcapillary venules. Another interesting finding is that, although resuscitation with 10 g% AUB led to an increase of the leukocyte-endothelial cell/cell interactions, the macromolecular leakage of FITC-Dextran as an indicator of the local endothelial integrity showed no significant changes. Therefore it must be concluded, that a decrease of leukocyte/endothelial cell/cell interactions does not translate into a better biological function of microvascular parameters such as described above.
Local tissue oxygenation was most effectively restored by resuscitation with autologous blood in the striated skin muscle. However, the increase of tissue pO2 seen after DCLHb was significantly higher than following resuscitation with Dextran. These findings are in good accordance with other experimental animal studies: DCLHb demonstrated a significant improvement of local tissue oxygenation when compared to Dextran, however, without reaching baseline values [Citation[39], Citation[40]]. In extreme hemodilution, DCLHb contributes to local tissue oxygenation and oxygen delivery, although tissue hypoxia is present in the skeletal muscle [Citation[41]]. Furthermore, DCLHb is known to increase the local tissue oxygenation by inducing a more homogenous oxygen distribution and a reduced shift of the histogram profile to lower tissue oxygen levels [Citation[40]]. The efficacy of autologous blood to restore local tissue oxygenation in the early reperfusion period might be explained by the hem diluting effect after resuscitation. Comparable results are seen after acute normovolemic hemodilution in the skeletal muscle of dogs using a polarographic platinum surface electrode and the radioactive microspheres technique [Citation[42]].
The most likely explanation for the inferior efficacy of DCLHb to restore local tissue oxygenation in striated skin muscle when compared to whole blood appears to be the reduction of functional capillary density (FCD). FCD is an indicator for local tissue perfusion [Citation[26]] and survival following hemorrhagic shock was found to correlate with the recovery of FCD after resuscitation [Citation[43]]. The FCD is defined as the length of RBC-perfused capillaries per observation area [Citation[26]]. Since DCLHb and Dextran are non-corpuscular solution, a reduction of FCD would be expected. Surprisingly, DCLHb led to a significant decrease of FCD 10 and 30 minutes after resuscitation, whereas the local tissue oxygenation showed a significant increase at 30 minutes following resuscitation. Acute toxic effects of DCLHb to endothelial cells as a possible explanation for this observation can be ruled out since no increase of macromolecular leakage or increase of leukocyte-endothelial cell/cell interactions were detected. These findings suggest a higher number of capillaries perfused by plasma and DCLHb but not by RBC. Obviously this is an impediment of the methodology to measure the perfusion of capillaries with non-corpuscular oxygen carrying solutions. Other experimental data demonstrated similar effects of DCLHb on FCD after isovolemic exchange transfusion [Citation[20], Citation[44]]. In these studies FCD was reduced up to 48% from baseline whereas local tissue oxygenation was not impaired. The reduced FCD might be the consequence of a specific action of DCLHb since pre-clinical data on a polyethylene glycol-modified hemoglobin with a hemoglobin content of 4 g/dL demonstrated an increase of the FCD after resuscitation from hemorrhagic shock in the same animal model using a similar experimental protocol [Citation[45]]. The heterogeneity of the microcirculation under physiological and pathophysiological conditions due to the spatial variance of local tissue perfusion may also contribute to varying results [Citation[46]]. The mechanisms regulating the local microcirculation are not fully understood, however our data might suggest an interaction of DCLHb with organ-specific factors modulating the tissue's microcirculation.
The experimental setup used in our study did not access the outcome of animals after resuscitation and therefore it remains unclear whether the reduction of leukocyte-endothelial cell/cell interactions in animals treated with DCLHb would positively influence mortality and lethality.
Previous experimental studies described the induction of arterial vasoconstriction after administration of stroma free hemoglobin solutions, e.g. DCLHb [Citation[31]]. DCLHb might therefore contribute to a decrease in the peripheral local tissue oxygen tension, an effect not seen after resuscitation with whole blood. Although no vasoconstriction was found in our studies, the vasoconstriction might take place in the larger upstream vessels not visible in the dorsal skin fold chamber preparation. Studies using radioactive labeled microspheres revealed an increased microvascular blood flow to the heart after infusion of DCLHb, whereas this phenomenon was absent after infusion of unmodified hemoglobin [Citation[47]]. This may indicate an organ specific action of DCLHb on the microcirculation. Intravital microscopy studies in the dorsal skin fold chamber of the hamster using DCLHb for isovolemic exchange transfusion and hypervolemic infusion demonstrated an increase of the local RBCV and a very short lasting arteriolar vasoconstriction for a maximum period of 120 seconds after hypervolemic infusion [Citation[20]]. However, this short lasting arteriolar vasoconstriction did not explain the prolonged increase of the RBCV at 30 minutes of hemorrhagic shock.
The novel finding of the present study is the efficacy of diluted autologous blood to restore the local tissue oxygenation immediately after resuscitation from hemorrhagic shock. When compared to previous studies, hemodiluted whole blood turned out to be as effective as non-diluted whole blood in restoring macro- and microcirculatory parameters [Citation[39]]. Isovolemic hemodilution has been shown to be efficient to increase oxygen delivery to tissues with a peak at 30% hematocrit [Citation[48]]. In the present study, the dilution of the reinfused autologous blood did not decrease the systemic hematocrit at the end of the observation period. The hypothesis that hemodilution contributes to a faster recovery of tissue pO2 is also objected by the experimental group using colloidal infusion solution. Although Dextran led to a decrease of systemic hematocrit after resuscitation, Dextran was less effective in restoring the local tissue pO2.
Despite the promising data reported from pre-clinical and clinical trials, there are still impediments for HBOCs, such as DCLHb, for a large-scale clinical use. The published data on post hoc mortality analysis of the clinical trauma trial revealed a higher mortality in patients treated with DCLHb than expected by survival analysis [Citation[15]]. These data led to speculations about the safety of modified hemoglobin solutions. These solutions do not represent simple oxygen carriers, but interact with a wide variety of factors, such as nitric oxide and endothelin, that are involved in the regulation of the local microcirculation of ischemic and reperfused organs. This study demonstrates the capability of DCLHb to substitute red blood cells but not to replace them.
REFERENCES
- Fratantoni, J.C. (1991). Transfusion. 31: 369–371. [CSA]
- Przybelski, R.J., Daily, E.K., Micheels, J., Sloan, E.P., Mols, P., Corne, L., Koenigsberg, M.D., Bickell, W.H., Thompson, D.R., Harviel, J.D., Cohn, S.M. (1999). Prehospital. Disaster. Med. 14: 251–264. [INFOTRIEVE], [CSA]
- Sloan, E.P., Koenigsberg, M., Gens, D., Cipolle, M., Runge, J., Mallory, M.N., Rodman, G.J. (1999). JAMA. 282: 1857–1864. [INFOTRIEVE], [CSA], [CROSSREF]
- Feola, M., Simoni, J., Tran, R., Canizaro, P.C. (1990). Biomater. Artif. Cells Artif. Organs. 18: 233–249. [INFOTRIEVE], [CSA]
- Feola, M., Simoni, J., Dobke, M., Canizaro, P.C. (1988). Circ. Shock. 25: 275–290. [INFOTRIEVE], [CSA]
- Zuck, T.F., Riess, J.G. (1994). Crit. Rev. Clin. Lab. Sci. 31: 295–324. [INFOTRIEVE], [CSA]
- Farmer, M., Ebeling, A., Marshall, T., Hauck, W., Sun, C.S., White, E., Long, Z. (1992). Biomater. Artif. Cells. Immobilization. Biotechnol. 20: 429–433. [INFOTRIEVE], [CSA]
- Chatterjee, R., Welty, E.V., Walder, R.Y., Pruitt, S.L., Rogers, P.H., Arnone, A., Walder, J.A. (1986). J. Biol. Chem. 261: 9929–9937. [INFOTRIEVE], [CSA]
- O'Hara, J.F.J., Colburn, W.A., Tetzlaff, J.E., Novick, A.C., Angermeier, K.W., Schubert, A. (2001). Anesth. Analg. 92: 44–48. [INFOTRIEVE], [CSA], [CROSSREF]
- Schultz, S.C., Powell, C.C., Burris, D.G., Nguyen, H., Jaffin, J., Malcolm, D.S. (1994). J. Trauma. 37: 408–412. [INFOTRIEVE], [CSA]
- Schultz, S.C., Hamilton, I.N.J., Malcolm, D.S. (1993). J. Trauma. 35: 619–625. [INFOTRIEVE], [CSA]
- van Iterson, M., Sinaasappel, M., Burhop, K., Trouwborst, A., Ince, C. (1998). J. Lab. Clin. Med. 132: 421–431. [INFOTRIEVE], [CSA], [CROSSREF]
- von Dobschuetz, E., Hoffmann, T., Messmer, K. (1999). Anesthesiology. 91: 1754–1762. [INFOTRIEVE], [CSA], [CROSSREF]
- Cohn, S.M., Farrell, T.J. (1995). J. Trauma. 39: 210–217. [INFOTRIEVE], [CSA]
- Sloan, E.P., Koenigsberg, M., Brunett, P.H., Bynoe, R.P., Morris, J.A., Tinkoff, G., Dalsey, W.C., Ochsner, M.G. (2002). J. Trauma. 52: 887–895. [INFOTRIEVE], [CSA]
- Faassen, A.E., Sundby, S.R., Panter, S.S., Condie, R.M., Hedlund, B.E. (1988). Biomater. Artif. Cells Artif. Organs. 16: 93–104. [INFOTRIEVE], [CSA]
- Sadrzadeh, S.M., Graf, E., Panter, S.S., Hallaway, P.E., Eaton, J.W. (1984). J. Biol. Chem. 259: 14354–14356. [INFOTRIEVE], [CSA]
- Menger, M.D., Lehr, H.A., Messmer, K. (1991). Klin. Wochenschr. 69: 1050–1055. [INFOTRIEVE], [CSA], [CROSSREF]
- Pickelmann, S., Nolte, D., Messmer, K. (1996). Artif. Cells Blood Substit. Immobil. Biotechn. 24: 405. [CSA]
- Nolte, D., Botzlar, A., Pickelmann, S., Bouskela, E., Messmer, K. (1997). J. Lab. Clin. Med. 130: 314–327. [INFOTRIEVE], [CSA], [CROSSREF]
- Simoni, J., Feola, M., Canizaro, P.C. (1990). Biomater. Artif. Cells Artif. Organs. 18: 189–202. [INFOTRIEVE], [CSA]
- Estep, T.N., Bechtel, M.K., Miller, T.J., Bagdasarian, A. (1989). Blood Substitutes, Marcel Dekker: New York, pp. 129–134.
- Endrich, B., Asaishi, K., Götz, A., Messmer, K. (1980). Res. Exp. Med. Berl. 177: 125–134. [INFOTRIEVE], [CSA], [CROSSREF]
- Sack, F.U., Funk, W., Hammersen, F., Messmer, K. (1987). Progress in Applied Microcirculation, Karger: Basel, 12, pp. 282–288.
- Zeintl, H., Sack, F.U., Intaglietta, M., Messmer, K. (1989). Int. J. Microcirc. Clin. Exp. 8: 293–302. [INFOTRIEVE], [CSA]
- Nolte, D., Zeintl, H., Steinbauer, M., Pickelmann, S., Messmer, K. (1995). Int. J. Microcirc. Clin. Exp. 15: 244–249. [INFOTRIEVE], [CSA]
- Kessler, M., Grunewald, W.A. (1969). Prog. Resp. Res. 3: 147–152. [CSA]
- Lübbers, D.W. (1969). Int. Symposium on Oxygen Pressure Recording, Nijmegen 1968, Karger: Basel, pp. 112–123.
- Messmer, K., Görnandt, L., Sinagowitz, E., Sunder Plassmann, L., Jesch, F., Kessler, M. (1973). Bibl. Anat. 12: 327–332. [INFOTRIEVE], [CSA]
- Lombard, E. (1952). Am. J. Physiol. 171: 189–193. [INFOTRIEVE], [CSA]
- MacDonald, V.W., Motterlini, R. (1994). Artif. Cells Blood Substit. Immobil. Biotechn. 22: 565–575. [CSA]
- Nolte, D., Menger, M.D., Messmer, K. (1995). Int. J. Microcirc. Clin. Exp. 15: 9–16. [INFOTRIEVE], [CSA]
- Pickelmann, S., Nolte, D., Leiderer, R., Schutze, E., Messmer, K. (1998). Am. J. Physiol. 275: H361–H368. [CSA]
- Kamler, M., Jakob, H., Lehr, H.A., Gebhard, M.M., Hagl, S. (1997). Eur. J. Cardiothorac. Surg. 11: 973–980. [INFOTRIEVE], [CSA], [CROSSREF]
- Pincemail, J., Detry, O., Philippart, C., Defraigne, J.O., Franssen, C., Burhop, K., Deby, C., Meurisse, M., Lamy, M. (1995). Free Radic. Biol. Med. 19: 1–9. [INFOTRIEVE], [CSA], [CROSSREF]
- Gulati, A., Sen, A.P., Sharma, A.C., Singh, G. (1997). Am. J. Physiol. 273: H827–H836. [CSA]
- Jia, L., Bonaventura, J., Stamler, J.S. (1996). Nature. 380: 221–226. [INFOTRIEVE], [CSA], [CROSSREF]
- Chen, L.E., Seaber, A.V., Nasser, R.M., Stamler, J.S., Urbaniak, J.R. (1998). Am. J. Physiol. 274: R822–R829. [CSA]
- Nolte, D., Steinhauser, P., Pickelmann, S., Berger, S., Härtl, R., Messmer, K. (1997). J. Lab. Clin. Med. 130: 328–338. [INFOTRIEVE], [CSA], [CROSSREF]
- Nolte, D., Pickelmann, S., Botzlar, A., Messmer, K. (1996). Artif. Cells Blood Substit. Immobil. Biotechn. 24: 394. [CSA]
- Meisner, F.G., Kemming, G.I., Habler, O.P., Kleen, M.S., Tillmanns, J.H., Hutter, J.W., Bottino, D.A., Thein, E., Meier, J.M., Wojtczyk, C.J., Pape, A., Messmer, K. (2001). Crit. Care. Med. 29: 829–838. [INFOTRIEVE], [CSA], [CROSSREF]
- Hutter, J., Habler, O., Kleen, M., Tiede, M., Podtschaske, A., Kemming, G., Corso, C., Batra, S., Keipert, P., Faithfull, S., Messmer, K. (1999). J. Appl. Physiol. 86: 860–866. [INFOTRIEVE], [CSA]
- Kerger, H., Waschke, K.F., Ackern, K.V., Tsai, A.G., Intaglietta, M. (1999). Am. J. Physiol. 276: H2035–H2043. [CSA]
- Tsai, A.G., Friesenecker, B., Winslow, R.M., Intaglietta, M. (1994). Artif. Cells Blood Substit. Immobil. Biotechn. 22: 841–847. [CSA]
- Wettstein, R., Tsai, A.G., Erni, D., Winslow, R.M., Intaglietta, M. (2003). Crit. Care. Med. 31: 1824–1830. [INFOTRIEVE], [CSA], [CROSSREF]
- Kleen, M., Habler, O., Zwissler, B., Messmer, K. (1998). Comput. Methods. Programs. Biomed. 55: 51–57. [INFOTRIEVE], [CSA], [CROSSREF]
- Gulati, A., Sharma, A.C., Burhop, K.E. (1994). Life. Sci. 55: 827–837. [INFOTRIEVE], [CSA], [CROSSREF]
- Sunder Plassmann, L., Kessler, M., Jesch, F., Dieterle, R., Messmer, K. (1975). Bibl. Haematol. 44–53. [CSA]