Abstract
This paper describes the approaches we have taken to construct a) mutant hemoglobins with different oxygen affinities, and b) mutant hemoglobins and myoglobins that polymerize to high molecular weight aggregates in an effort to prevent extravasation and the associated vasoactivity. In vivo testing indicates that exchange transfusion of polymeric hemoglobins in mice does not result in vasoactivity and that polymeric hemoglobins are effective oxygen carriers to ischemic tissues irrespective of their oxygen affinity and cooperativity.
HEME POCKET MODIFICATIONS
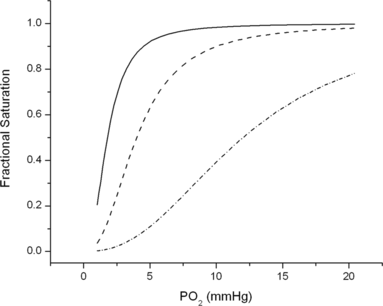
The functional properties of the heme pocket are sensitive to the polar character of the amino acid side chains that line the pocket [Citation[1]]. Accordingly, we modified the polarity of the heme pocket and obtained three mutant hemoglobins with a range of oxygen affinities () [Citation[2]].
Figure 1 Oxygen binding curves of βL28N (-----), HbA (— · · —), βV67T (· · · ·), and α V63T (— — —). Buffer, pH 7.4, 50 mM Hepes + 100 mM NaCl; Temperature, 25°C.
In the B-helix of the β-chains, we replaced leucine 28, a highly conserved residue in the hydrophobic cluster on the heme distal side, with the isosteric, but polar, asparagine. This residue, by interacting with bound O2, stabilizes the oxygenated R-state, resulting in a ten-fold increase in oxygen affinity (P50 = 0.65 torr). HbA under the same conditions has a P50 of 6.5 torr.
In the E-helix of the α or β chains, we replaced valine at position E11 with the isosteric, but polar, threonine. When this mutation was introduced in the β-chains, a two-fold decrease in oxygen affinity (P50 = 12 torr) with respect to HbA was observed. However, when this mutation was introduced at position E11 of the α-chains, the functional effects were strikingly different. The oxygen affinity of αV62T was decreased four-fold (P50 = 25 torr). The significantly decreased oxygen affinity measured in αV62T was attributed to stabilization of the water molecule present in the distal heme pocket of deoxy, T-state, which must dissociate prior to O2 bonding to the heme-Fe. Cooperativity was present in the mutants βL28N (n = 1.8) and βV67T (n = 2.2), but was essentially absent in αV62T (n = 1.1). A troublesome aspect of this approach was the increased autoxidation rate observed in all of the heme-pocket mutants, although in vivo, the reducing systems present in plasma would be expected to minimize the amount of circulating methemoglobin [Citation[3]].
SURFACE MUTATIONS
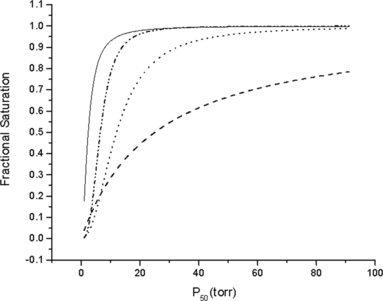
We also used surface mutations to modify the oxygen affinity of human Hb. By increasing the hydrophobic interactions between the A-helix and the hydrophobic core of the β-subunits,we constructed an Hb molecule with a three-fold decrease in oxygen affinity with respect to HbA. The amino acid substitutions introduced in the β-chain of HbA are Val1 → Met, His2 → deleted, Thr4 → Ile, Pro5 → Ala, and Ala 76 → Lys. In 50 mM Hepes buffer at pH 7.5 and at 25°C, this mutant had an oxygen affinity two-fold lower than that of HbA. In the presence of 100 mM Cl− (the concentration present in blood plasma), it exhibited an additional three-fold decrease in oxygen affinity () [Citation[4], Citation[5]]. Moreover, these surface mutations increased the resistance to autoxidation, which is contrary to expectation, as low oxygen affinity is often associated with an increased rate of autoxidation.
POLYMERIC HEMOGLOBINS
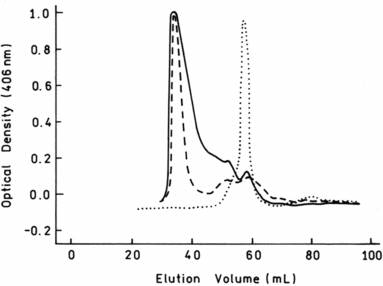
Problems related to the use of Hb solutions for transfusion are the rapid loss of tetrameric Hb through the kidneys and extravasation through the endothelium. The latter is associated with an increase in arterial blood pressure, thought to be due to scavenging of NO [Citation[6]]. To prevent Hb loss/extravasation and reduce the associated negative effects [Citation[7], Citation[8]], we explored the use of recombinant technology to obtain polymers of tetrameric Hb molecules. Polymerization has been obtained by introducing, on the protein surface, a cysteine at position β9 to promote the formation of intermolecular S-S bonds. Polymers of human Hb [Citation[9]] and of a human/bovine hybrid, Hb Minotaur, have been obtained [Citation[2], Citation[3]]. The latter has the functional characteristics of the HbA polymer and is endowed with a high expression level that has allowed preparation of the necessary amounts for in vivo experimentation. Polymers of different MW have been obtained. One homogeneous polymer, Hb Polytaur, is shown in illustrating the size exclusion chromatography of a polymer with MW-500KDa (Ve 48 ml), in comparison to that of HbA with a MW-64 KDa (Ve 60 ml). The two peaks have symmetrical shapes, indicating the presence of a major homogeneous fraction. The others were very large heterogeneous polymers of tetrameric hemoglobins, Hb (Polytaur)n, and of monomeric myoglobin, PolyMb [Citation[2]]. The size exclusion chromatography of these proteins, as compared to tetrameric HbA, is shown in . The elution volume (Ve = 37 ml) of the main fraction corresponds to the void volume of the column, indicating the presence of polymers with MW ≥ 1 million. PolyMb was obtained by introduction of the substitutions Gln8 → Cys, Lys50 → Cys, and Lys76 → Cys.
Figure 3 Size exclusion chromatography of HbA (Ve 60 ml, as for a protein with MW 64 kDa) and Poly-500 (Ve 48 ml, as for a protein with MW 500 kDa).
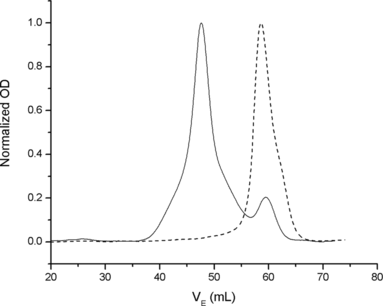
Figure 4 Size exclusion chromatography of HbA (Ve 60 ml, as for a protein with MW 64 kDa) and Hb (Polytaur)n (Ve 38 ml = exclusion volume of the column, as for proteins with MW > 1 million).
Polymerized Hbs have a high affinity for heme and are stable toward the reducing agents present in circulation [Citation[3]]. In the presence of whole blood, the half time of autoxidation (T1/2) is 46 h [Citation[3]]. This value is compatible with the retention time measured for chemically modified polymeric hemoglobins (T1/2 = 24 h) [Citation[10], Citation[11]]. Polymerization does not modify the functional characteristics of monomeric myoglobin [Citation[2]], and additional mutations could be introduced to modulate oxygen affinity.
IN VIVO TESTING
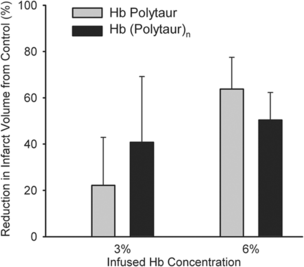
The hemoglobin polymers were tested in vivo in a mouse model. shows the time course of mean arterial blood pressure after hypervolemic exchange transfusion with albumin, Hb Polytaur, and Hb (Polytaur)n. The increase in pressure was similar in the three groups, indicating that it was related to blood volume expansion. The slight increase associated with the use of Hb polymers was probably due to the small amount of tetrameric Hb still present in the solution ( and ). Under physiologic conditions, the Pso of Hb Polytaur, and Hb (Polytaur)n are 18 torr and 2.0 torr, respectively [Citation[3], Citation[2]]. shows the dose-response reduction of cerebral infarct volume, with exchange transfusion of 3% and 6% solutions of Hb Polytaur, and Hb (Polytaur)n 2 h after onset of middle cerebral artery occlusion in mice. The data indicate that, under ischemic conditions, these polymers can act as effective oxygen carriers. Notably, the difference in oxygen affinity between the two polymers does not appear to be a factor in oxygen delivery. These results suggest the possible therapeutic application of polymerized Mb.
Figure 5 Change in mean arterial blood pressure (±SD) after hypervolumetric exchange transfusion with either 5% albumin, 3% Hb Polytaur, or 3% Hb (Polytaur)n in anesthetized mice. The increase in pressure with Hb polymers was largely related to the increase in blood volume. A small portion of the increase might have been related to residual tetrameric Hb in the infusate.
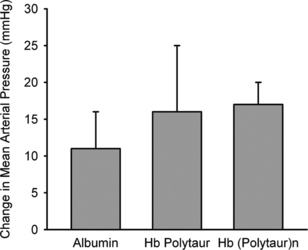
Figure 6 Percent reduction in infarct volume (±SD) with hypervolumetric exchange transfusion of 3% and 6% solutions of Hb Polytaur and Hb (Polytaur)n during 2 h of middle cerebral artery occlusion in mice. Infarct volume, measured at 22 h of reperfusion, is expressed as a percent reduction from infarct volume in control groups transfused with 5% albumin.
CONCLUSIONS
Molecular biology offers the opportunity to construct hemoglobin molecules as blood substitutes tailored to specific therapeutic applications. Oxygen affinity can be manipulated by amino acid substitutions in the heme pocket [Citation[2]] or on the protein surface [Citation[4], Citation[5]]. A response to the concentration of plasma-Cl− that mimics the effect of 2,3-DPG was also introduced in human Hb. Polymerization of tetrameric Hb prevents the vasoconstriction associated with the infusion of Hb solutions [Citation[7], Citation[8]]. Polymers have been obtained through the formation of intermolecular S—S bonds between cysteine residues introduced on the Hb surface [Citation[2], Citation[3], Citation[9]]. S—S bonds are approximately 2.5 Å long, and their accessibility to the reducing agents present in blood is hindered by the numerous intermolecular interactions that result from the crosslinks.
In a mouse model, transfusion of polymeric hemoglobins reduced the volume of cerebral infarction. This effect was also evident with Hb (Polytaur)n, which has a high oxygen affinity (P50 ≤ ∼ 2.0 torr) and no cooperativity (n = 1) [Citation[2]]. These are the same functional characteristics of myoglobin. Polymers of myoglobin have been constructed and could be a viable alternative to the use of polymeric hemoglobins in some pathologic conditions.
These data indicate the enormous flexibility offered by molecular engineering approaches to the manipulation of the conformational and functional properties of heme-proteins. The rapid development of bioengineering techniques will make possible the large-scale production of recombinant heme-proteins suitable for transfusion free of mammalian infectious agents.
This work was supported by a grant from the National Institute of Neurological Disorders and Stroke (NS-386684) and by the Eugene and Mary B. Meyer Center for Advanced Transfusion Practice and Blood Research at the Johns Hopkins University School of Medicine. The authors are grateful to Tzipora Sofare, MA, for editorial assistance.
REFERENCES
- Karavitis, M., Fronticelli, C., Brinigar, W.S., Vasquez, G.B., Militello, V., Leone, M., Cupane, A. (1998). Properties of human hemoglobin with increased polarity in the α or β heme pocket. Carbonmonoxy derivatives. J. Biol. Chem. 237: 23740–23749.
- Fronticelli, C., Bellelli, A., Brinigar, W.S. (2004). Approaches to the engineering of hemoglobin-based oxygen carriers. Trans. Alt. in Trans. Med. 5: 516–520.
- Bobofchak, K.M., Mito, T., Texel, S.J., Bellelli, A., Nemoto, M., Traystman, R.J., Koehler, R.C., Brinigar, W.S., Fronticelli, C. (2003). A recombinant polymeric hemoglobin with the conformational, functional, and physiological characteristics of an in vivo O2 transporter. Am. J. Physiol. Heart Circ. Physiol. 285: H549–H561.
- Fronticelli, C., Sanna, M.T., Perez-Alvarado, G.C., Karavitis, M., Lu, A.-L., Brinigar, W.S. (1995). Allosteric modulation by tertiary structure in mammalian hemoglobins. Introduction of the functional characteristics of bovine hemoglobin into human hemoglobin by five amino acid substitutions. J. Biol. Chem. 270: 30588–30592.
- Fronticelli, C., Bobofchak, K.M., Karavitis, M., Sanna, M.T., Brinigar, W.S. (2002). Introduction of a new regulatory mechanism into human hemoglobin. Biophys. Chem. 98: 115–126.
- Doherty, D.H., Doyle, M.P., Curry, S.R., Vali, R.J., Fattor, T.J., Olson, J.S., Lemon, D.D. (1998). Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat. Biotechnol. 16: 672–676.
- Sakai, H., Hara, H., Yuasa, M., Tsai,A.G., Takeoka, S., Tsuchida, E., Intaglietta, M. (2000). Molecular dimensions of Hb-based O2 carriers determine constriction of resistance arteries and hypertension. Am. J. Physiol. (Heart Circ. Physiol.) 279: H908–H915.
- Matheson, B., Kwansa, H.E., Bucci, E., Rebel, A., Koehler, R.C. (2002). Vascular response to infusion of non extravasating hemoglobin polymer. J. Appl. Physiol. 93: 1479–1486.
- Fronticelli, C., Arosio, D., Bobofchak, K.M., Vasquez, G.B. (2001). Molecular engineering of a polymer of tetrameric hemoglobins. Proteins 44: 212–222.
- Hughes, G.S., Antal, E.J., Locker, P.K., Francom, S.F., Adams, W.J., Jacobs, E.E. (1996). Physiology and pharmacokinetics of a novel hemoglobin-based oxygen carrier in humans. Crit. Care. Med. 24: 756–764.
- Hughes, G.S., Francom, S.F., Antal, E.J., Adams, W.J., Locker, P.K., Yancey, E.P., Jacobs, E.E. (1995). Hematologic effects of a novel hemoglobin-based oxygen carrier in normal male and female subjects. J. Lab. Clin. Med. 126: 444–451.