Abstract
Hemoglobin vesicle (HbV), a liposomal oxygen carrier containing human hemoglobin, was intravenously infused into rats. After the infusion of saline, the HbV or empty vesicle (EV), numbers of red cells, leukocytes and platelets in peripheral blood were unchanged during the observation period of one week in addition to each time point among three groups. However, the lymphocyte ratio transiently decreased and the granulocyte ratio increased in the HbV and EV groups at 6 h after the infusion. Those changes returned to the initial value one day after the infusion and those were maintained for the subsequent observation period. No dramatic change was seen in the ratio of CD4+/CD8+ T cells.
A transient decrease of the complement titer was observed three days after the infusion of HbV and EV, although the consumption of complement titer was not detected in rat serum by mixing HbV or EV in vitro, indicating that the transient decrease of complement titer in vivo was not due to the consumption of complement due to the interaction with HbV or EV. Multiple infusions of HbV caused the decrease of complement titer only after the first infusion and no allergic reaction was observed. No anaphylactic shock was observed in rats administered with EV several times, while ovalbumin (OVA) sensitized rats died with symptoms of respiratory distress after the second OVA administration. These results indicate that HbV could be administered without serious clinical symptoms or adverse reactions.
INTRODUCTION
Artificial oxygen carriers as a red cell substitute have been developed for their emergency use, capacity for long-term storage, being no requirement of cross-match and pathogen-free blood components. Hemoglobin itself, however, is not applicable as an artificial oxygen carrier because it easily dissociates from a tetramer into a dimer and exhibits nephrotoxicity [Citation[1]]. In addition, hemoglobin is considered to induce vasoconstriction based on the nitric oxide (NO) scavenging mechanism at a site between endothelium cells and the vascular smooth muscle layer [Citation[2], Citation[3]]. Multiple approaches have been taken to modify Hb to resolve these limitations, including chemical crosslinking to prevent dissociation, and polymerization or polyethyleneglycol (PEG) conjugation to increase its molecular mass to avoid penetration into the endothelium gap junction [Citation[1], Citation[4-9]]. Recently, recombinant hemoglobin that has a lower affinity for NO has been developed and showed the absence of vasoconstriction [Citation[10]]. On the other hand, the potential use of microencapsulated-hemoglobin into polymer as a cellular type of artificial oxygen carrier was reported in 1964 [Citation[4]]. Since then, the encapsulation of hemoglobin into a lipid bilayer (i.e. liposome) (liposome-encapsulated hemoglobin; LEH) to make a cellular type oxygen carrier has been developed, and this type of oxygen carrier has been regarded as another approach to prevent side-effects and toxicity of Hb [Citation[11-15]].
The hemoglobin vesicle (HbV) is a human hemoglobin encapsulated into liposomes with a PEG surface modification. We have revealed that HbV exhibits less interaction with platelets [Citation[16], Citation[17]], neutrophils [Citation[18]] and plasma proteins including complement [Citation[19]] in vitro using human blood. HbV was revealed not to activate and consume complement in vitro using human serum, while a similar type of artificial oxygen carrier, LEH, did activate the complement and triggered pseudoallergic reactions [Citation[20-22]]. Therefore, it is of importance to observe whether HbV affects the complement titer in vivo. In addition, we wished to know whether repeated administration of HbV induces an allergic or anaphylactic reaction.
Several studies showed that HbV resuscitated against hemorrhagic shock equivalent to red cell transfusion in model rats [Citation[23], Citation[24]] and rabbits [Citation[25]]. HbV infusion into rats induced minor changes in aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase without deteriorative liver damage, a transient increase of cholesterol and phospholipids and reversible changes of amylase and lipase levels in the plasma [Citation[26]]. Thus, HbV infusion transiently or reversibly affects biochemical parameters in the rat plasma.
In this study, we have investigated the effects of HbV on the hematological characteristics and complement titer using rats. In addition, the immune response after HbV infusion was also examined.
METHODS
HbV
HbV was prepared as previously described [Citation[27], Citation[28]]. Briefly, hemoglobin solution prepared from out-dated red blood cells obtained from blood centers was heated under a CO gas atmosphere to inactivate any possibly contaminating viruses and to remove the stroma and non-hemoglobin proteins [Citation[29]]. After the centrifugation and filtration, hemoglobin solution was mixed with lipids and then extruded through membrane filters with a pore size of 0.22 µm to make liposomes. The lipid composition (mol%) was as follows: dipalmitoyl phosphatidylcholine (DPPC):cholesterol (CHOL):dipalmitoyl-L-glutamate-N-succinic acid (DPEA):polyethylene glycol-conjugated distearoyl phosphatidylethanolamine (PEG5000-DSPE) = 5:5:1:0.033. The mean particle size was 250 nm. All lipids were purchased from Nippon Fine Chemical Co. (Osaka, Japan) except PEG5000-DSPE, which was from NOF Co. (Tokyo, Japan). HbV was suspended in saline and contained 10 g of hemoglobin/dl, 5.7 g lipids/dl and < 0.1 endotoxin unit of lipopolysaccharide/ml. An empty vesicle (EV), which consisted of the same lipid composition as HbV without hemoglobin encapsulation, was also prepared.
Animals and HbV Infusion
WKAH male rats, 8–10 weeks old and weighing 220–300 g, were purchased from Japan SLC (Shizuoka, Japan). Under ether anesthesia, HbV or EV was intravenously infused into rats from the tail vein at top-load. As a control, saline was infused into the rats. The injection volume was 20% of the whole blood volume according to the estimation as follows that the whole blood volume is 56 ml per kg of body weight in rats. For the experiments investigating the anaphylactic reaction, Brown Norway male rats, 8 weeks old and weighing 200–220 g (Japan SLC), were used because of their highly sensitive behavior to allergic and anaphylactic reactions [Citation[30]].
Hematological Analysis
Before and after the infusion of HbV, EV or saline, peripheral blood was collected from the tail vein into a plastic tube coated with EDTA. Numbers of red blood cells (RBC), white blood cells (WBC) and platelets (PLT) were measured using an automatic cell counter (AcT diff; Beckman Coulter, Miami, FL, USA). The leukocyte population was measured with a flow cytometer (EPICS XL; Beckman Coulter) based on forward-and side-scatter plot analysis. The lymphocyte subset was analyzed with the flow cytometer after staining with monoclonal antibodies; PE-labeled anti-CD4 (Beckman Coulter), FITC-labeled anti-CD8b (BD Biosciences, San Jose, CA, USA).
Complement Study
Before and after the infusion of HbV, EV or saline, peripheral blood was collected into the glass tube from the tail vein. Blood was clotted by standing for 1 h at room temperature and then for 1 h at 4°C. Serum was separated from the blood clot by centrifugation at 2,000 g for 20 min at 4°C, followed by additional centrifugation at 15,000 g for 45 min at 4°C to separate the serum from HbV or LE, and then stored at − 80°C until assay.
In another complement experiment in vivo, HbV or saline was administered four times at two-day intervals from the first injection. Blood was collected before each injection and at subsequent time intervals. The serum was prepared as described above and stored at − 80°C until assay.
In the in vitro study, rat serum was prepared from five non-treated rats. The serum was incubated with HbV, EV or saline at a ratio of 80:20 or 60:40 (v/v) at 37°C for 1 h. After centrifugation at 15,000 g for 45 min at 4°C, the supernatants were stored at − 80°C until assay.
The complement titer was measured with a 50% hemolysis assay based on Mayer's method using a commercial kit (New One point CH50 (KW); Japan BCG Supply Co., Tokyo, Japan), which was approved to be applicable to the measurement of rat serum complement [Citation[31]]. The unit of complement titer was expressed as CH50 (U/ml).
Examination of Anaphylactic Reaction
EV was used in this study to avoid interfering heterologous immune responses against human hemoglobin in HbV. Brown Norway rats were intravenously administered with EV (1–2 ml). The second infusion was carried out at 2 weeks, the third infusion was at 4 weeks, and the fourth infusion was at 8 weeks after the first infusion. As a positive control of the anaphylactic reaction model, Brown Norway rats were immunized subcutaneously with ovalbumin (OVA) in combination with the complete Freud's adjuvant. Two weeks after the immunization, OVA was intravenously infused from the tail vein. Clinical behavior, such as fluffiness, tear, blood drain speed and respiration, was observed during and after the infusion.
Statistical Analysis
Experimental differences from the controls (i.e. saline group) among the three groups were assessed with Non-repeated Measures ANOVA followed by the Dunnett test as a post-hoc test. The comparison of two groups was made with the Unpaired Student's t-test. A computer statistics package was utilized for statistical analysis (ystat2004, IGAKUTOSYO Press Co. Ltd., Tokyo, Japan). Values of p < 0.05 were considered significant.
RESULTS
Hematological Changes After HbV Infusion
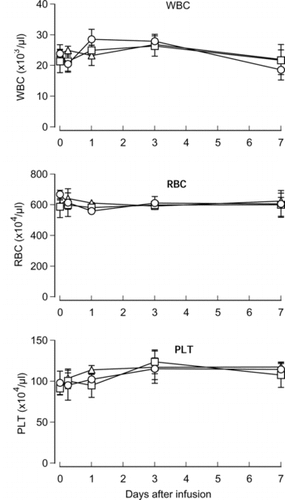
Hematological markers were investigated at 6 h and 1, 3 and 7 days after the infusion of HbV, EV or saline. During the observation period, numbers of RBC, WBC and PLT varied in three groups. However, no significant differences were observed among the three groups at any time point ().
Figure 1 Effects of HbV infusion on peripheral blood cells. HbV, EV or saline was infused into rats at top-load from the tail vein. Blood was sampled and numbers of red cells, white cells and platelets were counted with an automatic cell counter. Triangles, HbV; squares, EV; circles, saline. N = 3–4, mean±SD.
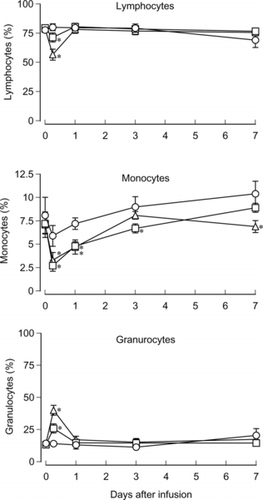
For the leukocyte population, the transient decrease of the lymphocyte ratio and the increase of the granulocyte ratio was significant in the HbV and EV groups at 6 h after the infusion. The 80.1% lymphocyte ratio in the control was decreased to 71.4% in EV and 56.7% in HbV. On the other hand, the 14.0% granulocyte ratio in the control was increased to 25.9% in EV and 40% in HbV. The degrees of the decrease and the increase were significantly greater in the HbV group than the EV group. The ratio of monocytes decreased 6 h after the infusion in three groups and significant differences were observed between the HbV and EV groups and the control group. The decrease of monocytes gradually returned to the initial level. The ratios varied from 2.7% to 10.4% ().
Figure 2 Effects of HbV infusion on the leukocyte population. HbV, EV or saline was infused into rats at top-load from the tail vein. Blood was sampled and ratios of lymphocytes, monocytes and granulocytes were analyzed with the flow cytometer. Triangles, HbV; squares, EV; circles, saline. N = 3–4, mean±SD. *p < 0.05.
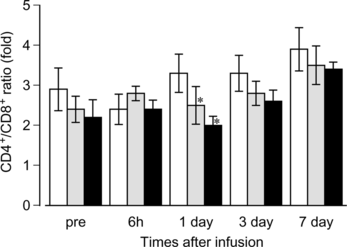
A subset of lymphocytes was analyzed by flow cytometry using monoclonal antibodies. Ratios of T cells and B cells were unchanged in all groups during the observation period (data not shown). As shown in , ratios of CD4+/CD8+ T cells varied from 2.0 to 3.9 of the mean value in the series of experiments. However, statistical significance was not observed in the HbV and EV groups versus control group except on day 1. The mean values of the CD4+/CD8+ ratios in the HbV and EV groups were generally lower than those of the control group.
Figure 3 Effects of HbV infusion on the T cell subset. HbV, EV or saline was infused into rats at top-load from the tail vein. Blood was sampled and ratios of CD4+ or CD8+ T cells were analyzed as described in the Materials and Methods section. Black bars, HbV; gray bars, EV; white bars, saline. N = 3–4, mean±SD. *p < 0.05.
Effects of HbV on the Complement Titer
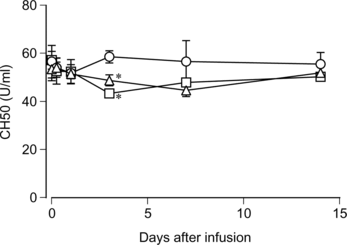
The complement titer in rat serum was assessed before and after the infusion of HbV, EV or saline. As shown in , the complement titer dropped significantly three days after the infusion of HbV or EV, then afterwards it gradually returned to the levels before infusion and of the saline-infused group on day 14. No significant difference was observed between the HbV-infused group and the EV-infused group regarding the degree of decrease.
Figure 4 Changes of the complement titer in the rat serum after HbV infusion. HbV, EV or saline was infused into the rats at top-load from the tail vein. Blood was sampled, and sera were prepared as described in the Materials and Methods section. The complement titer was measured and indicated as CH50. Triangles, HbV; squares, EV; circles, saline. N = 3–4, mean ±SD. *p < 0.05.
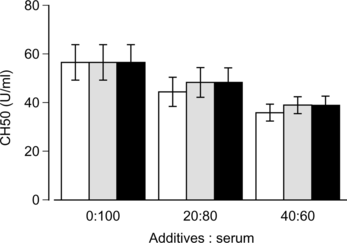
HbV, EV or saline was mixed with rat serum in vitro at a ratio of 20:80 or 40:60 and incubated for 1 h at 37°C. The complement titer decreased in accordance to the dilution of serum by mixing HbV, EV or saline (). No significant difference was observed among three subjects at any mixing ratios, indicating that both HbV and EV did not consume complement.
Figure 5 Effects of HbV on the rat complement in vitro. HbV, EV or saline was mixed with rat sera and incubated at 37°C for 1 hr. After centrifugation, the complement titer in the supernatant was measured. Black bars, HbV; gray bars, EV; white bars, saline. N = 5, mean±SD.
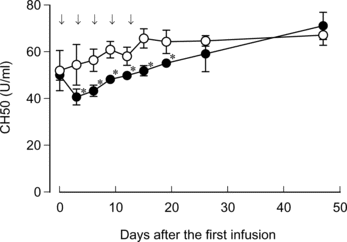
HbV was sequentially infused five times with two-day intervals. The complement titer dropped on day 3 after the first injection (). However, the additional infusion of HbV with 2-day intervals no longer further decreased the complement titer. Two weeks after the final injection, on day 26, no significant difference was observed in the complement titer between two groups.
Figure 6 Effects of repeated infusion of HbV on the rat serum complement. HbV or saline was infused into rats at top-load from the tail vein at the time point as indicated by arrows. Blood sampling, sera preparation and complement measurement were carried out as described in the Materials and Methods section. Closed circles, HbV; open circles, saline. N = 3–5, mean±SD. *p < 0.05.
Assessment of Anaphylactic Reaction Caused by EV
Because HbV contains human hemoglobin, which may act as an antigen in rats, EV was used to assess the potential to induce anaphylactic reaction caused by liposome components. No abnormal behavior, such as fluffiness and tear, was observed in the EV-infused rats and the respiration was kept normal. All animals were alive in the EV-infused group even after the final injection with symptomless (). OVA administration was performed to evaluate whether this rat model is appropriate to assess anaphylactic reaction. Rats were sensitized subcutaneously with OVA usi'plete Freud's adjuvant to obtain complete sensitization. Two weeks after the sensitization, OVA was intravenously infused from the tail vein. Immediately after the infusion, all rats were affected with respiratory distress and the blood drain speed dropped, resulting in death.
Table 1. Lack of anaphylactic shock in rats infused with EV
DISCUSSION
In the previous study, Rabinovici et al. reported that LEH induced a hematological response in rats, such as an increase of WBC and a decrease of PLT within 5–25 min after the infusion, but these changes returned to basal levels 2 h after the infusion [Citation[32]]. However, we did not observe the effects of HbV infusion in such a short period, and the numbers of RBC, WBC and PLT were unchanged in the three groups during the observation period of one week . Although the numbers of WBC did not differ among the three groups, a significant change of the ratios of lymphocytes and granulocytes was observed at 6 h after the infusion (). The degree of these changes was greater in the HbV group than in the EV group. Similarly, Rudolph et al. reported that the polymorphonuclear leukocyte counts were increased and lymphocyte counts were decreased 2 h after the LEH infusion, except that their change was correlated with the increase of WBC [Citation[33]]. We also observed that the ratios of monocytes dropped 6 h after the infusion, even in the saline group, and gradually returned to the initial level. However, the reasons for these phenomena are unexplainable at present.
The ratio of CD4+ T cells and CD8+ T cells is an important indicator of the immune system in subjects. In immune compromised patients, such as AIDS, an inversion of the CD4+/CD8+ ratio is a typical event due to an increase in the absolute number of CD8+ cells [Citation[34]]. In this study, the CD4+/CD8+ ratio was not inverted and was constant in each group during the observation period (). Although a significant difference between the HbV and EV groups and control group was observed on day 1, the implication of this significance for an immune response may be less important because the CD4+/CD8+ ratio was not inverted and lower in HbV and EV groups than in the control group, even at the pre-infusion time, due to unknown reasons.
As mentioned in the previous study [Citation[19]], the influence of liposomes on the complement is a considerable subject to assess the safety of liposomal therapeutics. The complement plays a role in subjects not only as a defense system, but also as causal agents for adverse reactions when it is activated in excess. The considerable characters of liposomes and other things are as follows: negatively charged surface [Citation[35-37]], cholesterol contents [Citation[35], Citation[38]], and the existence of natural anti-phospholipid antibodies in subjects [Citation[20]]. Complement activation induces a pseudoallergic reaction in the pig model [Citation[21], Citation[22]], and hypotension, flushing, respiratory distress, decrease of mean arterial pressure and chest pain in humans [Citation[39], Citation[40]]. We previously reported that HbV does not activate the complement in vitro using human serum [Citation[19]]. In this study, we examined the effect of HbV on the complement using rats in vivo and in vitro. The in vitro study showed that the complement titer in the HbV group was equivalent to that in the control group, indicating that HbV did not consume the complement of rat serum (). The in vivo study, however, showed that the complement titer dropped by 9% on day 3 and 14% on day 7 after the HbV infusion, and then returned to the initial level (). No allergic reaction was observed in these rats. In contrast, another study reported that complement consumption in vivo occurred immediately (i.e. within 10–120 min) in rats after the infusion of LEH, which has the ability to activate complement in vitro [Citation[41]]. Therefore, the gradual drop of complement titer in rats in this study is unlikely to be due to the complement consumption, but some impairment in the metabolic or synthetic function of complement may be underlying the mechanisms of this phenomenon.
To further examine the decline of the complement titer by HbV, a repeated-infusion study was performed. Additional HbV was infused at two-day intervals because the decline of complement titer was remarkable on day 3 after the HbV infusion. Very interestingly, only the first infusion reduced the complement titer and, thereafter, the recovery of the complement titer occurred gradually despite additional HbV being infused (). Namely, the additional HbV infusion did not induce the further decline of the complement titer. These results also indicate that the transient drop of complement titer is not due to the complement consumption that resulted from the direct interaction between HbV and the complement components. As mentioned above, the gradual drop of the complement titer is probably due to yet unknown mechanisms other than consumption. However, such unknown mechanisms were not enhanced by the additional HbV infusion. Therefore, the precise mechanisms of the decrease of the complement titer induced by HbV infusion remain to be clarified with great interest.
To investigate whether HbV would induce anaphylactic or pseudoallergic reactions, rats were sensitized several times with EV at appropriate intervals. Because HbV would be administered intravenously in clinical settings, the first administration of EV was performed intravenously. On the other hand, OVA as a positive control was subcutaneously administered with the adjuvant to obtain a complete immunization. Although three administrations were carried out after the first EV infusion, no clinical modulation was observed while the OVA-sensitized rats resulted in death after the second administration. These results suggest that HbV may induce neither anaphylactic nor allergic reaction in subjects, possibly also including humans.
Finally, HbV induced a transient change in the leukocyte population, but not in the RBC, WBC or PLT counts. In addition, the CD4+/CD8+ ratio was maintained at a reasonable level without the inversion of the ratio. Although the complement titer transiently dropped on day 3 after the HbV infusion, the decline was not considered to be the complement consumption and the degree of the decline is unlikely to affect host defense. In addition, repeated HbV infusion also revealed that the influence of HbV on complement was transient and an additional four HbV infusions did not induce the further decline of the complement titer. Furthermore, no anaphylactic reaction was observed in multiple EV infusions. In conclusion, from a clinical point of view, HbV induced no serious side-effects regarding the subjects in this study, and is a promising material to be safely administrable as an artificial oxygen carrier.
This study was supported, in part, by a Health Science Research Grant (Artificial Blood Project) from the Ministry of Health and Welfare, Japan, and by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports and Culture.
REFERENCES
- Bunn, H.F., Esham, W.T., Bull, R.W. (1969). The renal handling of hemoglobin. I. Glomerular filtration. J. Exp. Med. 129: 909–923.
- Martin, W., Villani, G.M., Jothianandan, D., Furchgott, R.F. (1985). Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J. Pharmacol. Exp. Ther. 232: 708–716.
- Hess, J.R., MacDonald, V.W., Brinkley, W.W. (1993). Systemic and pulmonary hypertension after resuscitation with cell-free hemoglobin. J. Appl. Physiol. 74: 1769–1778.
- Chang T.M. (1964). Semipermeable microcapsules. Science. 146: 524–525.
- Chang T.M. (1971). Stabilisation of enzymes by microencapsulation with a concentrated protein solution or by microencapsulation followed by cross-linking with glutaraldehyde. Biochem. Biophys. Res. Commun. 44:1531–1536.
- Chatterjee, R., Welty, E.V., Walder, R.Y., Pruitt, S.L., Rogers, P.H., Arnone, A., Walder, J.A. (1986). Isolation and characterization of a new hemoglobin derivative cross-linked between the a chains (lysine 99a1-lysine 99a2). J. Biol. Chem. 261: 9929–9937.
- DeVenuto, F., Zegna, A. (1983). Preparation and evaluation of pyridoxalated-polymerized human hemoglobin. J. Surg. Res. 34: 205–212.
- Ajisaka, K., Iwashita, Y. (1980). Modification of human hemoglobin with polyethylene glycol: a new candidate for blood substitute. Biochem. Biophys. Res. Commun. 97: 1076–1081.
- Lieberthal, W., Fuhro, R., Andry, C., Valeri, C.R. (2000). Effects of hemoglobin-based oxygen-carrying solutions in anesthetized rats with accute ischemic renal failure. J. Lab. Clin. Med. 135: 73–81.
- Raat, N.J., Liu, J.F., Doyle, M.P., Burhop, K.E., Klein, J., Ince, C. (2005). Effects of recombinant-hemoglobin solutions rHb2.0 and rHb1.1 on blood pressure, intestinal blood flow, and gut oxygenation in a rat model of hemorrhagic shock. J. Lab. Clin. Med. 145: 9–11.
- Djordjevich, L., Miller, I.F. (1980). Synthetic erythrocytes from lipid encapsulated hemoglobin. Exp. Hematol. 8: 584–592.
- Gaber, B.P., Farmer, M.C. (1984). Encapsulation of hemoglobin in phospholipid vesicles: Preparation and properties of a red cell surrogate. Prog Clin Biol Res. 165: 179–190.
- Hunt, C.A., Burnette, R.R., MacGregor, R.D., Strubbe, A.E., Lau, D.T., Taylor, N., Kiwada, H. (1985). Synthesis and evaluation of a prototypal artificial red cell. Science 230: 1165–1168.
- Rudolph, A.S., Klipper, R.W., Goins, B., Phillips, W.T. (1991). In vivo biodistribution of a radiolabeled blood substitute: 99mTc-labeled liposome-encapsulated hemoglobin in an anesthetized rabbit. Proc Natl Acad Sci USA. 88: 10976–10980.
- Phillips, W.T., Klipper, R.W., Awasthi, V.D., Rudolph, A.S., Cliff, R., Kwasiborski, V., Goins, B.A. (1999). Polyethylene glycol-modified liposome-encapsulated hemoglobin: A long circulating red cell substitute. J. Pharmacol Exp. Ther. 288: 665–670.
- Wakamoto, S., Fujihara, M., Abe, H., Sakai, H., Takeoka, S., Tsuchida, E., Ikeda, H., Ikebuchi, K. (2001). Effects of poly(ethyleneglycol)-modified hemoglobin vesicles on agonist-induced platelet aggregation and RANTES release in vitro. Artif. Cells Blood Substit. Immobil. Biotechnol. 29: 191–201.
- Wakamoto, S., Fujihara, M., Abe, H., Yamaguchi, M., Takeoka, S., Tsuchida, E., Azuma, H., Ikeda, H. (2005). Effects of hemoglobin vesicles on resting and agonist-stimulated human platelets in vitro. Artif. Cells Blood Substit. Biotechnol. 33: 101–111.
- Ito, T., Fujihara, M., Abe, H., Yamaguchi, M., Wakamoto, S., Takeoka, S., Sakai, H., Tsuchida, E., Ikeda, H., Ikebuchi, K. (2001). Effects of poly(ethyleneglycol)-modified hemoglobin vesicles on N-formyl-methionyl-leucyl-phenylalanine-induced responses of polymorphonuclear neutrophils in vitro. Artif. Cells Blood Substit. Immobil. Biotechnol. 29: 427–437.
- Abe, H., Fujihara, M., Ikebuchi, K., Takeoka, S., Tsuchida, E., Harashima, H., Azuma, H., Ikeda, H. (2006). Interaction of hemoglobin vesicles, a cellular-type artificial oxygen carrier, with human plasma: Effects on coagulation, kallikrein-kinin, and complement systems. Artif. Cells Blood Substit. Biotechnol. 34: 1–10.
- Szebeni, J., Wassef, N.M., Rudolph, A.S., Alving, C.R. (1996). Complement activation in human serum by liposome-encapsulated hemoglobin: The role of natural anti-phospholipid antibodies. Biochim. Biophys. Acta 1285: 127–130.
- Szebeni, J., Baranyi, L., Savay, S., Bodo, M., Morse, D.S., Basta, M., Stahl, G.L., Bunger, R., Alving, C.R. (2000). Liposome-induced pulmonary hypertension: Properties and mechanism of a complement-mediated pseudoallergic reaction. Am. J. Physiol. Heart Circ. Physiol. 279: H1319–H1328.
- Laverman, P., Boerman, O.C., Oyen, W.J.G., Corstens, F.H.M., Storm, G. (2001). In vivo applications of PEG liposomes: Unexpected observations. Crit. Rev. Ther. Drug Carrier Syst. 18: 551–566.
- Sakai, H., Takeoka, S., Wettstein, R., Tsai, A.G., Intaglietta, M., Tsuchida, E. (2002). Systemic and microvascular responses to hemorrhagic shock and resuscitation with Hb vesicles. Am. J. Physiol. Heart Circ. Physiol. 283: H1191–1199.
- Sakai, H., Masada, Y., Horinouchi, H., Yamamoto, M., Ikeda, E., Takeoka, S., Kobayashi, K., Tsuchida, E. (2004). Hemoglobin-vesicles suspended in recombinant human serum albumin for resuscitation from hemorrhagic shock in anesthetized rats. Crit. Care Med. 32: 539–545.
- Yoshizu, A., Izumi, Y., Park, S., Sakai, H., Takeoka, S., Horinouchi, H., Ikeda, E., Tsuchida, E., Kobayashi, K. (2004). Hemorrhagic shock resuscitation with an artificial oxygen carrier, hemoglobin vesicle maintains intestinal perfusion and suppresses the increase in plasma tumor necrosis factor-alpha. ASAIO J. 50: 458–463.
- Sakai, H., Horinouchi, H., Masada, Y., Takeoka, S., Ikeda, E., Takaori, M., Kobayashi, K., Tsuchida, E. (2004). Metabolism of hemoglobin-vesicles (artificial oxygen carriers) and their influence on organ functions in a rat model. Biomaterials. 25: 4317–4325.
- Sakai, H., Takeoka, S., Park, S.I., Kose, T., Nishide, H., Izumi, Y., Yoshizu, A., Kobayashi, K., Tsuchida, E. (1997). Surface modification of hemoglobin vesicles with poly(ethylene glycol) and effects on aggregation, viscosity, and blood flow during 90% exchange transfusion in anesthetized rats. Bioconjug. Chem. 8: 23–30.
- Sou, K., Naito, Y., Endo, T., Takeoka, S., Tsuchida, E. (2003). Effective encapsulation of proteins into size-controlled phospholipid vesicles using freeze-thawing and extrusion. Biotechnol. Prog. 19: 1547–1552.
- Abe, H., Ikebuchi, K., Hirayama, J., Fujihara, M., Takeoka, S., Sakai, H., Tsuchida, E., Ikeda, H. (2001). Virus inactivation in hemoglobin solution by heat treatment. Artif. Cells Blood Substit. Immobil. Biotechnol. 29: 381–388.
- Singh, P., Daniels, M., Winsett, D.W., Richards, J., Doerfler, D., Hatch, G., Adler, K.B., Gilmour, M.I. (2003). Phenotypic comparison of allergic airway responses to house dust mite in three rat strains. Am. J. Physiol. Lung Cell Mol. Physiol. 284: L588–L598.
- Kurebayashi, Y., Honda, Y. (1991). Protection by 16,16-dimethyl prostaglandin E2 and dibutyryl cyclic AMP against complement-mediated hepatic necrosis in rats. Hepatology 14: 545–550.
- Rabinovici, R., Rudolph, A.S., Feuerstein, G. (1989). Characterization of hemodynamic, hematologic, and biochemical responses to administration of liposome-encapsulated hemoglobin in the conscious, freely moving rat. Circulatory Shock 29: 115–132.
- Rudolph, A.S., Cliff, R.O., Spargo, B.J., Spielberg, H. (1994). Transient changes in the mononuclear phagocyte system following administration of the blood substitute liposome-encapsulated haemoglobin. Biomaterials 15: 796–804.
- Cooper, D.A., Tindall, B., Wilson, E.J., Imrie, A.A., Penny, R. (1988). Characterization of T lymphocyte responses during primary infection with human immunodeficiency virus. J. Infect. Dis. 157: 889–896.
- Cunningham, C.M., Kingzette, M., Richards, R.L., Alving, C.R., Lint, T.F., Gewurz, H. (1979). Activation of human complement by liposomes: A model for membrane activation of the alternative pathway. J. Immunol. 122: 1237–1242.
- Chonn, A., Cullis, P.R., Devine, D.V. (1991). The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. J. Immunol. 146: 4234–4241.
- Devine, D.V., Wong, K., Serrano, K., Chonn, A., Cullis, P.R. (1994). Liposome-complement interactions in rat serum: Implications for liposome survival studies. Biochim. Biophys. Acta 1191: 43–51.
- Alving, C.R., Richards, R.L., Guirguis, A.A. (1977). Cholesterol-dependent human complement activation resulting in damage to liposomal model membranes. J. Immunol. 118: 342–347.
- Laing, R.B., Milne, L.J., Leen, C.L., Malcolm, G.P., Steers, A.J. (1994). Anaphylactic reactions to liposomal amphotericin. Lancet 344: 682.
- Ringden, O., Andstrom, E., Remberger, M., Svahn, B.M., Tollemar, J. (1994). Allergic reactions and other rare side-effects of liposomal amphotericin. Lancet 344: 1156–1157.
- Szebeni, J., Wassef, N.M., Spielberg, H., Rudolph, A.S., Alving, C.R. (1994). Complement activation in rats by liposomes and liposome-encapsulated hemoglobin: Evidence for anti-lipid antibodies and alternative pathway activation. Biochem. Biophys. Res. Commun. 205: 255–263.