Abstract
Exogenous surfactant therapy based on animal lung extract preparations has been developed successfully for the treatment of neonatal respiratory distress syndrome. However, because of the inherent limitations of these natural preparations, the development of new synthetic surfactants is a major objective. We report here that a perfluorocarbon gas (perfluorooctyl bromide, gPFOB) inhibits the formation of the semi-crystalline domains that occur during compression of a Langmuir monolayer of dipalmitoyl phosphatidylcholine (DPPC), taken as a simplified model of lung surfactant. gPFOB also facilitates the re-spreading of the DPPC monolayer. These results suggest that PFOB, a fluorocarbon already investigated for oxygen delivery, may be useful in lung surfactant replacement compositions.
INTRODUCTION
The absence of lung surfactant (LS) or an alteration of the quantitative or qualitative composition result in numerous severe conditions such as neonatal respiratory distress syndrome (NRDS) or acute respiratory distress syndrome (ARDS) [Citation[1]]. LS is a lipoprotein complex that covers the alveolar surface of all mammalian lungs. By strongly reducing the surface tension at the air/water interface, LS allows alveolar ventilation and gas exchange at physiological transpulmonary pressures and prevents alveoli from collapsing during expiration [Citation[2-4]]. LS is secreted into the alveolar space by epithelial type II pneumocytes via exocytosis [Citation[5], Citation[6]]. It is a complex mixture of ∼ 85% phospholipids, ∼ 5% neutral lipids and ∼ 10% proteins (SP-A, SP-B, SP-C and SP-D) [Citation[7], Citation[8]]. Dipalmitoyl phosphatidylcholine (DPPC) is the most abundant phospholipid of LS and is responsible for surface tension reduction. However, DPPC is a poor LS when used alone because it forms, upon compression (expiration), semi-crystalline domains of liquid condensed (LC) phase at the alveolus/air interface, and these LC domains do not re-spread during expansion (inspiration) [Citation[2-4]]. The unsaturated and anionic phospholipids (such as phosphatidylglycerols, PGs) and hydrophobic proteins (SP-B, SP-C) compensate for these limitations by decreasing the surface viscosity of the DPPC monolayer [Citation[9]]. SP-B was also shown to induce a reversible folding transition at monolayer collapse, allowing all the components of LS to remain at the interface during inspiration [Citation[4]].
Several LS substitutes based on animal lung extract preparations have been developed for the treatment of NRDS [Citation[10]]. However, the development of such natural LS substitutes for the treatment of other pulmonary diseases, such as ARDS, is limited, due to inherent immunological risks. Other drawbacks include potential viral contamination and a costly purification procedure. Therefore, there is a clear need for alternative, synthetic LS substitutes [Citation[2-4], Citation[9], Citation[11]].
Because of their high biological inertness, remarkable ability to solubilize oxygen and extremely low solubility in water, perfluorocarbons (PFCs) have potential in medicine and biomedical research [Citation[12-16]]. PFCs have been investigated for intravascular oxygen transport and for the stabilization of gaseous microbubbles used as contrast agents in ultrasound imaging [Citation[13], Citation[14], Citation[14a]]. Partial liquid ventilation (PLV) with PFCs has been explored as a treatment of the respiratory distress syndrome (RDS) [Citation[17-20]]. Improved oxygenation and lung compliance were achieved in preterm animal models [Citation[17]], as well as in premature infants [Citation[18], Citation[19]]. PFC-based PLV was also reported to have an anti-inflammatory effect in the alveolar environment of trauma patients that may contribute to the protective role of PFCs in injuries associated with local activation of inflammatory processes [Citation[21], Citation[22]]. Delivery of vaporized PFCs to oleic acid-injured ARDS sheeps resulted in significant and sustained improvements of gas exchange and of lung compliance [Citation[23], Citation[24]]. Although these results suggest that PFCs may be useful in pulmonary disease therapy, no study aiming at determining the influence of PFCs on lung surfactant or lung surfactant models appears to have been reported so far.
We have investigated the effects of gaseous perfluorooctyl bromide (gPFOB) on the physical state of a Langmuir DPPC monolayer taken as a simplified model of LS. Compression isotherms, fluorescence microscopy (FM) and grazing incidence X-ray diffraction (GIXD) were used to determine the influence of gPFOB on the morphology and degree of order of the semi-crystalline domains that form upon compression in the DPPC monolayer [Citation[25], Citation[26]]. The PFC selected for this study, PFOB, was chosen among the PFCs most thoroughly investigated for biomedical applications, in particular for intravascular oxygen transport [Citation[16]]. This proceeding is based on a lecture given at the Xth Int. Symp. on Blood Substitutes in Providence in June 2005.
MATERIALS AND METHODS
Materials
PFOB was kindly provided by Alliance Pharmaceutical Corp. (San Diego, CA, USA). L-α-1,2-dipalmitoyl-sn-3-glycero-phosphatidylcholine (DPPC, purity > 99%) was purchased from Sigma. Water was purified using a Millipore system (pH = 5.5; surface tension: 72.1 mN m−1 at 20°C, resistivity: 18 MΩcm). The fluorescent dye (2-[6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino] hexanoyl-1-hexadecanoyl-sn-glycero-3-phosphocholine, NBDC6-HPC) was purchased from Molecular Probes (Eugene, OR, USA).
Methods
Compression of DPPC Monolayers Under gPFOB
Surface pressure, π, versus molecular area, A, isotherms were recorded on a Langmuir minitrough (Riegler & Kirstein, Potsdam, Germany) equipped with two movable barriers (speed: 2.0 mm min−1). The surface pressure was measured using the Wilhelmy plate method. The trough was enclosed in a gas-tight box (volume = 9 L). The gas-tight box was flushed either with pure N2 or with N2 saturated with PFOB. In the latter case, N2 was allowed to bubble at room temperature through the liquid PFOB before being flushed into the gas-tight box. The flow rate of the gas phase (N2 or N2 saturated with PFOB) was set to 1.2 L min−1. The evaporation rate of PFOB was then ∼ 6 mL h−1. The errors on π and A were ±0.5 mN m−1 and ±1 Å2, respectively. Spreading solutions of DPPC were prepared in chloroform (analytical grade).
Fluorescence Microscopy
Fluorescence microscopy (FM) was achieved with the above balance equipped with an Olympus fluorescence microscope (20 × -power objective) mounted on a xy translation stage, which allows scanning of the trough over different regions. An Olympus 100 W high-pressure mercury lamp was used for excitation. A dichroic mirror/barrier filter assembly was used to filter and direct the excitation light onto the monolayer (450–490 nm) and to filter out the emitted fluorescence (520 nm). The emitted fluorescence was collected by the objective and detected via a Hamamatsu intensified camera. The microscope was linked to the gas-tight box through an extensible gusset. The surface pressure was kept constant during FM experiments. The fluorescent dye NBDC6-HPC was used at a lipid mole ratio of 1%. The fluorescent dye segregates from ordered to disordered regions [Citation[27]]. As a result, LC domains appear dark on the fluorescence images.
Grazing Incidence X-ray Diffraction (GIXD)
GIXD experiments were achieved at the D41B beamline of the LURE-DCI synchrotron source (Orsay, France). The X-ray wavelength λ = 1.646 Å of the incoming X-ray beam was selected using a focusing Ge (111) monochromator. The grazing angle of incidence θi = 2.08 mrad was set slightly below the critical angle for total external reflection of the X-rays at the air/water interface (about 2.8 mrad at 1.646 Å). Under these conditions, an evanescent wave is formed that is scattered by the monolayer. If the monolayer is ordered (i.e. crystalline) the wave is diffracted and Bragg peaks are obtained [Citation[28]]. For the acquisition of the diffracted intensity, we used a new setup composed of a two-dimensional detector and a single vertical slit positioned between the sample and the detector [Citation[29]]. The resulting qxy resolution was 0.007 Å−1 for the q-range explored here. The shape of the Bragg rods gave information about the tilt angle t and tilt azimuth φ [Citation[30]]. In the following, the rectangular description of the chain lattice will be used. The diffraction pattern exhibits two peaks. The peak located at low qxy corresponds to the degenerate [Citation[11]] and [i1] out-of-plane reflections, and the other peak to the [02] in-plane reflection. Since the maximum of intensity along the Bragg rods [Citation[11]] and [i1] is located out of the plane and in the scattering plane along the Bragg rod [02], the chains are tilted to the nearest neighbor. Thus, the observed phase is L2d (t ≠ 0, φ = 0), according to the nomenclature introduced in reference [Citation[28]].
RESULTS AND DISCUSSION
The isotherms of the DPPC monolayer compressed at 26°C on a pure water subphase under an atmosphere of N2 or N2 saturated with gPFOB are shown in . When compressed under pure N2, DPPC undergoes a first order phase transition from a liquid-expanded (LE) phase to a liquid-condensed (LC) phase at a surface pressure π ∼ 13 mN m−1.The LE/LC coexistence region is evidenced by the presence of a plateau on the isotherm and by the formation of discrete domains of LC phase within a continuous LE phase, as visualized by FM (, insert a). This observation is in agreement with previous work [Citation[31]]. When π increases, the LC domains increase in size, become more numerous and progressively merge into a continuous LC phase.
Figure 1 Compression isotherm (surface pressure (π)/molecular area (σ)) of DPPC measured at 26°C under an atmosphere of N2 (dashed line) and N2 saturated with PFOB (solid line). Fluorescence images of a) the DPPC monolayer at 15 mN m−1, showing the crystalline domains and b) the DPPC monolayer in contact with gPFOB (30 mN m−1).
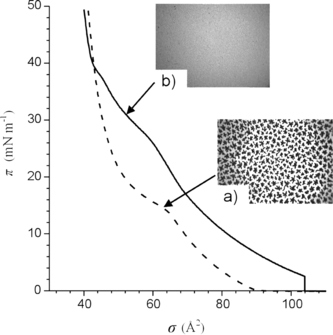
Compressing the DPPC monolayer under a N2 atmosphere saturated with gPFOB changes the phase behavior drastically. The transition observed at ∼ 13 mN m−1 () has disappeared. The compression isotherm is now characterized by two kinks at ∼ 28 and ∼ 38 mN m−1. Below ∼ 38 mN m−1, the isotherm is shifted towards the large molecular areas, which indicates that PFOB molecules are incorporated into the DPPC monolayer. It is also noteworthy that, even at the beginning of the compression, the surface pressure is not zero, as in the case of pure DPPC, but ∼ 2 mN m−1, which also shows that FC molecules are inserted into the DPPC monolayer. The new transition seen at ∼ 28 mN m−1 is no longer of the LE / LC type, as assessed by the fluorescence images that are bright and featureless, not only at 30 mN m−1 (, insert b), but up to 38 mN m−1. This suggests a conformational change of the PFOB molecules inserted in the DPPC monolayer. For π higher than ∼ 38 mN m−1, the isotherm becomes steeper and the limiting area (∼ 50 Å2) is very comparable to the limiting area of DPPC compressed in absence of gPFOB, which indicates that the PFOB molecules are progressively squeezed out on the top of the DPPC monolayer. At such high surface pressures, FM images show the presence of very small crystalline domains, suggesting that the LE/LC transition occurred at ∼ 38 mN m−1. It is noteworthy that for π higher than 38 mN m−1, i.e., after the squeeze out of the PFOB molecules, only small LC domains are to be seen, as compared to the practically continuous LC phase observed for DPPC under pure N2 at the same π values. It is likely that the presence of a thin liquid film of PFOB on top of the DPPC monolayer disorganizes the DPPC molecules and contributes to the fluidization process at high surface pressures. These experiments demonstrate that gPFOB molecules interact with DPPC molecules, prevent the formation of the LC phase until high values of lateral pressure, and hence induce a fluidizing effect in the monolayer.
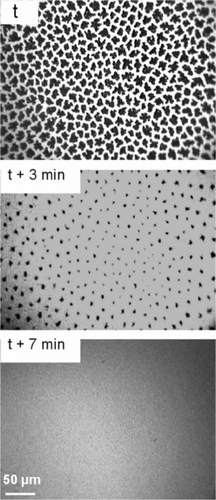
In order to assess the effect of gPFCs on LC domains that are already formed, a DPPC monolayer was first compressed to 13 mN m−1,gPFOB-saturated N2 was then allowed to flush the gas-tight box that encloses the through, the surface pressure being maintained at 13 mN m−1. The fluorescence images () clearly show that, 3 min after the introduction of gPFOB, the LC domains have become significantly smaller. After 7 min, these domains have totally disappeared, indicating that the DPPC monolayer has become totally fluid.
Figure 2 Fluorescence images of a DPPC monolayer compressed at 13 mN m−1 under N2 (upper image). At time t, the atmosphere of N2 above the monolayer started to be saturated with gPFOB. One can see that the LC domains progressively disappear with time. After 7 min, the monolayer is totally homogenous and fluid.
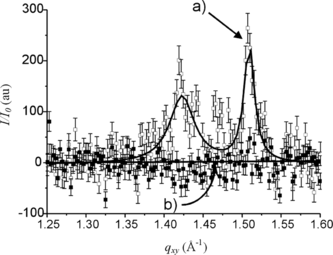
Fluorescence microscopy provides information on a local scale. Small crystalline domains present at the interface may escape from the investigation field and be not taken into account. In order to have a definitive, independent assessment of any possible crystalline regions present in the monolayer, we therefore performed grazing incidence X-ray diffraction experiments (GIXD) using synchrotron radiation. a shows the integrated diffracted intensity (I/I0) as a function of the in-plane component of the scattering wave vector (qxy) of a DPPC monolayer compressed at 20 mN m−1. At this surface pressure, two Bragg peaks were obtained indicating a rectangular unit cell (NN tilted, L2d phase). The calculated area per chain of ∼ 23Å2 and the tilt angle obtained from Bragg rod analysis are in agreement with published data for DPPC. When the He atmosphere was saturated with gPFOB, the diffraction peaks disappeared within a few minutes (b), establishing the dissolution of the LC domains and the rapid re-spreading of the DPPC molecules. Furthermore, when pure He was subsequently allowed to flush the gastight box, the surface pressure being maintained at 20 mN m−1, the two diffraction peaks were seen to slowly build up, evidencing the re-formation of the LC domains. This establishes that PFOB molecules are adsorbed into the DPPC monolayer and that they inhibit the organization of DPPC molecules into LC domains. When the inflow of gPFOB is stopped while the flow of He is maintained, the PFOB molecules adsorbed at the interface are removed, and the DPPC monolayer then recovers its normal behavior, i.e. crystallizes.
Figure 3 Diffracted intensity of the grazing X rays, I/I0, as a function of the in-plane scattering wave vector, qxy. When compressed at 20 mN m−1, the DPPC monolayer shows the two Bragg peaks characteristic of the tilted LC phase at 1.42 and 1.51 Å−1 (a). When He is saturated with gPFOB the peaks disappear rapidly (b), demonstrating the dissolution of the crystalline LC domains and the rapid re-spreading of the DPPC molecules.
As a conclusion, we have shown that gPFOB demonstrated a highly effective fluidizing effect on the DPPC monolayer. Other fluorocarbons, including perfluorooctylethane, bis(perfluoroalkyl)ethane and perfluorodecalin, were also effective. The insights provided by this study suggest that combinations of PFCs and DPPC may be useful as lung surfactant substitute compositions. Fluorocarbons can easily be delivered to the lungs in the form of reverse emulsions using a metered dose inhalor [Citation[33]]. Studies on Langmuir monolayers of mixtures of DPPC with fluorinated surfactants are also available [Citation[34]35].
The authors thank Alliance Pharmaceutical Corp. (San Diego, CA, USA) for the gift of PFOB.
REFERENCES
- Günther, A., Ruppert, C., Schmidt, R., Markart, P., Grimminger, F., Walmrath, D., Seeger, W. (2001). Respir. Res. 2: 353.
- Zasadsinski, J.A., Ding, J., Warriner, H.E., Bringezu, F., Waring, A.J. (2001). Curr. Opin. Colloid Interface Sci. 6: 506.
- Notter, R.H. (2000). Lung Surfactants: Basic Science and Clinical Applications, Vol. 149. Marcel Dekker: New York.
- Ding, J., Takamoto, D.Y., von Nahmen, A., Lipp, M.M., Lee, K.Y.C., Waring, A.J., Zasadsinski, J.A. (2001). Biophys. J. 80: 2262.
- Wright, J.R., Dobbs, L.G. (1991). Annu. Rev. Physiol. 53: 395.
- Rooney, S.A. (2001). Comp. Bioch. and Physiol. A 129: 233.
- Goerke, J. (1998). Biochim. Biophys. Acta 1408: 79.
- Postle, A.D., Heeley, E.L., Wilton, D.C. (2001). Comp. Bioch. and Physiol. A 129: 65.
- Veldhuizen, R., Nag, K., Orgeig, S., Possmayer, F. (1998). Biochim. Biophys. Acta 1408: 90.
- McDonald, C.L., Ainsworth, S.B. (2004). Curr. Pediatrics 14: 284.
- Kattwinkel, J. (2005). Pediatrics 115: 1075.
- Krafft, M.P. (2001). Adv. Drug Del. Rev. 47: 209–228.
- Riess, J.G. (2001). Chem. Rev. 101: 2797.
- Schutt, E.G., Klein, D.H., Mattrey, R.M., Riess, J.G. (2003). Angew. Chem. Int. Ed. 42: 3218.
- Krafft, M.P., Chittofrati, A., Riess, J.G. (2003). Curr. Opin. Colloid Interface Sci. 8: 251.
- Riess, J.G. (2003). Curr. Op. Colloid Interface Science 8: 259–266.
- Riess, J.G. (2004). in Handbook of Fluorous Chemistry, J.A. Gladysz, I. Horváth, D.P. Curran, Eds. Wiley, VCH, Weinheim, pp. 521–573
- Wolfson, M.R., Kechner, N.E., Roache, R.F., DeChadarevian, J., Friss, H.E., Rubenstein, S.D., Shaffer, T.H. (1998). J. Appl. Physiol. 84: 624.
- Sukumar, M., Bommaraju, M., Fisher, J.E., Morin, F.C., Papo, M.C., Fuhrman, B.P., Hernan, L.J., Leach, C.L. (1998). J. Appl. Physiol. 84: 327.
- Leach, C.L., Greenspan, J.S., Rubenstein, D., Shaffer, T.H., Wolfson, M.R., Jackson, J.C., DeLemos, R., Fuhrman, B.P. (1996). New Engl. J. Med. 335: 761.
- Rotta, A.T., Gunnarsson, B., Hernan, L.J., Fuhrman, B.P., Steinhorn, D.M. (2000). J. Crit. Care 28: 202.
- Croce, M.A., Fabian, T.C., Patton, J.H.J., Melton, S.M., Moore, M., Trenthem, L. (1998). J. Trauma. 45: 273.
- Koch, T., Ragaller, M., Haufe, D., Hofer, A., Grosser, M., Albrecht, D.M., Kotzsch, M., Luther, T. (2001). Anesthesiology 94: 101.
- Bleyl, J.U., Ragaller, M., Tschöh, U., Regner, M., Hübler, M., Kanzow, M., Vincent, O., Albrecht, M. (2002). Crit. Care Med. 30: 1340.
- von der Hardt, K., Schoof, E., Kandler, M.A., Dötsch, J., Rascher, W. (2002). Pediatr. Res. 51: 177.
- Gerber, F., Krafft, M.P., Vandamme, T.F., Goldmann, M., Fontaine, P. (2005). Angew. Chem. Int. Ed. 44: 2749.
- Gerber, F., Krafft, M.P., Vandamme, T.F., Goldmann, M., Fontaine, P. Biophys. J. 90: 3184–3192.
- Knobler, C.M., Desai, R.C. (1992). Annu. Rev. Phys. Chem. 43: 207.
- Kaganer, V.M., Möhwald, H., Dutta, P. (1999). Rev. Mod. Phys. 71: 779.
- Fontaine, P., Goldmann, M., Bordessoule, M., Jucha, A. (2004). Rev. Sci. Instrum. 75: 3097.
- Als-Nielsen, J., Jacquemain, D., Kjaer, K., Leveiller, F., Lahav, M., Leiserowitz, L. (1994). Phys. Rep. 251: 246.
- Lösche, M., Sackmann, E., Möhwald, H. (1983). Ber. Bunsenges Phys. Chem. 87.
- Butz, N., Porté, C., Courrier, H., M.P. Krafft, M.P., Vandamme, T.F. (2002). Int. J. Pharmaceutics 238: 257–269.
- Hiranita, T., Nakamura, S., Kawachi, M., Courrier, H.M., Vandamme, T.F., Krafft, M.P., Shibata, O. (2003). J. Colloid Interface Sci. 265: 83–92.
- Courrier, H.M., Vandamme, T.F., Krafft, M.P., Nakamura, S., Shibata, O. (2003). Colloids Surfaces A. 215: 33–41.