Abstract
The main objective of this study is to develop and evaluate the immobilization of Staphylococcal Protein A on magnetic cellulose microspheres (SPA-MCMS) for immunological capture of IgG. After cloning, expression and separation, SPA was immobilized onto MCMS to prepare a magnetic affinity media subject to the purification of IgGs. The binding capacity, binding time, leakage of SPA and its reproducibility were optimized to improve the binding efficiency with an appropriate amount and recovery of IgG. Rabbit IgG was successfully purified from serum in a single-step by SPA-MCMS with an overall recovery of 73.18% and purity of 90.27%. Therefore, this study effectively illustrated the advantages of magnetic microcarriers for rapid and efficient purification of antibodies. The separation media shows a high potential for the future development of affinity isolation and immunodiagnostic application.
INTRODUCTION
Staphylococcal Protein A (SPA), a cell-wall protein of Staphylococcus aureus, plays an important role in biotechnology and bioscience owing to its specific binding to the Fc portion of Immunoglobulin gamma (IgG) from many mammals [Citation[1]]. SPA technology especially immobilized SPA has the advantages of interacting between immobilized SPA and IgG molecule without interrupting its antigen-binding ability, which exploits great developments in immunological applications [Citation[2], Citation[3]]. Since the introduction of encapsulation of magnetic materials in the 1960s [Citation[4]], there have been commercialized SPA immobilized MMS (SPA-MMS) products applied to a wide variety of biological fields. However, high price and low binding capacity of MMS products limited the large-scale industrial application in affinity chromatography [Citation[5], Citation[6]]. Commercial immobilized Protein A gel displayed excellent specificity as a tool for antibodies purification, but incapable of exerting the applications in immunoassay due to enslaving to affinity column [Citation[7]]. Hence, it is desirable to develop and evaluate an applicable SPA-MMS to achieve good performance/price effective for both analysis and isolation.
Magnetic cellulose microspheres (MCMS) exhibit good characteristics as biological materials with highly favorable biocompatibility and preferred efficiency [Citation[8]]. Abundant free hydroxyl groups appeared on MCMS can be easily activated and coupled with adequate ligands for the long-lasting and repetitive use. At present, MCMS are mainly applied for the immobilization of enzymes in enzymatic oxidation of substrates [Citation[9], Citation[10]], medical immunoassays [Citation[11], Citation[12]] and affinity purification of biomaterials [Citation[13]]. In this article, we prepared and characterized SPA immobilized MCMS (SPA-MCMS) for the purification of antibodies. Favorable price/performance of SPA-MCMS predicted the potential application of immobilization SPA technology for further development of immunological and biochemical research.
MATERIALS AND METHODS
Materials
Staphylococcus aureus Cowan I (ATCC 6538) was kindly provided by Prof. JinYing Chen (Tianjin Medical University, Tianjin China). Restriction enzymes were purchased from Sino–American Biotechnology Co., Ltd (Luoyang, China). All other reagents for cloning and expression of Protein A were obtained from Beijing DingGuo Biotechnology Development Center (Beijing, China). Purified cotton was obtained from a local store in Tianjin. Standard IgG from different species, bovine serum albumin (BSA), O-phenylenediamine (OPD), diaminobenzidine (DAB) and other immunological reagents were purchased from Tianjin Haoyang Biotechnology Manufacture (Tianjin, China)
SPA Cloning
The full-length SPA gene was amplified using the primers bearing different restriction sites for the construction of pET 21a (+). The sequences of forward and reverse primers were 5′ CGCGAATTCATGTTGAAAAAGAAAAACATTTATTC 3′ and 5′ TATCTCGAGTTATTTTGGTGCTTGAGCATCGT 3′ bearing EcoR I and Xho I restriction sites (underlined in bold), respectively. The PCR were carried out using thermal profile starting with 5 min denaturation at 95°C, followed by 30 cycles consisting of 94°C (1 min), 52°C (1 min), 72°C (1 min) with an additional extension time at 72°C (10 min) on a T-gradient PCR thermocycler (Biometra, Germany).
The amplified 981-bp products were inserted into multiple cloning sites of pET 21a (+) and the recombinant plasmid was transformed into E. coli DH 5α. The inserts from the obtained clones were sequenced using an automatic DNA sequencer (ABI prism, USA). Without mutations, the recombinant plasmid was then introduced into E. coli BL21 (DE3), and selected on Luria-Bertani (LB) agarose plates supplemented with ampicillin (100 µg/mL) for the selection of pET 21a (+) derivatives, pET-SPA.
Expression and Purification Studies
After optimization of various process parameters including subculture time, amount of IPTG added and induction time for maximal SPA production, the transformed E. coli cells were cultured at 37°C in 5 mL of fresh LB medium containing 100 µg/mL of ampicillin for 10–12 h. The freshly grown cultures were subcultured in larger volumes until an optical density (OD) value of 0.5 at 600 nm was obtained. The addition of IPTG to a final concentration of 0.03 mM induced the culture, which was continuously shaken at 37°C for about 3 h. The induced cells were harvested by centrifugation at 4, 000 g for 20 min at 4°C, and suspended in phosphate-buffered saline (PBS) (pH 7.4) for ultrasonic treatment with Ultrasonic Cell Disrupter System (JY88-II, Scientz, China) to obtain a clear lysate. The lysate was centrifuged at 20, 000 g for 20 min at 4°C. The supernatant was applied to IgG-conjugated sepharose affinity column (Amersham, UK) at a flow rate of 0.5 mL/min and the column was washed with 5 bed volumes of PBS. Elution of the bound SPA was carried out with 0.1 M Gly-HCl buffer (pH 2.5) and the eluates were pooled, dialyzed and freeze-dried and then stored at 4°C for future use.
Sandwich ELISA for Detection of SPA
SPA was determined by a sandwich ELISA [Citation[14]]: 96-well plates (Costar, USA) were coated with 100 µL of rabbit IgG (10 µg/mL) for 2 h at 37°C in 50 mM NaHCO3–NaCO3 (pH 9.6). Then the plates were washed twice with 0.1% Tween 20 in PBS (PBST) and the wells were blocked overnight at 4°C with 200 µL 1% BSA in PBS. After two washes with PBST, the plates were incubated with 100 µL per well of SPA standard dilution samples (0.001–10 µg/mL) or serially diluted test samples for 30 min at 37°C. After six washes with PBST, the plates were finally incubated with mouse IgG conjugated horseradish peroxidase (HRP) at 1: 2000 dilution in PBS at room temperature for 30 min. The plates were finally washed again and 100 µL OPD substrate solution was added to each well. After stopping the reaction with 50 µL 1 M H2SO4, the absorbance at 490 nm was determined using a microplate reader (ELX800, Biotech, USA).
SDS-PAGE and Western Blot
The qualitative measurement of purified SPA was conducted by sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE), using 10% resolving gel and 4% stacking gel under denaturing and reducing conditions, according to the method of Laemmli [Citation[15]]. The purified SPA was resolved on SDS-PAGE and stained with Coomassie brilliant blue R-250.
Following the separation by SDS-PAGE, proteins were transferred onto nitrocellulose membrane for western analysis [Citation[16]]. The separated SPA was visualized with mouse IgG conjugated HRP at 1:2000 dilution and finally developed with DAB staining.
Preparation of MCMS
MCMS were prepared by a suspension embedding procedure [Citation[17]]. Briefly, purified cotton was reacted with NaOH and carbon disulfide to prepare a viscose solution. 0.5 g potassium oleate was dissolved in 180 mL chlorobenzene and 40 mL tetrachloromethane, and then 70 mL of viscose solution containing ferrofluids was added to the mixture. The thermostat was adjusted to 90°C. The mixture was stirred for 2 h, and MCMS were isolated on a sieve, which were washed with ethanol and finally with water.
Activation and Immobilization Procedures of MCMS
The procedure used to prepare SPA immobilized MCMS was performed according to the previously described method [Citation[13]]. Briefly, MCMS were washed three times with PBS, and mixed with an equal volume of epichlorohydrin (ECH) in 3 M NaOH solution at 40°C for 3 h. After being washed thoroughly with acetone and distilled water, ECH-activated MCMS were treated with 5% ammonia hydroxide for 6 h and then 1% glutaraldehyde (GA) for 2 h. 1 mL GA-activated MCMS were incubated with 3 mL of 0.01 M Na2CO3–NaHCO3 buffer (pH 9.6) containing 10 mg SPA for 12 h at 4°C. After coupling, MCMS were blocked with 5 mL of 1 M glycine and underwent an Amadori rearrangement to form a stable linkage with sodium borohydride. After being washed with distilled water, the SPA-MCMS was stored at 4°C for future use. The SPA concentration in the samples was determined using Lowry method [Citation[18]].
Purification of IgG by SPA-MCMS
Different parameters for characterizing of SPA-MCMS focused on the performance in antibody binding capacity of SPA-MCMS. The binding efficiency influenced by the amount of IgG captured, binding time, leakage of SPA and reproducibility were demonstrated with rabbit IgG samples including rabbit standard IgG and serum. After appropriate dilution with PBS, the sample was added to a test tube containing 100 µL SPA-MCMS and incubated on a shaker at room temperature for 1 h. After magnetic separation, the microspheres were washed with PBS till the content of proteins in the washing dropped to zero. Elution was carried out twice with 400 µL of 0.1 M Gly-HCl buffer (pH 2.5) for 10 min and the collected eluates were dialyzed against PBS at 4°C. Finally SPA-MCMS were washed with PBS containing 1‰ sodium hydrazoate and stored at 4°C for reuse.
Sandwich ELISA for Quantification of Rabbit IgG
The immunologic quantification of rabbit IgG was carried out by sandwich ELISA. 96-well plates were coated with an optimal concentration of 10 µg/mL goat anti-rabbit IgG. Standard dilution rabbit IgG (0.01–10 µg/mL) or serially diluted eluate were tested simultaneously. Goat anti-rabbit IgG conjugated HRP was used as a tracer at 1:2000 dilution in PBST and final developed with OPD substrate.
High-Performance Size-Exclusion Chromatography Analysis
High-performance size-exclusion chromatography (HPSEC) was used to analyze the purified product. Shimadzu Model LC-10ATVP HPLC apparatus (Shimadzu, Japan) with a SPD-10AV detector was utilized for processing 20 µL aliquots of purified IgG on a TSK-GEL G2000SWXL column (30 cm × 7.8 mm i.d.). The mobile phase was 0.15 M NaCl, 0.02 M sodium phosphate, pH 7.0, with a flow rate of 0.8 mL/min. The detection was performed by measuring the absorbance at 280 nm, and the purity was calculated by analyzing the peak area of IgG.
RESULTS AND DISCUSSIONS
Construction of Plasmid pET-SPA
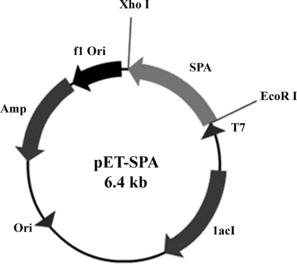
An amplified fragment of SPA gene (981 bp) was doubly digested with the enzymes EcoR I and Xho I, and ligated to the pET 21a (+) vector (downstream of T7 promoter) (). The clones were verified by restriction analysis for the inserts, and sequencing was done to confirm the clone. The nucleotide sequence of the gene was matched with the SPA sequences of Staphylococcus aureus Cowan I ATCC 6538 in GenBank. Alignment by standard methods revealed that it had 99% identity at the amino acid level with standard SPA. The recombinant plasmid (pET-SPA) was then introduced into E. coli BL21 (DE3) for expression.
Expression and Purification of SPA
Recombinant E. coli BL21 (DE3) cells harboring plasmids pET-SPA were grown in LB medium containing ampicillin and induction strategy was optimized to give the maximal expression amount. Nearly 15% expression amount was achieved when 0.03 mmol/L final concentration of IPTG was added in the medium after OD600 value of subculture reached 0.5, following by 3-hour induction (A, lane 2).
Figure 2 (A) SDS-PAGE and (B) western blot analysis of purified SPA. Lane M: low range protein marker; Lane 1: control E. coli BL21 without recombinant plasmids; Lane 2 and 4: induced E. coli BL21 transformed with recombinant plasmid pET-SPA; Lane 3 and 5: affinity purified SPA.
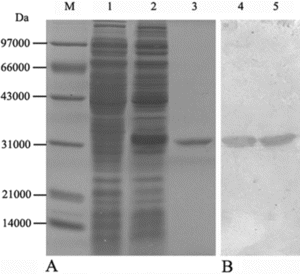
The protein lysate after ultrasonic treatment was subjected to IgG sepharose affinity column. The recombinant SPA, was eluted with 0.1 M Gly-HCl buffer (pH 2.5), as evidenced by SDS-PAGE and western blot (). After dialysis and freeze-dried, the total yield of the purified proteins was estimated to be around 182.5 mg from 1 liter of recombinant E. coli culture in a fermenter (wet weight of pellets around 9.750 g).
Preparation of SPA-MCMS
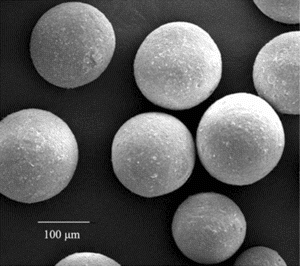
MCMS were successfully synthesized by suspension embedding technique. The particular size distribution (100 to 200 µm) was chosen because it enabled good magnetic response and suitable protein coupling [Citation[8]]. The morphology and intensity of microspheres were evaluated by environmental scanning electro microscopy (ESEM Quanta 200, FEI, USA). As shown in , MCMS had a very homogenous distribution and good spherical geometry with a good intensity.
MCMS are the most suitable carriers as biological materials with highly favorable biocompatibility and preferred efficiency [Citation[9], Citation[10]]. After activation, MCMS immobilized with SPA were used for future characterization, which revealed that 7.82 mg SPA was presented on 1 mL MCMS calculated by comparing the content of SPA before and after coupling.
Characterization of SPA-MCMS
The prepared SPA-MCMS was used as magnetic affinity adsorbents for specific binding with IgG from different species. Above all, a number of parameters were studied to improve the efficiencies of matrices and the requirements of application [Citation[19]]. Binding capacity, binding time, leakage of SPA and reproducibility were investigated as characterization parameters for optimal preparation of SPA-MCMS. IgG-binding was determined by Lowry method and sandwich ELISA, and leakage of SPA in the eluate was measured by sandwich ELISA for SPA quantification. Furthermore, the maximal binding capacity of SPA-MCMS on IgGs from different species was investigated.
Amount of IgG Captured
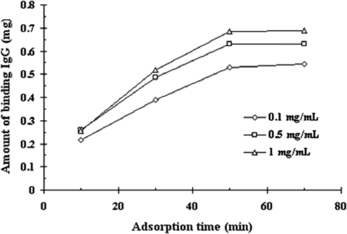
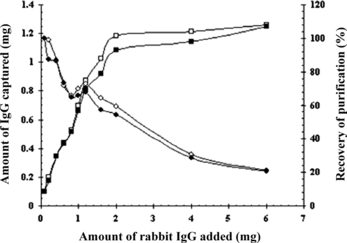
A comparative study of amount of IgG captured at different amount of rabbit standard IgG and serum from 100 µg to 6 mg was shown in . With 100 µL SPA-MCMS, the adsorption amount of IgG rapidly increased as the amount of IgG increased to 2 mg, and then remained at 1.1–1.2 mg for higher antibody quantity. It indicated that IgG up to 2 mg, the surface area of the SPA-MCMS available for binding to IgG is the maximum, thus becoming saturated. Contrarily the recovery of purification displayed a depression as the antibody amount increased and exhibited a slight reversal of 72.6% at 1.2 mg of IgG added. So an appropriate binding result was achieved with 0.86 mg IgG captured from 1.2 mg IgG for both standard IgG and serum (about 0.12 mL) by 100 µL SPA-MCMS.
Figure 4 Amount and recovery of IgG captured to the SPA-MCMS as a function of increased amounts of IgG added. The amount of rabbit IgG and serum in the sample was increased, as the total reaction – volume was kept at 1 mL. 100 µL of SPA-MCMS were used per sample. ▪and □ indicated amount of IgG captured from rabbit IgG and serum; ♦ and □ indicated recovery from rabbit IgG and serum.
Binding Time
There is no doubt that adsorption time has an important influence on the dynamics of protein binding including loss of capacity and rate of protein uptake [Citation[20]]. As demonstrated in , maximum amount of rabbit IgG binding was obtained after 50 minutes incubation. The adsorbed IgG kept constant even after prolonged binding time, which indicated that the equilibrium was reached and the time of 50 min was confirmed to be adequate for binding and strong interaction between IgG and SPA-MCMS. Furthermore, the amount of IgG captured was dependent on the concentration of IgG in the starting sample, which confirmed the theory of protein-protein interactions at high protein concentrations [Citation[21]], where such interactions involved weak binding forces leading to multiple IgG layers to be formed on the beads surface and increasing the quantity of binding efficiency.
Leakage and Reproducibility of SPA
Leakage of SPA, which should primarily due to nonspecific adsorption of protein A onto MCMS, is utterly important because it cause difficulties in the repeated use of SPA-MCMS, and also reduces the purity of products [Citation[22]]. Up to date, some SPA mimetic ligands such as TG19318 were under applications in order to overcome detection interference with leaking substances, but still seriously influenced repetitive use of immobilized matrix [Citation[23]]. In this study, no trace of leakage of SPA from SPA-MCMS could be detected by sandwich ELISA in the limit of 1 ng/mL. Further investigations showed that SPA-MCMS could be reused for the isolation of rabbit IgG after 10 cycles, without showing any capacity decrease (data not shown). Good performance of SPA-MCMS predicted long-lasting and repeated use during immunological analysis and isolation.
Binding Capacity for Different Species of IgGs
Binding strength of SPA-MCMS to different types of IgGs also indicated the binding specificity of SPA to mammalian IgG at their Fc regions. The IgGs from rabbit, mouse, rat, goat and human were investigated for their extensive and potential application value in biochemistry and immunology. As shown in , SPA-MCMS exhibited strong binding strength with IgG from rabbit, mouse and human, but weak interaction with rat and goat types of IgG, which are basically in agreement with the results of Richman et al [Citation[24]].
Table 1. Binding capacity of SPA-MCMS for various types of IgGs
The results provided a guidance to quantify binding capacity. The binding capacity of SPA-MCMS is 9.12 mg human IgG/mL beads, which is obviously superior to commercially available protein A magnetic beads (reported to range from 0.25 to 0.4 mg of an average human IgG per milliler with 1–3 µm of bead diameter (Dynal or New England Biolab)). In addition, 9.26 mg mouse IgG/mL SPA-MCMS is significant in comparison to Pierce commercial product, Immunopure Immobilized Protein A Gel, having an capacity of 6–8 mg/mL beads. Good performance resulted from high immobilizing quantity of SPA on MCMS further intensified and extended the development of MCMS in the application of biomacromolecules purification.
Purification of Rabbit IgG from Serum Using SPA-MCMS
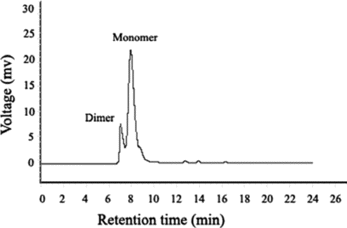
Single-step purification of rabbit IgG from serum after characterization of different parameters was extensively studied. To achieve a considerable incorporation of amount and recovery during magnetic separation, 1.2 mL rabbit serum containing 11.82 mg IgG was subjected to 1 mL SPA-MCMS. The results shown in suggested that 73.18% protein activity could be recovered with an amount of 8.65 mg IgG using SPA-MCMS. Product purity of 98.63% could be identified by Lowry method and ELISA. As shown in , HPSEC was applied to further investigate the purity of protein. Two peaks indicated the presence of both rabbit IgG and its altered form, dimmer, which should be ascribed to elution buffer of 0.1 M Gly-HCl buffer (pH 2.5). Low pH required for elution of antibodies from Protein A column often causes aggregation of certain antibodies [Citation[25]]. Both monomer and dimmer of rabbit IgG purified by SPA-MCMS could reach an overall purity of 90.27%.
Table 2. Purification of rabbit IgG by SPA-MCMS from rabbit serumFootnotea
CONCLUSIONS
Separation media which combines the selectivity of affinity chromatography with the convenience of magnetic properties during recovery has been developed in this study. After cloning and expression, the purified recombinant SPA was immobilized onto MCMS to prepare and formulate a magnetic affinity media of SPA-MCMS. According to characteristic analysis, SPA-MCMS performed better in the isolation of IgG from different species as an applicable facility, which is quite significant in comparison to commercially available immobilized Protein A used in IgG purification. Preliminary studies predict that it holds a potential in the development of efficient affinity chromatography and exercisable immunoassay with economical aspects and promising magnetic quality.
REFERENCES
- Jungbauer, A., Hahn, R. (2004). Engineering protein A affinity chromatography. Curr. Opin. Drug Discov. Devel. 7: 248–256.
- Shpigel, E., Goldlust, A., Eshel, A., Ber, I.K., Efroni, G., Singer, Y., Levy, I., Dekel, M., Shoseyov, O. (2000). Expression, purification and applications of staphylococcal protein A fused to cellulose-binding domain. Biotechnol. Appl. Biochem. 31: 197–203.
- Kruger, N.J., Hammond, J.B.W. (1988). Purification of immunoglobulins using protein A-Sepharose, in Methods in Molecular Biology. New Protein Techniques, J.M. Walker, Ed., Humana Press: Clifton, New Jersey, pp. 363–371.
- Chang, T.M.S. (1966). Semipermeable aqueous microcapsules (artificial cells): With emphasis on experiments in an extracorporeal shunt system. Trans. Am. Soc. Artif. Intern. Organs 12: 13–19.
- Tsushima, S. (1988). Production of human monoclonal autoantibodies that react with islet-cell-surface antigens by EBV-transformed lymphocytes: new method of preselection of ICSA producing lymphocyte by protein A magnetic microspheres. Hokkaido Igaku Zasshi (Japan). 63: 573–588.
- Worlock, A.J., Sidgwick, A., Horsburgh, T., Bell, P.R. (1991). The use of paramagnetic beads for the detection of major histocompatibility complex class I and class II antigens. BioTechniques 10: 310–315.
- Huse, K., Bohme, H.J., Scholz, G.H. (2002). Purification of antibodies by affinity chromatography. J. Biochem. Biophys. Methods 51: 217–231.
- Shinkai, M., Ito, A. (2004). Functional magnetic particles for medical application. Adv. Biochem. Eng. Biotechnol. 91: 191–220.
- Bilkova, Z., Slovakova, M., Lycka, A., Horak, D., Lenfeld, J., Turkova, J., Churacek, J. (2002). Oriented immobilization of galactose oxidase to bead and magnetic bead cellulose and poly(HEMA-co-EDMA) and magnetic poly(HEMA-co-EDMA) microspheres. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 770: 25–34.
- Bilkova, Z., Slovakova, M., Horak, D., Lenfeld, J., Churacek, J. (2002). Enzymes immobilized on magnetic carriers: Efficient and selective system for protein modification. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 770: 177–181.
- Brandl, M., Hartmann, J., Posnicek, T., Ausenegg, F.R., Leitner, A., Falkenhagen, D. (2005). Detection of fluorescently labeled microparticles in blood. Blood Purif. 23: 181–189.
- Hollung, K., Gabrielsen, O.S., Jakobsen, K.S. (1994). Enrichment of DNA-binding proteins from crude tissue for electrophoretic mobility shift assay using magnetic phospho cellulose particles. Nucleic Acids Res. 22: 3261–3262.
- Cao, Y., Bai, G., Chen, J.Q., Tian, W., Wang, S.Q., Yang, W.B. (2006). Preparation and characterization of magnetic microspheres for the purification of interferon alpha-2b. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 833: 236–244.
- Nielsen, U.B., Geierstanger, B.H. (2004). Multiplexed sandwich assays in microarray format. J. Immunol. Methods 290: 107–120.
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., Struhl, K. (1995). Protein analysis methods, in Short Protocols in Molecular Biology, 3rd Ed., John Wiley & Sons, Inc.: New York, Chap. 10, pp. 366–373.
- Fang, H., Wei, J., Yu, Y.T. (2004). In vivo studies of endotoxin removal by lysine-cellulose adsorbents. Biomaterials 25: 5433–5440.
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. (1951). Protein measurement with the Folin Phenol Reagent. J. Biol. Chem. 193: 265–275.
- Gupta, V., Eshwari, A.N., Panda, A.K., Agarwal, G.P. (2003). Optimization of IMAC for single step purification of recombinant ovine growth hormone from inclusion bodies of E coli. J. Chromatogr. A 998: 93–101.
- Albarghouthi, M., Fara, D.A., Saleem, M., El-Thaher, T., Matalka, K., Badwan, A. (2000). Immobilization of antibodies on alginate-chitosan beads. Int. J. Pharm. 206: 23–34.
- Deshpande, S.S. (1996). Protein-protein interaction. Enzyme Immunoassays: From Concept to Product Development, Chapman and Hall: New York, pp. 302–304.
- Godfrey, M.A., Kwasowski, P., Clift, R., Marks, V. (1993). Assessment of the suitability of commercially available spa affinity solid-phases for the purification of murine monoclonal-antibodies at process scale. J. Immunol. Methods 160: 97–105.
- Godfrey, M.A., Kwasowski, P., Clift, R., Marks, V. (1992). A sensitive enzyme-linked immunosorbent assay (ELISA) for the detection of staphylococcal protein A (SpA) present as a trace contaminant of murine immunoglobulins purified on immobilized protein A. J. Immunol. Methods 149: 21–27.
- Richman, D.D., Cleveland, P.H., Oxman, M.N., Johnson, K.M. (1982). The binding of staphylococcal protein A by the sera of different animal species. J. Immunol. 128: 2300–2305.
- Martsev, S.P., Kravchuk, Z.I., Vlasov, A.P., Lyakhnovich, G.V. (1995). Thermodynamic and functional characterization of a stable IgG conformer obtained by renaturation from a partially structured low pH-induced state. FEBS Lett. 361: 173–175.