Abstract
Candida rugosa lipase has been covalently immobilized on ferromagnetic azide polyethyleneterepthalate (Dacron) with specific activity retention of 16% for 4-nitrophenyl palmitate and 24% for hydrolysis of triolein in hexane. The immobilized enzyme was more thermal stable than the soluble one, retaining 78.8% of the activity after 1 h at 60°C. Also, this immobilized derivative was stable at the storage at 4°C. It has been used 5 cycles for pNPP hydrolysis without loss of activity. Soluble and immobilized Candida rugosa lipase showed a Michaelian behavior for fatty acid 4-nitrophenyl esters and different apparent KM values: 0.110 mM and 0.124 mM (4-nitrophenyl palmitate – C16); 0.193 mM and 0.235 mM (4-nitrophenyl laurate – C12) and 0.206 mM and 0.119 mM (4-nitrophenyl butyrate – C4), respectively. The immobilized lipase was more efficient for catalyzing the hydrolysis of 4-nitrophenyl esters with short chain length fatty acid (4-NPB – C4) than soluble enzyme. The ferromagnetic Dacron-lipase derivative was able to catalyze the synthesis of triolein from glycerol and oleic acid with 50% of conversion after 72 h at 40°C.
INTRODUCTION
Lipases are enzymes that, in general, exert their biocatalytic action at organic/aqueous interfaces. In the last decade, much use of this class of enzyme has been made for the manufacture of commercial products ranging from food products (oils and fats bioconversion) and textile detergency to pharmaceutical drugs. It has gone from academic curiosity to the point where today a manufacturing plant producing 50 ton per year of chiral intermediate methyl methoxyphenyl glycidate is in operation [Citation[1], Citation[2]]. Lipases are spontaneously soluble in aqueous solution, but their natural substrates are not. Although the use of a proper organic solvent or an emulsifier helps overcome the contact problem between substrate and enzyme, the practical use of lipase in such pseudohomogeneous reaction systems poses technological and economical difficulties. If lipase is immobilized, then it becomes an independent phase within the reaction system, which may be easily retained in the reactor via mechanical means with concomitant advantages in preventing contamination of the products and extending its useful active life [Citation[3]].
Candida lipases are of special interest, having proved to be a nonspecific type of catalyst of many (commercial) interesting reactions such as the modification of oils and fats, reactions in organic solvents, and resolution of racemic mixtures [Citation[2], Citation[4]]. Such lipases have been immobilized by covalent binding on different supports such as PEG, PVC, chitin, agarose, Chitosan, Sepharose and Trisacryl for olive oil hydrolysis using batch stirred-tank or packed-bed reactors [Citation[3]]. In addition, Candida cylindracea lipase has been covalently immobilized on agarose by the tosylation method and on silica by the trichlorotriazine method, chitosan beads activated with carbodiimide, epoxy groups of the poly (GMA-HEMA-EGDMA) microspheres [Citation[4–6]]. The use of magnetic particles can also reduce the capital and operation costs. Because of this, many magnetic materials have been developed such as nanostructured superparamagnetic magnetite in hydrophobic sol-gel materials, magnetic poly (VAc-DVB), superparamagnetic nanoparticles, magnetic poly(methacrylate-divinylbenzene) microsphere and superparamagnetic silica [Citation[7–11]]. This work has shown the use of a non-expensive support, ferromagnetic Dacron films, which provided a derivative of easy separation from a reaction system by using a magnet coated stirred-tank reactor, in the hydrolysis of fatty acid 4-nitrophenyl esters. Also, the physical-chemistry properties, kinetic and application in synthesis reaction of the ferromagnetic-dacron-lipase derivative were studied.
MATERIALS AND METHODS
Materials
Candida rugosa Lipase type VII (lyophilized powder), Triton X-100 and 4-nitrophenyl esters were supplied by the Sigma Chemical Co. (St. Louis, MO, USA). Dacron films were kindly donated by Rhodia Polymer Industry-Brazil. All of the other chemicals were of analytical grade and were purchased from Merck AG or Sigma Chemical Co.
Immobilization of Candida rugosa Lipase
Lipase was immobilized on ferromagnetic azide polyethyleneterepthalate (Dacron) as follows according to Carneiro-Leão et al. [Citation[12]] and Bruno et al. [Citation[13]].
Hydrazinolysis of Dacron
Films of dacron (4 g) were cut into strips and incubated in 100 mL of methanol containing 10 mL of hydrazine hydrate at 40°C for 48 h with stirring. Afterward, the hydrazine-dacron (powder) was washed and filtered a vaccum twice with 50 mL of methanol and 50 mL of 90% v/v methanol. After this process, 2 g of the hydrazide-dacron was mixed with 100 mL of distilled water in a tube test for eliminating of fine particles by decantation. PET bottles processed by this method showed similar results.
Magnetization of Hydrazine-Dacron
Hydrazine-dacron (2 g) was stirred in deionized water (100 mL) and an aqueous solution (10 mL) containing FeCl3 ⋅ 6H2O (300 mg/mL) and FeCl2 ⋅ 4H2O (121 mg/mL) was added dropwise. Under vigorous stirring, the mixture was adjusted to pH 8.3 by addition of (28% v/v) ammonium hydroxide solution and then incubated at 60°C for 30 minutes. The material obtained was filtered using a vacuum pump and washed exhaustively with distilled water.
Conversion of Groups from Hydrazide to Azide on Magnetized Dacron
The ferromagnetic hydrazide-dacron (2 g) resulting was converted to ferromagnetic azide-dacron by incubating the material in 0.6 M HCl (16 mL) containing 5% w/v sodium nitrite (2 mL) at approx. 30°C for 30 minutes with stirring. Then, the ferromagnetic azide-dacron was isolated from the solution by using a magnet and washed with deionized water (twice), 1 M NaCl (twice) and deionized water (twice).
Covalent Coupling of Lipase to Ferromagnetic Azide-Dacron
5 mL of lipase solution (0.33 U/mL) containing 0.41 mg/mL of protein (2.04 mg protein and 1.65 U) was incubated with 0.1 g of ferromagnetic azide-dacron for 3 h under constant agitation at 4°C. Then, the immobilized derivative (enzyme-ferromagnetic-dacron) was isolated from the solution using a magnet and washed with 50 mL of 1 M NaCl, 50 mL of 0.05 M Tris-HCl buffer pH 7.2, 100 mL of 0.5% Triton X-100 w/v, 50 mL of more Tris-HCl buffer and 50 mL of 1 M NaCl. The immobilized enzyme was stored in 0.05 M Tris-HCl buffer pH 7.2 at 4°C.
Lipase Activity
The lipolytic activity was measured by 4-nitrophenyl palmitate method [Citation[14–16]] at 30°C and pH 8.0. Soluble Candida rugosa lipase (0.1 mL, 0.33 U/mL, 0.41 mg protein/ml) was added in the cuvette containing 0.9 ml of uniform emulsion of 4-nitrophenyl palmitate. The absorbance change was read at 410 nm over 1 minute using a spectrophotometer. The molar coefficient absorbance of 4-nitrophenyl was considered 1.32 × 104 M−1 ⋅ cm−1 to calculate the enzyme activity. For immobilized lipase was used 0.1 g of derivative and 10 mL of 4-nitrophenyl esters microemulsions removing samples (1 mL) of reaction mixture to read the absorbance at 410 nm and after read the sample was returned to the reaction tube. The same experimental conditions were used for the all 4-nitrophenyl esters: 4-nitrophenyl butyrate (4-NPB – C4); 4-nitrophenyl laurate (4-NPL – C12) and 4-nitrophenyl palmitate (4-NPP – C16).
Lipase activity using triolein (0.113 M) in hexane and the amount of free fatty acid was measured by cupric acetate method [Citation[17], Citation[18]]. 5 mL of triolein solution (0.113 M) was mixed rapidly with 0.1 g of immobilized lipase under magnetic stirrer. Samples (0.2 mL) was removed from triolein/hexane layer and received in a test tube containing 0.1 ml of 5 N HCl solution, mixed in vortex for 15 sec, and then isooctane (2.0 mL) was added, mixing for 30 sec, and cupric acetate-pyridine reagent (0.5 mL), mixing for 90 sec. The absorbance of the isooctane upper layer was read at 715 nm. A calibration curve was made using oleic acid as standard (0.1 to 10 µmol).
Protein Determination
Protein concentration was measured according to Lowry et al. [Citation[19]] in both soluble and immobilized lipase (after acid hydrolysis using 1 mL of immobilized lipase suspension with 0.2 mL of HCl in closed tubes at 100°C for 1 h, after neutralization with sodium hydroxide). The amount of bound protein on the support was determined using the dried weight of the immobilized lipase suspension.
Effect of Isopropanol on the Lipase Activity
This study was carried out using 4-NPP in a concentration range from 10 up to 800 µM containing isopropanol from 0.1% to 10% (v/v), respectively. The amount of others mixture reaction components was kept constant in the microemulsion.
Thermal Stability
The thermal stability of soluble and immobilized lipase was carried out incubating both enzymes suspended in 0.05 M Tris-HCl buffer pH 7.2, at the temperature range from 30 to 80°C for 1 h. After this treatment, the activities were measured by 4-NPP method.
Storage Stability
The immobilized derivative (ferromagnetic dracon-azide-lipase) was stored at 4°C in 0.05 M Tris-HCl buffer pH 7.2 and its activity was measured periodically using 4-NPP method as described previously.
Synthesis of Triglycerides
Synthesis reaction was tested according to Park and Pastore [Citation[20]] using a reaction mixture containing oleic acid (0.9 g) and glycerol (8 g) in a closed tube, which was incubated at 40°C under orbital stirring, followed by the addition of 0.4 mL of immobilized enzyme suspension. Samples (0.1 mL) were transferred at defined time intervals to tubes containing isooctane and the amount of free fatty acids was measured by cupric acetate method as described previously.
RESULTS AND DISCUSSION
Candida rugosa lipase (0.33 U/mL or 0.81 U/mg of protein) has been immobilized by covalent linkage on ferromagnetic azide polyethyleneterepthalate (Dacron) was stable retaining 16% of specific activity or 24% for hydrolysis of triolein in hexane with 12 mg of protein/g of support. The results can be compared to those reported by several researchers, using microbial lipases immobilized on several supports ().
Table 1. Immobilization of lipase on various supports
There was no change in the values of the optimal temperature (45°C) and pH (7.2) after immobilization (datas not shown). According to the literature, the change in optimum pH of soluble and immobilized enzyme can be caused by conformation changes (exposure of catalytic site) of lipase molecules after immobilization, changing the dissociation state of the catalytic site. The effect of temperature on enzymatic activity is related to activation energy, so an increase of optimum temperature, for example, means increase in the activation energy, which implies an increase of energy barrier [Citation[8]]. Then, according to the results, the immobilization of lipase on magnetized Dacron did not affect the lipase conformation, leaving the catalytic site accessible to H+ or OH− ions, and neither the activation energy of enzymatic reaction.
The immobilized derivative showed higher thermal stability than the soluble enzyme, retaining the whole activity after 1 h at 50°C and 50% of the initial one at 65°C for the same time, while the soluble enzyme retained only 10% at the same conditions ().
Figure 1 Thermal stabilities of soluble (▪) and immobilized (▴) on ferromagnetic dacron-azide lipases. The initial specific activities for soluble and immobilized lipases were 0.81 U/mg protein and 0.13 U/g support, respectively.
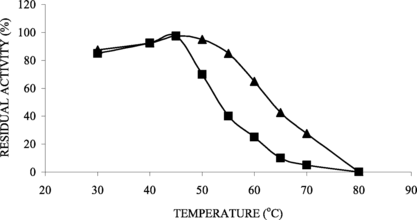
The increase on thermal stability showed that the immobilization seems to protect the enzyme activity [Citation[21]]. According to Balcão et al. [Citation[3]], because immobilization provides a more rigid external backbone for lipase molecules, the effect of higher temperatures in breaking the interactions that are responsible for the proper globular, thermal stability is expected to increase.
Thermal stability study was carried out incubating the enzyme at each temperature for 1 h, which showed that there was no activity loss for the immobilized lipase up to a temperature of 40°C. However, the free enzyme was found to be stable only up to 40°C. At 60°C, the immobilized lipase on dry and wet chitosan beads retained an activity of about 45% and 60%, respectively, whereas the activity retained by the free enzyme was only 20% [Citation[22]].
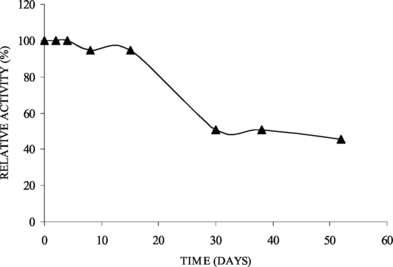
Immobilized lipase on ferromagnetic Dacron-azide showed a good storage stability at 4°C, maintaining complete activity for 15 days, with 50% of initial activity present after 50 days (). This stability could be related to hydrophobic caracter of the support keeping the lipase free from the hydrophylic substances.
Hung et al. [Citation[5]] found for Candida rugosa lipase immobilized on chitosan beads via carbodiimide a storage stability of 67% of activity retention after 7 days of storage. However, Candida rugosa lipase covalently attached on poly (GMA-HEMA-EGDMA) microspheres preserved about 67% of its initial activity during 84 days at 4°C [Citation[6]]. According to Kilinç et al. [Citation[23]], operational stability assays showed that lipase on CL-PVA had a half-life of 8 h and the storage stability at 4°C for the soluble enzyme was 6 days while the immobilized one in the same period retained 85% of initial activity decreasing slowly.
The storage stability in aqueous medium showed that the activity of free and immobilized lipase on dry chitosan beads decreased considerably in 5 and 3 days, respectively, but the immobilized lipase on wet chitosan beads did not exhibit any loss in activity up to 30 days at 25°C [Citation[22]]. The immobilized derivative was reused 5 cycles for hydrolysis of 4-NPP without loss of activity.
Immobilized Candida rugosa lipase on dry and wet chitosan beads-carbodiimide retained 78% and 85% of its initial activity after 10 batch hydrolytic cycles, respectively [Citation[22]]. Magnetic polysiloxane-polyvinyl alcohol – Mucor meihei lipase derivative was recycled by 7 assays keeping 10% of its initial activity [Citation[13]]. The activity of the Candida cylindracea lipase covalently immobilized on magnetic poly(methacrylate-divinylbenzene) microspheres decreased significantly after 4 cycles [Citation[11]].
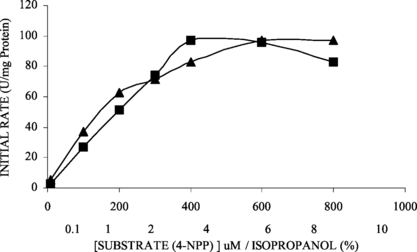
Because the solvent at concentration higher than 2% (v/v) led to a progressive loss of activity of both soluble and immobilized Candida rugosa lipase on microporous polypropylene, that depended on the polarity of the solvent. So, the higher the polarity of the solvent used, the greater the loss of enzyme activity [Citation[24]]. The isopropanol effect has been studied (0.1–10% v/v) on the activity of lipase, which was used to dissolve the hydrophobic substrate fat esters of 4-nitrophenyl. The result showed that there was no significant effect on the lipase activity (). However, Garcia-Alles and Gotor [Citation[25]] found that the Candida antarctica lipase was inhibited by organic solvent, mainly with isopropanol, a fact explained by dependence on solubility properties of the alcohol.
Figure 3 Effect of solvent (isopropanol) on the activity of on soluble (▪) and immobilized (▴) lipase activities. The initial specific activities for soluble and immobilized lipases were 0.81 U/mg protein and 0.13 U/g support, respectively.
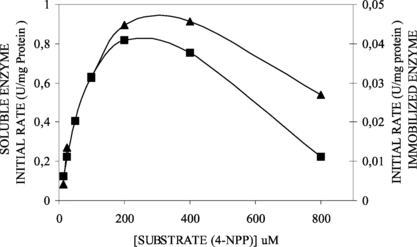
Candida rugosa lipase soluble and immobilized have shown a Michaelis-Menten behavior when tested for kinetic studies using different fatty acid 4-nitrophenyl esters (0 – 800 µM). shows that the inhibition by high substrate concentration (800 µM) for immobilized derivative was lighter than the soluble enzyme.
Figure 4 Effect of substrate concentration (4-NPP) on soluble (▪) and immobilized (▴) lipase activities. The initial specific activities for soluble and immobilized lipases were 0.81 U/mg protein and 0.13 U/g support, respectively.
Kinetic studies about esterase activity of Candida cylindracea lipase covalently immobilized on agarose or silica were carried out using 4-nitrophenyl acetate at 40 to 400 µM [Citation[4]]. Candida rugosa lipase immobilized on chitosan carbodiimide beads was used for kinetic study of hydrolytic activity using (0–0.5% w/v or 13200 µM) of 4-NPP in ethanol [Citation[22]]. Both reports did not show an inhibition effect of high substrate concentration. Similar results were reported by Knight et al. [Citation[26]] for Fusarium solani FS1 lipase immobilized on magnetized dacron.
and show the kinetic behavior of soluble and immobilized lipases for each 4-nitrophenyl fatty ester. The difference of specificity constant (VMAX/KM) values from soluble (4.77 min−1 ⋅ g−1 support) and immobilized derivative (0.21 min−1 ⋅ g−1 support), using pNPP as substrate, showed that the immobilization can cause diffusion limitations or structural changes in the enzyme. The results obtained for 4-nitrophenyl esters of fatty acids with 4 C, 12 C or 16 C suggested that the linkage of lipase on support without an arm-spacer caused low accessibility of the substrate to the active sites. Because of this, the lipase derivative was more efficient in catalyzing the hydrolysis of 4-nitrophenyl esters with short chain length fatty acid (4-NPB – C4) and the soluble enzyme showed higher efficiency for long chain length fatty acid (4-NPP – C16) (Tables and ). Similar results involving changes in kinetic behavior have been reported in the literature [Citation[22], Citation[27]].
Figure 5 Effect of various fatty acid 4-nitrophenyl esters, 4-NPB (▴), 4-NPL (▪), 4-NPP (•), concentration on the activity of soluble lipase.
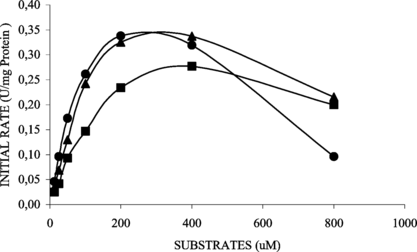
Figure 6 Effect of various fatty acid 4-nitrophenyl esters, 4-NPB (▴), 4-NPL (▪), 4-NPP (•), concentration on the activity of immobilized lipase. Immobilized derivative retained 16% of soluble specific activity.
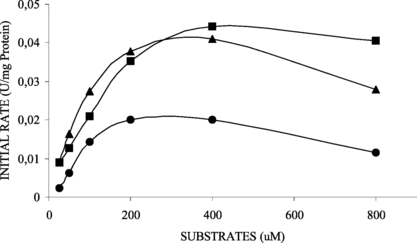
Table 2. The apparent Michaelis-Menten kinetics and efficiency for the hydrolysis of 4-nitrophenyl esters by soluble lipase
Table 3. The apparent Michaelis-Menten kinetics and efficiency for the hydrolysis of 4-nitrophenyl esters by immobilized lipase
Preliminary assay showed that the immobilized derivative was also able to catalyze the synthesis of triolein from glycerol and oleic acid in 72 h at 40°C with 50% of conversion measured by disappearance of the fatty acid (). This result was not good, maybe due to the low accessibility of the substrate (oleic acid – C16).
Figure 7 Synthesis of triglyceride (oleic acid + glycerol) catalysed by immobilized lipase on ferromagnetic dacron-azide (0.13 U/g support).
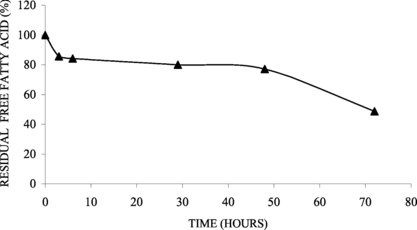
It has been reported that Candida rugosa lipase immobilized on nylon using glutaraldehyde as spacer was able to catalyze the synthesis of various short and long chain, saturated and unsaturated fatty acid esters. Also, it was shown that the esterification rates of oleic acid with oleyl alcohol are smaller than those with butanoic acid, indicating some effect of the length and structure of the acid molecule [Citation[28]].
The lower KM value of the coated lipase means an increased affinity between the enzyme and substrate. And the increase in VMAX implies that the rate of reaction between the enzyme and substrate was increased due to the decreased diffusion limitation [Citation[29]]. Also, it could be caused by the new conformational structure of lipase on the support after immobilization.
This increase in KM value might be due to structural changes in the enzyme induced by immobilization, which leads to a lower accessibility of substrate to the active sites [Citation[22]].
CONCLUSION
Candida rugosa lipase immobilized on ferromagnetic Dacron-azide by covalent linkage showed biotechnological potential using a cheap support material Dacron, which can be replaced by PET bottles and can be separated from the reaction system easily. The immobilized derivative showed improved physical chemical and kinetic properties compared to another immobilized lipases. Use of waste plastic (PET) in this way may help to reduce the environmental impact of this plastic.
REFERENCES
- Braun, B., Klein, E., López, J.L. (1995). Immobilization of Candida rugosa lipase to nylon fibers using its carbohydrate groups as the chemical link. Biotech. Bioeng. 51: 327–341.
- Villeneuve, P. et al. (2000). Customizing lipases for biocatalysis : A survey of chemical, physical and molecular biological approaches. J. Mol. Catal. B: Enz. 9: 113–148.
- Balcão, V.M., Paiva, A.L., Malcata, X. (1996). Bioreactors with immobilized lipases: State of the art. Enzyme Microb. Technol. 18: 392–416.
- Sánchez, E.M. et al. (1996). Kinetic and enantioselective behavior of the lipase from Candida cylindracea: A comparative study between the soluble enzyme and the enzyme immobilized on the agarose and silica gels. Enzyme Microb. Technol. 18: 468–476.
- Hung, T.C. et al. (2003). Binary immobilization of Candida rugosa lipase on chitosan. J. Mol. Catal. B: Enz. 26: 69–78.
- Bayramoğlu, G., Kaya, B., Arica, M.Y. (2005). Immobilization of Candida rugosa lipase onto spacer-arm attached poly (GMA-HEMA-EGDMA) microspheres. Food Chem. 92: 261–268.
- Reetz, M.T. et al. (1998). Entrapment of lipases in hydrophobic magnetite-containing sol-gel materials: Magnetic separation of heterogeneous biocatalysts. J. Mol. Cat. A: Chem. 134: 251–258.
- Guo, Z., Bai, S., Sun, Y. (2003). Preparation and characterization of immobilized lipase on magnetic hydrophobic microspheres. Enzyme Microb. Technol. 32: 776–782.
- Bai, S. et al. (2005). Resolution of (±)-menthol by immobilized Candida rugosa lipase on superparamagnetic nanoparticles. Food Chem. (In press).
- Liu, X. et al. (2004). Immobilization of lipase onto micron-size magnetic beads. J. Chrom. B. 822: 91–97.
- Liu, X. et al. (2005). Synthesis of amino-silane modified superparamagnetic silica supports and their use for protein immobilization. Colloids Surf. A. 238: 127–131.
- Carneiro-Leão, A.M.A., Oliveira, E.A., Carvalho-Jr, L.B. (1991). Immobilization of protein on ferromagnetic dacron. Appl. Biochem. Biotech. 31: 53–58.
- Bruno, L.M. et al. (2005). Characterization of Mucor meihei lipase immobilized on polysiloxane-polyvinyl alcohol magnetic particles. World J. Microb. Biotechnol. 21: 189–192.
- Winkler, U.K., Stuckmann, M. (1979). Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J. Bacteriol. 138: 663–670.
- Pimentel, M.C.B. et al. (1994). Lipase from a Brazilian strain of Penicillium citrinum. Appl. Biochem. Biotech. 49: 59–73.
- Pimentel, M.C.B. et al. (1997). Lipase from a Brazilian strain of Penicillium citrinum cultured in a simple and inexpensive medium: Heat-denaturation, kinetics and pH stability. Appl. Biochem. Biotechnol. 66: 185–195.
- Ju, Y.H., Huang, F.C. (1995). Lipase immobilized on hydrophobic microporous polypropylene for the hydrolysis of palm kernel olein. Appl. Biochem. Biotechnol. 55: 17–26.
- Kwon, D.Y., Rhee, J.S. (1986). A simple and rapid colorimetric method for determination of free fatty acids for lipase assay. JAOCS 63: 89–92.
- Lowry, O.H. et al. (1951). Protein measurement with the folin phenol reagent. J. Biol. Chem. 193: 265–275.
- Park, Y., Pastore, G. (1989). Esterificação de ácido graxo com glicerol por lipases microbianas, in Ciência e Tecnologia de Alimentos, Campinas University Publishers: Campinas, São Paulo, Brasil, 9: 163–171.
- Nadruz, W. et al. (1994). Characterization of Candida rugosa lipase immobilised on alkylamine glass beads. Genetic Eng. Biotechnol. 14: 143–148.
- Chiou, S.-H., Wu, W.-T. (2004). Immobilization of Candida rugosa lipase on chitosan with activation of the hydroxyl groups. Biomaterials 25: 197–204.
- Kilinç, A., Önal, S., Telefoncu, A. (2002). Chemical attachment of porcine pancreatic lipase to crosslinked poly(vinyl alcohol) by means of adipoyldichloride. Process Biochem. 38: 641–647.
- Virto, M.D. et al. (1995). Kinetic properties of soluble and immobilized Candida rugosa lipase. Appl. Biochem. Biotechnol. 50: 127–136.
- Gárcia-Alles, L.F., Gotor, V. (1998) Alcohol inhibition and specificity studies of lipase B from Candida antarctica in organic solvent. Biotechnol. Bioeng. 59: 163–170.
- Knight, K. et al. (2000). Immobilization of lipase from Fusarium solani FS1. Braz. J. Microbiol. 31: 1–6.
- Janssen, A.E.M., Vaidya, A.M., Halling, P.J. (1996). Substrate specificity and kinetics of Candida rugosa lipase in organic media. Enzyme Microb. Technol. 18: 340–346.
- Zaidi, A. et al. (2002). Esterification of fatty acids using nylon-immobilized lipase in n-hexane: Kinetic parameters and chain-length effects. J. Biotechnol. 93: 209–216.
- Wu, J.-C. et al. (2003). Hydrolytic reactions catalyzed by surfactant-coated Candida rugosa lipase in an organic-aqueous two phase system. Process Biochem. 39: 233–238.