Abstract
In this study, we prepared a tri-copolymer porous matrices by natural polymer, collagen (Col), Chitosan (Chi) and Chondroitin (CS). Rabbit articular chondrocytes were isolated from the shoulder articular joints of a rabbit, seeded in Col-Chi-CS scaffold, and implanted subcutaneously in the dorsum of athymic nude mice to tissue engineer articular cartilage in vivo. In vitro studies show that Chondrocytes adhered to the scaffold, where they proliferated and secreted extracellular matrices with time, filling the space within the scaffold. The results of hematoxylin and eosin staining scanning electron microscopy revealed that most of the chondrocytes maintained their typically rounded morphology. After 28 days of culture within Col-Chi-CS scaffold in vitro, the results of histological staining showed forming of cartilage-specific morphological appearance and structural characteristics such as lacunae. Subcutaneous implantation studies in nude mice demonstrated that a homogeneous cartilaginous tissue, which was similar to those of natural cartilage, formed when chondrocytes were seeded in Col-Chi-CS matrix after implant 12 weeks. The tri-copolymer matrix could therefore have potential applications as a three-dimensional scaffold for cartilage tissue engineering.
INTRODUCTION
Articular cartilage has a limited ability to repair after injury because of its low mitotic activity and the nature of avascular tissue. Most of the current therapies to repair damaged cartilage have focused on the abrasion arthroplasty, excission, and the use of perichondreal or periosteal autografts. But the results have some limitations, and all fail to produce long-lasting repair tissue [Citation[1-4]]. In recent years, tissue engineering techniques have been extensively used on tissue repair. These techniques involve the isolation of articular chondrocytes or their precursor cells that may be expanded in vitro and then seeded into a biocompatible matrix, or scaffold, for cultivation and subsequent implantation into the joint [Citation[5-7]].
Various materials have been explored as tissue engineering scaffolds, including natural components such as collagen [Citation[8-11]], hyaluronate [Citation[12]], alginate [Citation[13]], and chitosan [Citation[14]], as well as synthetic polymers such as polylactide, polyglycolide (PGA), and their copolymers [Citation[15], Citation[16]]. Scaffolds should have adequate biocompatibility, biodegradation, porous structure, and mechanical strength [Citation[17]].
A three-dimensional biodegradable porous scaffold plays a vital role in a tissue engineering approach. The advantages of incorporating scaffold into this approach are that the scaffold matrix can provide the initial structural support and retain cells in the defective area and can act as a delivery system for nutrients and metabolites.
One of the considerable characteristics in the cartilage tissue is that a small number of chondrocytes are embedded in the rich ECM. Therefore, cell-matrix interactions is a crucial factor in the development and regeneration of the cartilage tissue. So, scaffold plays an important role as the extracellular matrix (ECM) during engineered tissue development.
To successfully achieve cartilage tissue regeneration, an ideal cell-carrier substance (matrix) should be the one that most closely mimics the naturally occurring environment in the articular cartilage matrix.
The two main extracelluar macromolecles that form the natural ECM are proteoglycans and fibrous protein. Collagen is a structural fibrous protein, which provides the tensile strength of the ECM, whereas proteogycans provide compressive strength in cartilage tissue.
Collagen (Col) is one of the most common ECM materials used for culturing cells in vitro. Porous collagen matrices with defined physical, chemical, and biological characteristics have a great potential in tissue engineering [Citation[18]].
Chitosan (Chi) is a (1–4)2-amino-2-deoxy-B-D-glucan, a unique polysaccharide derived from chitin that has structure characteristics similar to glycosaminoglycans [Citation[19], Citation[20]]. Previous research has revealed that chitosan has bacteriosatic [Citation[21]] and hemostatic properties [Citation[22]]. Furthermore, it has excellent biocompatibility, and is enzymatically degraded to absorbable oligosaccharides [Citation[23]]. In addition, it can form insoluble complexes with anionic polymers such as collagen, which may be attributed to its cationic nature [Citation[24]].
Chondroitin-sulfate (CS) also is the main GAG in native articular cartilage. Furthermore, CS is involved in the adhesion, migration, proliferation and differentiation of cells [Citation[25-27]].
The reason for choosing the collagen, Chitosan and CS to prepare a tri-copolymer scaffold in this study was to mimic the natural cartilage matrix and to try to meet the requirement for vivo natural environment for cartilage tissue engineering. The vitro and in vivo behaviors of rabbit articular chondrocytes seeded in the collagen-chitosan-chondroitin sulfate (Col-Chi-CS) tri-Copolymer Scaffold were evaluated.
MATERIALS AND METHODS
Preparing of Col-Chi-CS Porous Scaffolds
Native insoluble type I collagen was isolated from bovine achilles tendon and treated by the method of enzyme digestion described by Zhang et al. [Citation[28]].
The blended Col-Chi-CS scaffolds were made by the freeze-drying method. Briefly, a 0.6% (w/v) collagen solution (0.3% dilute acetic media) was blended with a 1% (w/v) chitosan solution (0.2 M acetic acid solution), resulting in 20% Chi/Col (w/w) solution. These blends were then frozen at − 40°C and lyophilized for 63 h in a freeze dryer.
These scaffolds were cross-linked by 1-ethyl-3-(3-dimethy inaminoproyl) carbodiimide (EDC). Matrices of 50 mg dry weight were incubated for 0.5 h in 20 ml of 40% ethanol containing 50 mmol/L 2-morpholinoethanesulphonic acid (MES) (pH 5.5). Subsequently, the matrices were cross-linked by immersion in the 19.5 ml of 40% ethanol containing 50 mM MES (pH 5.0), 33 mM EDC and 8 mM N-hydroxysuccinimide (NHS) and 2% CS. After reaction for 4 h, the matrices were washed in 0.1 M Na2HPO4 (pH 9.1) for 1 h. Finally, the matrices were washed with 1 M NaCl and 2 M NaCl for 2 h and 1 day, respectively, followed by washing with distilled water and lyophilization.
Matrix morphology and porosity were analyzed using scanning electron microscopy.
Chondrocytes Isolation and Seeding
Chordroncytes were obtained from the articulars of New Zealand white rabbits. The cartilage was minced and washed three times in phosphate-buffered saline (PBS; pH 7.4). The minced cartilage was digested with 0.25% trypsin for 45 minutes, and then digested with 0.2% collagenase for 4 h in 37°C. The digestion solution was filtrated through a sterile 70-µm nylon mesh to remove any undigested fragments, and the chondrocytes were subsequently collected by centrifugation and washed twice with PBS. The cells' number and viability were determined by using a hemocytometer and the trypan bule dye exclusion. The collected cells were suspended in culture medium (DMEM containing 10% fetal bovine serum, 4500 mg/l glucose, 584 mg/l glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 10 mM HEPES, and 50 mg/l ascorblic acid), seeded in a 25-cm2 flask at a cell density of 1 × 105 cells/cm2 and subcultured at an atmosphere of 5% CO2 at 37°C.
The third passages' chondrocytes were trypsinized and re-suspended at a concentration of 1 × 107 cells/ml DMEM. Crosslinked collagenous matrices were sterilized. Matrices, 10 mm in diameter and approximately 1.5-mm thick, were placed in 48-wells plates, and about 100 µl of cell suspension was injected into each scaffold, then were cultured at 37°C, 5% CO2, and 95% humidity up to 28 days. The medium (1 ml) in plates was changed every day. Samples were collected on 3rd, 14th, 21st and 28th days, respectively.
Scanning Electron Microscopy (SEM)
Samples were prepared for SEM, cell-seeded matrices (n = 2) were fixed in 0.1 M phosphate buffer (pH 7.4) containing 2%(v/v) glutaraldehyde for at least 24 h at 4°C. Matrices were studied using a XL30 environment scanning electron apparatus.
In Vivo Implantation
The animal experiment was conducted according to the committee guidelines of the National Institute of Advanced Industrial Science and Technology for animal experiments. Athymic male mice were obtained at 5 weeks and acclimated for 1 week before use. The mice were anesthetized with 3% pentobarbital sodium. The scaffold-cell constructs were implanted subcutaneously in the dorsum of athymic nude mice mouse. The implants were harvested after in vivo incubation of 4 and 12 weeks.
Histological Staining
The cell-seeded matrices and the implant samples were fixed in a 0.1 M phosphate-buffersolution (pH 7.4) containing 4% paraformaldehyde, dehydrated in alcohol and embedded with paraffin, and sections (7µm) were stained with haematoxylin-eosin.
RESULTS
Matrix Characterization
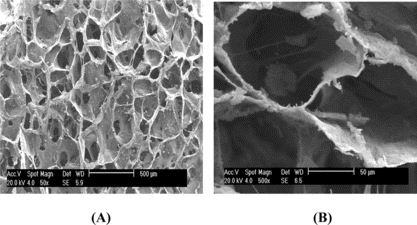
A shows a uniform distribution with porous structures for the Col-Chi-CS matrice. The scaffolds had interconnected pores of mean diameter about 80 ∼ 150 µm (B).The size of porous structure would have allowed a substantial number of cells to migrate to the interior region of the scaffold.
Histologic Appearance of Cell-Scaffolds In Vitro
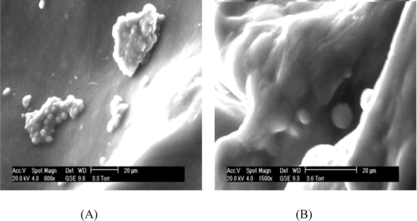
SEM micrographs of chondrocytes cultured on the Col-Chi-CS scaffold for 3 days and 21 days are shown in . The chondrocytes aggregates grew and adhered to the pores of the Co-Chi-CS scaffold with spherical shape (A). Cells proliferated and regenerated cartilaginous extracellular matrices to fill the void spaces in the scaffold with culture time (B).
Figure 2 Scanning electron micrographs chondrocytes seeded in Col-Chi-CS scaffold. A. Cell aggregates grew and adhered to scaffold after 3 days. B. Cells are surround by synthesized matrix after 21 days cell-seeding.
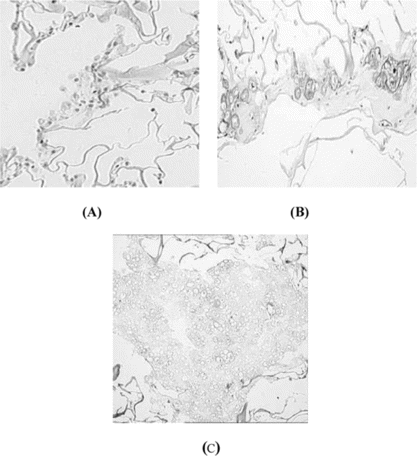
Histological staining is shown in . After 3 days, the majority of the cells had maintained chondrocytes phenotypically round morphology (A). At the superficial area of the scaffold, cartilaginous tissue had been formed after 14 days culture time (B). There are more homogeneous cartilage-like tissue at interior scaffold after 28 days; cells presented large, oval, and trapped in cartilage-specific lacunae (C). Undegraded scaffold could be seen around the cartilage-like tissue (C).
In Vivo Culture
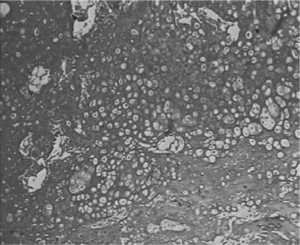
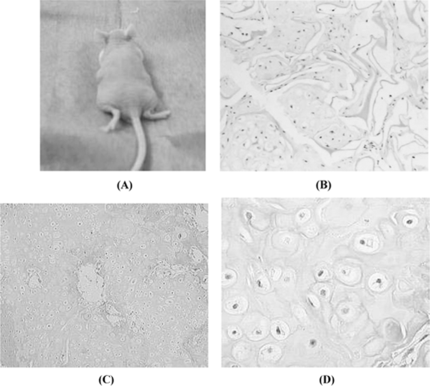
The scaffold-cell constructs were implanted subcutaneously in the dorsum of athymic nude mice after the constructs had been cultured in vitro for 7 days. The implants were harvested after 4 and 12 weeks. No evidence of superficial infection was demonstrated in the experimental mice. The constructs were easily dissected from the subcutaneous tissue (A). Gross examination of these implants showed no evidence of malignant invasion in any of the specimens. The constructs preserved their original shapes during implantation period. Histological examination of these specimens using hematoxylin and eosin staining revealed that chondrocytes-scaffold showed a cartilage-like tissue within the interconnecting pores after being implanted 4 weeks (B). A near-complete formation of cartilage-like tissue, which was similar to those of natural cartilage, was formed after implanting for 12 weeks (C, and D); toluidine blue stained sections of constructs demonstrated that abundance GAG was presented around chondorcytes ().
Figure 4 A. Chondrocyte-scaffold constructs implanted subcutaneously in the back of athymic nude mouse. Photographs of mouse immediately after implantation after 12 weeks. B. The H&E staining of Chondrocytes/Col-Chi-CS scaffold implanted in vivo for 4 weeks (× 100); C. The H&E staining of Chondrocytes/Col-Chi-CS scaffold implanted in vivo for 12 weeks (× 100). D. The H&E staining of Chondrocytes/Col-Chi-CS scaffold implanted in vivo for 12 weeks (× 400).
DISCUSSION
The choice of biomaterial is critical to successfully achieve cartilage repair using tissue engineering approaches. A variety of biomaterials, naturally occurring and synthetic, have been introduced as potential scaffold materials for cartilage repair. Collagen is a natural extracellular matrix (ECM) of many tissues and has a great potential in tissue engineering for its low antigenicity, biodegradability and good cell-blinding properties cartilage tissue engineering; however, its poor mechanical stability and rapid biodegradation limited its usefulness. Chitosan have been reported to be a useful alternative as a scaffold for catlilage tissue engineering [Citation[29], Citation[30]]. Collagen had shown more excellent biocompatibility property than chitosan matrix. Chondroitin-sulfate is the main GAG in native articular cartilage and inclusion of chondroitin sulfate in scaffold may promote adhesion, migration, proliferation and differentation of cells. In addition, CS can increase the water-binding capacity of matrice.
In this study, the tri-copolymer scaffold was produced from collagen, chitosan and CS to culture rabbit articular chondrocytes in vitro and in vivo. Chondrocytes could be seeded into the Col-Chi-CS scaffold by directly dropping a cell suspension onto the scaffold. Subsequently, the cells diffuse quickly throughout the scaffold. SEM sections of the following 3 days dynamic seeding showed rapid cell-aggregate attachment onto and throughout the scaffolds (A). Chondrocytes proliferation and secreted extracelluar increased with culture time on the scaffold (). This suggested that the proliferation and vitality of the adhered chondrocytes were excellent, indicating the fine biocompatibility of the scaffold. Maintenance of the chondrocytic phenotype and differentiation is a critical factor in cartilage tissue engineering. A suitable scaffold should be cell compatible, guarantee the homogenous distribution of the cells and allow matrix synthesis.
In vivo study, a histological examination of the implants with haematoxylin and eosin staining showed that there was near-complete formation of cartilage-like tissue within the interconnecting pores after implanted 4 weeks (B). A homogeneous cartilaginous tissue, which was similar to those of natural cartilage, formed when chondrocytes were seeded in Col-Chi-CS matrix after implanting for 12 weeks (C). But only the few cells leaked out were shown; this may be caused by unhomogeneous pore size.
Scaffolds for cartilage tissue engineering must have a highly porous and interconnected pore structure to ensure a biological environment conducive to cell attachment and proliferation as well as tissue growth, and structural stability of scaffolds are also critical for their practical use in tissue engineering. It was also reported that copolymerization of GAG with collagen yields sponges that are more degradation-resistant and have higher elastic moduli and higher fracture energy than collagen alone [Citation[31]]. The incorporation of chitosan into a collagen scaffold is known to increase its mechanical strength, as it forms an ionic complex between the positively charged chitosan and the negatively charged collagen [Citation[32]]. Some remnant scaffold materials could be found after 4 weeks culture period in vivo (B). Similar to other reports, a major limitation in using collagen alone porous scaffolds is that it is rapid biodegradation. As shown by our tests, the Col-Chi-CS may maintain structure integrity as compared to collagen alone under fluid flow. New tissue formed in scaffold maintained the same shape as that of scaffold. The strong mechanical property may prevent the materials' collapse, and thus would provide easy surgical handling and maintain a good fit in an implant site over time.
Previous research focused on combine synthetic polymers (PLGA) with collagen in vivo for tissue engineering, in order to avoid collagen alone scaffold rapid collapses in vivo. In addition, collagen–chitosan-GAG composites have been used to grow chondrocytes in vitro [Citation[33]]. In the current study, we blended natural polymer, chitosan and chondroitin sulfate, which are a main component of the proteoglycans (PGAs) or similarity in the cartilage, were applied to collagen matrices to mimic three-dimensional scaffolds and constructed successfully tissue engineering artificial cartilage in vivo.
In conclusion, the results of the present research demonstrated that the polymers of Col-Chi-CS showed good biocompatibility to chondrocytes by supporting cell growth, promoting cell proliferation and facilitated the formation of articular cartilagious tissue formation in vitro and in vivo. Moreover, the degradation time of Col-Chi-CS scaffold was in accordance with the cartilage tissue forming time. So, EDC crosslinked Col-Chi-CS tri-scaffold may serve as a suitable scaffold for cartilage tissue engineering.
REFERENCES
- Minas, T., Nethrer, S. (1997). Current concepts in the treatment of articular cartilage defects. Orthopedics 20: 525–538.
- Rodrigo, J.J., Steadman, R.J., Silliman, J.F., Fullstone, H.A. (1994). Improvement of full-thickness chondral defect healing in the human knee after debridement and microfracture using continous passive motion. Am. J. Knee. Surg. 7(3): 109–116.
- Johnson, L.L. (1989). Arthroscopic abrasion arthroplasty historical and pathological perspective: Present status. Athroscopy 2: 54–69.
- Buckwalter, J.A., Mankin, H.J. (1997). Articular cartilage. Part II: Degeneration and osteoarthrosis, repair, regeneration, and transplantation. J. Bone Jt. Surg. 79-A: 12–32.
- Wakitani, S., Kimura, T., Hirroka, A. (1989). Repair of rabbit articular surfaces with allograft chondrocytes embedded in collagen gel. J. Bone Jt. Surg. 71-B: 74–80.
- Chu, C.R., Coutts, R.D., Yoshioka, M., Harwood, F.L., Monosov, A.Z., Amiel, D. (1995). Articular cartilage repair using allogenic perichondrocyte-seeded biodegradable porous polylactic acid (PLA): A tissue-engineering study. J. Biomed. Mater. Res. 29: 1147–1150.
- Butnariu-Ephrat, M., Robinson, D., Mendes, D.G., Halperin, N., Nevo, Z. (1996). Resurfacing of goat articular cartilage by chondrocytes derived from bone marrow. Clin. Orthop. Relat. Res. 330: 234–243.
- Toolan, B.C., Frenkel, S.R., Pachence, J.M., Yalowitz, L., Alexander, H. (1996). Effects of growth-factor-enhanced culture on a chondrocyte–collagen implant for cartilage repair. J. Biomed. Mater. Res. 31: 273–280.
- Frenkel, S.R., Toolan, B., Menche, D., Pitman, M.I., Pachence, J.M. (1997). Chondrocyte transplantation using a collagen bilayer matrix for cartilage repair. J. Bone Joint Surg. 79: 831–836.
- Grande, D.A., Halberstadt, C., Naughton, G., Schwartz, R., Manji, R. (1997). Evaluation of matrix scaffolds for tissue engineering articular cartilage. J. Biomed. Mater. Res. 34: 211–220.
- Pieper, J.S., van der Kraan, P.M., Hafmans, T., Kamp, J., Buma, P., van Susante, J.L., van den Berg, W.B., Veerkamp, J.H., van kuppervelt, T.H. (2002). Crosslinked type II collagen matrices: Preparation, characterization, and potential for cartilage engineering. Biomaterials 23: 3183–3192.
- Aigner, J., Tegeler, J., Hutzler, P., Campoccia, D., Pavesio, A., Hammer, C., Kastenbauer, E., Naumann, A. (1998). Cartilage tissue engineering with novel nonwoven structured biomaterial based on hyaluronic acid benzyl ester. J. Biomed. Mater. Res. 42: 172–181.
- Shapiro, L., Cohen, S. (1997). Novel alginate sponges for cell culture and transplantation. Biomaterials 18: 583–590.
- Madihally, S.V., Matthew, H.W.T. (1999). Porous chitosan scaffolds for tissue engineering. Biomaterials 20: 1133–1142.
- Freed, L.E., Marquis, J.C., Nohria, A., Emmanual, J., Mikos, A.G., Langer, R. (1993). Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J. Biomed. Mater. Res. 27: 11–23.
- Ishaug-Riley, S.L., Okun, L.E., Prado, G., Applegate, M.A., Ratcliffe, A. (1999). Human articular chondrocyte adhesion and proliferation on synthetic biodegradable polymer films. Biomaterials 20: 2245–2246.
- Thomson, R.C., Wake, M.C., Yaszemski, M.J., Mikos, A.G. (1995). Biodegradable polymer scaffolds to regenerate organs. Adv. Polym. Sci. 122: 245–274.
- Zhang, Q.Q., Yao, K., Liu, L.R. (1999). Evaluation of porous collagen membrane in guided tissue regeneration. Artif. Cells Blood Substitutes, Immobilization Biotechnol. 27: 245–253.
- Qin, Y., Agboh, O.C. (1998). Chitin and chitosan fibres. Med. Dev. Technol. 9: 24–28.
- Rathke, T.D., Hudson, S.M. (1994). Review of chitin and chitosan as fiber and film formers. Rev. Macromol. Chem. Phys. C34: 375–437.
- Cuero, R.G. (1999). Antimicrobial action of exogenous chitosan. EXS 87: 315–333.
- Malette, W.G., Quigley, H.J., Gaines, R.D., Johnson, N.D., Rainer, W.G. (1983). Chitosan: A new hemostatic. Ann. Thorac. Surg. 36: 55–58.
- Varum, K.M., Myhr, M.M., Hjerde, R.J., Smidsrod, O. (1997). In vitro degradation rates of partially N-acetylated chitosan in human serum. Carbohydr. Res. 299: 99–101.
- Taravel, M.N., Domard, A. (1993). Relation between the physicochemical characteristics of collagen and its interactions with chitosan: I. Biomaterials 14: 930–939.
- Van Susante, J.L.C., Pieper, J., Buma, P., van Kuppevelt, T.H., van Beuningen, H., van der Kraan, P.M., Veerkamp, J. H., van den Berg, W.B., Veth, R.P.H. (2001). Linkage of chondroitin-sulfate to type I collagen scaffolds stimulates the bioactivity of seeded chondrocytes in vitro. Biomaterials 22: 2359–2369.
- Piepera, J.S., van der Kraanb, P.M., Hafmansa, T., Kampap, J., Buma, P., van Susante, J.L., van den Berg, J.H., Veerkampa, J.H., van Kuppevelt, T.H. (2002). Crosslinked type II collagen matrices: Preparation, characterization, and potential for cartilage engineering. Biomaterials 23: 3183–3192.
- Sechriest, V.F., Miao, Y.J., Niyibizi, C., Westerhausen-Larson, A., Matthew, H.W., Evans, C.H., Fu, S.H., Suh, J.K. (2000). GAG-augmented polysaccharide hydrogel: A novel biocompatible and biodegradable material to support chondrogenesis. J. Biomed. Mater. Res. 49: 534–541.
- Zhang, Q.Q., Liu, L.R., Ren, L., Wang, F.J. (1997). Preparation and characterization of collagen-chitosan composites. J. Appl. Polym. Sci. 64: 2127–2130.
- Xia, W., Liu, W., Cui, L., Liu, Y., Zhong, W., Liu, D., Wu, J., Chua, K., Cao, Y. (2004). Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed. Mater. Res. B. Appl. Biomater. 71: 373–380.
- Nettles, D.L., Elder, S.H., Gilbert, J.A. (2002). Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 8: 1009–1116.
- Cahn, F. (2002). Modification of natural polymers: Collagen–glycosaminoglycan copolymers, in Methods in Tissue Engineering, A. Atala, R.P. Lanza, Eds., Academic Press: San Diego, pp. 515–523.
- Taravel, M.N., Domard, A. (1996). Collagen and its interactions with chitosan: III. Some biological and mechanical properties. Biomaterials 17: 451–455.
- Lee, J.E., Kim, K.E., Kwon, I.C., Ahn, H.J., Lee, S.H., Cho, H., Kim, H.J., Seong, S.C., Lee, M.C. (2004). Effects of the controlled-released TGF-b1 from chitosanmicrospheres on chondrocytes cultured in a collagen/chitosan/glycosaminoglycan scaffold. Biomaterials 25: 4163–4173.