Abstract
Urease and AlaDH enzymes immobilized on active PEG derivatives were encapsulated at different ratios within sheep erythrocytes and their activity, encapsulation yields and erythrocyte recovery levels were assessed. Encapsulated derivatives were administered at given dosages and at given intervals to sheep having raised blood urea levels as a result of addition of urea to their feed, and the lowering of their blood urea levels and the change in the amount of ammonia were followed. Results were analyzed using day related NPar. Wilcoxon Signet Ranks test. It was found that 1 ml of PEG-enzyme preparation comprising PEG-urease/PEG-AlaDH at an activity ratio of 3/9 U:U/ml remained active for a period of 2 days, whereas 1 ml erythrocyte preparation, prepared under the same conditions and containing PEG-urease/PEG-AlaDH at an activity ratio of 2.15/4.5 U:U/ml, showed activity for a period of 6 days. It was shown that a single dose achieved a daily decrease of 21.7–61.6 mg/L in the blood urea level, and created no significant increase in the blood ammonia levels. No antigenic effect was observed for the PEG-enzyme preparations in the immunological test carried out.
1. INTRODUCTION
The use of erythrocytes as alternative carriers in the encapsulation of pharmacological agents such as drugs, hormones and enzymes was accomplished by Ihler and co-workers for the first time in 1973 [Citation[1]] and this process was used in cancer therapy 3 years later [Citation[2]]. A rapid increase in the number of works involving encapsulation of enzymes within erythrocytes [Citation[4-10]] followed, after the discovery that proteolytic activity in the cell was very low [Citation[3]]. Although many different methods have been used in the preparation of the carrier erythrocytes [Citation[11-15]], the most frequently used method is hypotonic dialysis [Citation[16-20]]. In this method, hematocrite value is kept high, so that the majority of the volume used is intracellular and hence a greater amount of matter is introduced into the erythrocyte.
Urease is one of the most widely used enzymes in the enzyme immobilization studies [Citation[21-24]]. The aim of these efforts is to help patients with cronical renal failure to avoid the need to undergo the difficult and expensive process of hemodialysis. Generally, systems tested involved administration through extracorporeal blood circulation [Citation[25-28]], oral route [Citation[29-31]] and peritoneal injection [Citation[27]]. The use of these types of applications was necessitated due to the need to incorporate systems for avoiding toxification arising from urea hydrolysis, and the requirement of auxiliary enzymes, coenzymes and co-substrates to provide the biochemical trasformations [Citation[32-35]]. Recently, systems comprising urease and urease-alaninedehydrogenase encapsulated within human erythrocytes were developed by us [Citation[36-38]]. Enzymes were used as natural enzymes or as PEG-derivatives. Thus, an immobilized enzyme preparation was prepared that coverted urea into alanine and that could be directly introduced into the blood circulation. The success of this system lies in the fact that the required pyruvate and NADH are supplied by the erythrocyte metabolism, regenerating enzymes are not required, and erythrocytes are used as carriers. Use of PEG-enzyme derivatives instead of natural enzymes increases the stability of the enzyme, increases its resistance towards proteolysis, facilitates transport through membranes due to its hydrophobic character, removes immunological reactivity of the enzymes introduced into the circulation as a result of the hemolysis, increases their circulatory life and decreases their renal clearance rates [Citation[39-48]].
The aim of this study is to encapsulate the PEG-urease/PEG-AlaDH enzyme couple within sheep erythrocytes and to test its ability to lower blood urea levels in vivo.
2. MATERIALS AND METHODS
PEG(MW:5000), PEG-Succinimide, PEG-p-nitrophenylchloroformate, Urease (type III), AlaDH, piruvate, NADH, TNBS and FDNB were obtained from Sigma Chemicals; Urea and TsT were obtained from Merck Company. Activation of PEG with TsT was carried out in accordance with the literature methods [Citation[39], Citation[40]]. All other chemicals used in the experiments were of analytical purity.
2.1. Preparation of PEG-Enzyme Derivatives
In the preparation of PEG-enzyme derivatives, enzyme lysine content/active PEG ratio was taken as 1/3, and the immobilization process was effected according to literature methods [Citation[49]]. Commercial AlaDH enzyme stored in a medium of ammonium sulphate was eluted through a PD-10 gel filtration column before use. Degree of PEG-substitution was determined using TNBS method via measurement of the free amino groups [Citation[50]].
2.2. Determination of Enzyme Activity
Activities of the free and PEG-derivatized urease were determined by using a Valtek Berthelot kit and the activities were obtained from a standard ammonium chloride curve (0–10.5 µmol/ml). Activities of AlaDH and PEG-AlaDH were determined from the decrease of absorbans values of NADH at 340 nm [Citation[51]]. For the measurements of the initial reaction rates of AlaDH in the direction of reductive amination, standard test mixtures were prepared in a total volume of 3 ml from 200 µl (17.6 mg/ml) pyruvate, 50 µl(10 mg/ml) NADH, 150 µl(16.05 mg/ml) NH4Cl, 50 mM phosphate buffer (pH:8.0) and enzyme solutions. Encapsulation yields were calculated by determining enzymatic activities in the washing waters and in the erythrocyte conjugates.
2.3. Preparation of Sheep Erythrocytes
Freshly drawn heparinized sheep blood was centrifuged at 1000 × g for 10 minutes, the serum and the buffy-coat were separated, and the packed cells were washed 3 times with 10 ml of PBS buffer.
2.4. Enzyme Encapsulation
Encapsulation of the enzyme system within erythrocytes was carried out using the enzyme couple PEG-urease/PEG-AlaDH in the ratios of 0.5:1.5; 1:3; 1:5; and 3:9 U/U, according to the Kruse Method under the optimized conditions as determined in our previous study [Citation[36], Citation[37], Citation[52]]. A mixture of the enzyme composition having a suitable activity ratio was mixed in 1:1 (v/v) ratio with washed erythrocytes and dialysed in a dialysis bag, first at 4°C against 10 mM phosphate buffer, containing 4 mM MgCl2 at pH 7.2 for 60 minutes (1st dialysis), and then against PBSG buffer at 25°C for 30 minutes (2nd dialysis), and finally the content of the dialysis bag was transferred to a test tube and incubated at 37°C for 30 minutes (annealing). This preparation was washed three times with PBSG buffer and stored at + 4°C after addition of 0.14 ml of CDP buffer per 1 ml volume. Encapsulation yields were calculated by determining enzymatic activities in the wash waters and the erythrocyte conjugates. Hemogram analyses were carried out with an Hematil 2000 equipment.
2.5. In Vivo Lowering of Blood Urea Levels; Animal Experiments
A total of 20 kıvırcık type sheep, 16 of which were two years old and 4 of which were three years old, were purchased from Bornova Veterinary Control and Research Institute and were kept at the sheepfold belonging to the same Institute on a daily diet comprising 1 kg of hay, 0.5 kg of bran, 0.3 kg of commercial pelletised fodder and urea. Initial amount of the daily urea was 2 g/sheep, which was increased to 12 g/sheep in a period of 15 days and kept constant at this value. Urea was added to the mixture of hay and bran as an aqueous solution. Sheep were divided into three groups as a control group, a PEG-enzyme receiving group, and an erythrocyte-receiving group (8,6,6 sheep in each group, respectively). The dose control experiments were made with the PEG-enzyme receiving group and the erythrocyte sample including enzyme. By using these results obtained, the PEG-enzyme group of animals received injections of PEG-urease/PEG-AlaDH preparation at 3/9 U/U activity ratio, the erythrocyte group of animals received injections of 1 ml erythrocyte preparation prepared in a medium containing PEG-urease/PEG-AlaDH in a ratio of 3/9 U/U. Injections were administered at 9 hours before the morning feeding, once a day for 3 consecutive days to the PEG-enzyme group, whereas the erythrocyte group received 3 doses at 2 days intervals (i.e., on the 4th day after the first dose). Morning-starvation values and throughout-the-day values of the blood urea levels were measured. Ammonia levels were measured using a Valtek Berthelot kit and the urea levels were measured using a 3 H Chemical diacetylmonoxime kit. Measurements were made in the plasma samples and no corrections were carried out for the whole blood values. The samples that could not be analyzed on the same day were stored at −20°C. In these groups of experiments, all materials were subjected to autoclaving or to filtration through a 0.45 mm sterile filter in the case of solutions before use, and all processes were carried out in a sterile chamber. Measurements were made on duplicate samples, and each day two commercial control samples were added to the measurement set. 1:2 SD rule was taken as a criterion for correct measurement.
2.6. Immunological Tests
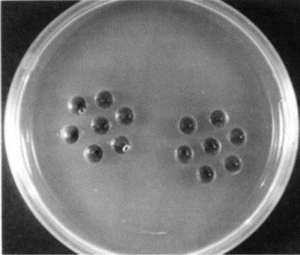
The probability of the PEG-enzyme preparations causing an immunological response was investigated at Manisa Animal Disease Research and Vaccine Production Institute by a gel precipitation test. Three sheep of the PEG-enzyme group were injected before being fed with a PEG-enzyme couple preparation having 3/9 U/U activity on three consecutive days and this procedure was repeated 4 times in total, with one-week intervals. The blood samples collected on days 21 and 34 were analyzed together with blood samples taken from 3 control group animals, against PEG-urease and PEG-AlaDH samples prepared by 2, 10 and 100 fold dilutions of the PEG-enzyme preparations.
3. RESULTS AND DISCUSSION
Properties of the PEG-enzyme derivatives, prepared using PEGs activated by different methods, are given in and their storage stabilities are given in . Enzyme activity values given in the tables do not correspond to the activity of 1 mg of modified derivative, but instead represent the activity of a modified derivative comprising 1 mg of enzyme, which corresponds approximately to 2 mgs of modified derivative [Citation[49]]. Degree of modification was determined via the standard curve of catalase, which is known to contain 108 lysine residues, and the urease activity was determined by the standard ammonia curve. Stabilities of the PEG-enzyme derivatives vary depending on their resistance to bacterial contamination and according to the type of linkage. The TsT derivatives were resistant to bacterial contamination, whereas a visible contamination was determined in the PEG-succimimide derivatives after one week. Instability of the PEG-pNPC derivatives may be due to the carbamate ester type bonding. In later studies, PEG-enzyme derivativies prepared by succinimide activation were used and stored at − 20°C, since a higher activity was obtained with AlaDH, which is about 320 times as expensive as a corresponding amount of urease of equal activity.
Table 1. Properties of PEG enzyme derivatives
Table 2. Storage stabilities of PEG enzyme derivatives at 4°C
Data relating to the encapsulation of enzyme couples within erythrocytes is given in . It can be seen from these values that approximately 2 U PEG-urease and about double the amount of PEG-AlaDH can be loaded into 1 ml of erythrocytes. Using the PEG-enzyme specific activity values given in , it can be seen that an amount of PEG-enzyme derivative corresponding to 108.5 µg of urease and 159.4 µg of AlaDH is encapsulated within 1 ml of erythrocytes. These data show that 1 ml of enzyme laden erythrocytes would be able to convert 2.88 mM (172.8 mg) urea per 24 hrs into alanine, assuming said enzymes react at maximum activity, and accumulation of ammonia due to degradation of urea may be avoided. The values shown in are not the maximum amounts that can be loaded into erythrocytes; on the contrary, it was shown in our previous work that urease of 19.2 U/ml activity could be encapsulated without deformation in the shape of the erythrocytes [Citation[36]]. This value is 5.3 times the maximum amount obtained when free enzyme is used [Citation[36]] and it is in agreement with the surface active character of PEG mentioned above.
Table 3. Encapsulation of PEG enzymes in erythrocytes
Changes taking place in the erythrocyte structure as a result of encapsulation were investigated by hemogram analysis (). It can be seen from the values in the table that erythrocyte indices for the encapsulated samples were different from control values. Calculated recovery values are 62.7% for the activity ratio of 1/3 and 54.5% for the activity ratio of 3/9; these values are considerably lower than the corresponding recovery value of 94.1% obtained for the encapsulation process at an activity ratio of 05/1.5 using human erythrocytes [Citation[37]]. The decrease observed in the McV, McH and McHc values show that erythrocytes have grown smaller and some stomatocyte formation has taken place.
Table 4. Hemogram analysis of erythrocytes encapsulated at various urease/AlaDH activity ratios
Sheep were chosen for the animal experiments because they are amenable for blood withdrawal of up to 3–4 times daily (each day on 9, 11, 13 and 15th hrs), it is possible to raise their blood urea levels simply by addition of urea into their feed, without the need for a surgical intervention, and because they are more resistant to toxification with urea. In fact, when the amount of urea reached 12 g per day level as a result of addition of increasing amounts urea to their feed, with the exception of 1–2 sheep, an increase of 30–85 mg/L was achieved in the blood urea levels. The blood ammonia levels in these sheep were in the range of 2–6.5 mg/L.
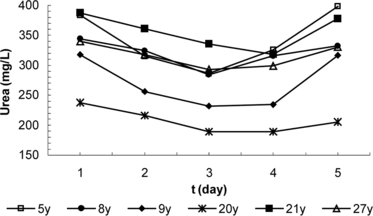
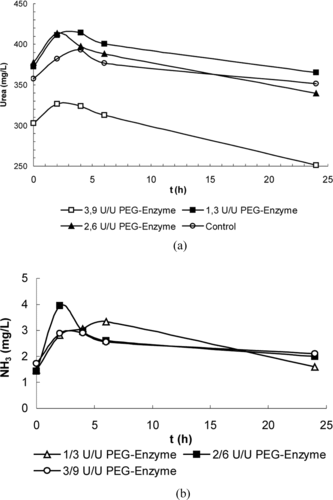
In dose control tests carried out on 6 sheep, blood urea level reductions averaging 7.1 mg/L, 38.1 mg/L and 51.6 mg/L were obtained for 1 ml of enzyme preparation obtained in media containing PEG-urease/PEG-AlaDH at activity ratios of 1/3, 2/6; 3/9 U/U, respectively. Results showing the changes in the urea and ammonia values within a day are given in a and 1b. No risk of ammonia toxification was established, since the blood ammonia levels ranged from 1.59–3.95 mg/L. Later experiments were conducted with enzyme and erythrocyte preparations prepared at an activity ratio of 3/9.
Figure 1 Variation of average (a) blood urea, (b) blood ammonia values during the day in the dose control group injected with PEG enzyme preparation.
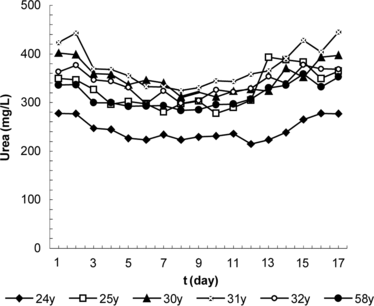
The average of blood urea values for the control group are shown in ; results obtained by injections of PEG-enzyme and erythrocyte preparation are shown in and . It was determined from the examination of these figures that a single dose was effective for 2 days in the case of PEG-enzyme preparations and for 6 days in the case of erythrocyte preparations, providing a drop in the blood urea levels in the range of 21.7–61.6 mg/L (6.4–19.4%). Statistical evaluation of the results using the time (days) dependent, nonparametric Wilcoxon Signet Ranks test produced the following values for α = 0.05: in the control group, p > 0.05 for a period of 5 days; in the PEG-enzyme group, p < 0.05(0.028) for 2nd–4th days and p > 0.05(0.249) for the 5th day; and in the erythrocyte group, p > 0.05 (0.752) on the 2nd day, p < 0.05 (0.028) for 3rd–12th days and p > 0.05 for 13th–17th days. In other words, although no significant changes were observed in the control group, a significant decrease in the blood urea level was provided during the 2nd–4th days in the PEG-enzyme group and during the 2nd–12th days in the erythrocyte group.
Table 5. Daily variations of the blood urea values in the control group
The blood ammonia values throughout the day remained below 10 mg/L even at the peak level, and in the case of erythrocyte preparations, dropped to undetectable values between the 8th and 11th days. Although attempts were made to assay alanine within the test samples stored at − 20°C, in the form of a dinitrophenyl derivative by HPLC, no measurable quantity could be determined. Data obtained is also in good agreement with the literature disclosures. For example, the plasma half-life of PEG-superoxidedismutase prepared from CDI activated PEG increased from 3.5 min. to 9 hrs [Citation[47]] and the half-life of PEG-superoxidedismutase prepared by TsT activation increased from 10 min. to 40 hrs [Citation[46]]. Furthermore, it was disclosed that the circulation half-lives of asparaginase [Citation[4]] and rhodanase [Citation[8]] also encapsulated in erythrocytes increased to 7 days and 8.5 days, respectively.
The fact that no precipitation bands were observed after 21 and 34 days in the gel precipitation tests () in which PEG-Urease and PEG-AlaDH were placed into central cavities proves that PEG-enzyme preparations have no antigenic effect, which is an expected result [Citation[39], Citation[40], Citation[46], Citation[47], Citation[49]].
4. CONCLUSION
In conclusion, it may be stated that an injection of 1 ml of PEG-enzyme preparation containing PEG-urease/PEG-AlaDH at a ratio of 3:9 U/U was effective for 2 days and 1 ml of erythrocyte preparation prepared in the presence of PEG-urease/PEG-AlaDH at a ratio of 3:9 U/U was effective for 6 days for each injection, and the latter could lower the blood urea levels significantly without leading to a dangerous increase in the blood ammonia levels, even at the maximum value reached during the day, and also that it did not invoke an immunological response. It is believed that the use of enzymes of higher purity, provision of means for separation of the enzyme loaded erythrocytes from the rest and preparation of erythrocyte systems with longer half-lives would increase the effectiveness of this system and provide for the production of a medicine useful in the treatment of patients suffering from chronic renal failure.
References
- Ihler, G.M., Glev, R.H., Schnure, F.W. (1973). Proc. Nat. Acad. Sci. 70(9): 2663–2666.
- Tyrrell, D.A., Ryman, B.E. (1976). Biochem. Soc. Trans. 4: 677–680.
- Ihler, G.M. (1983). Pharm. Ther. 20: 151–169.
- Updike, S.J., Wakamiya, R.T. (1983). J. Lab. Clin. Med. 101: 679–690.
- DeLoach, J.R. (1985). Biblthca Haemat. 51: 1–6.
- Ihler, G.M., Tsang, H.C.W. (1987). Methods Enzymol. 149: 221–229.
- DeLoach, J.R. (1987). Methods Enzymol. 149: 235–242.
- Leung, P., Cannon, E.P., Petricovics, I., Hawkins, A., Way, J.L. (1991). Toxicol. Appl. Pharm. 110: 168–274.
- Accorsi, A., Piatti, E., Piacentini, M.P., Fazi, A. (1992). In The Use of Resealed Erythrocytes as Carriers and Bioreactors, M. Magnani, J.R. DeLoach, Eds., Plenum Press: , NY, pp. 119–125.
- Pei, L., Omburo, G., McGuinn, W.D., Petricovics, I., Dave, K., Raushel, F.M., Wild, J.R., DeLoach, J.R., Way, J.L. (1994). Toxicol. Appl. Pharm. 124: 296–301.
- Magnani, M., Rossi, L., Ascenzo, M.D., Panzani, I., Bigi, L. (1998). Biotech. Appl. Biochem. 28: 1–6.
- Lizano, C., Sanz, S., Luque, J., Pinilla, M. (1998). Biochim. Biophys. Acta. 1425: 328–336.
- Kruse, C.A. (1991). Blood Cells 17: 177–189.
- Sprandel, U., Way, J.L. (1997). Erythrocytes as Drag Carriers in Medicine, Plenum Press: , NY.
- Green, R., Widder, K. (1987). Methods Enzymol. 149: 221–312.
- Alvares, F.J., Herraez, A., Tejedor, M.C., Diez, L.C. (1996). Biotech. Appl. Biochem. 23: 173–179.
- Droleskey, R.E., Andrews, K., Chiarantini, L., DeLoach, J.R. (1992). In The Use of Resealed Erythrocytes as Carriers and Bioreactors, M. Magnani, J.R. DeLoach, Eds., Plenum Press: , NY, pp. 73–80.
- DeLoach, J.R., Droleskey, R.E. (1993). Biotech. Appl. Biochem. 18: 83–92.
- DeLoach, J.R., Andrew, K. (1986). Biotech. Appl. Biochem. 8: 537–545.
- DeLoach, J.R., Ihler, G. (1977). Biochim. Biophys. Acta 496: 136–145.
- Onyelizi, F.N. (1988). J. Biochem. Biophys. Methods 16: 255–262.
- Krajewska, M.I., Zaborska, W. (1990). Angew. Makromol. Chem. 179: 21–33.
- Monshipouri, M., Neufeld, R.J. (1991). Enz. Microb. Tech. 13(4): 309–313.
- Huang, T.C., Chen, D.H. (1992). J.Chem. Tech. Biotech. 55: 191–199.
- Gordon, M.A., Greenhaum, L.B., Marantz, L.B., McArtur, M.S., Maxwel, M.D. (1969). Trans. Am. Soc. Artif. Intern. Organs 15: 347.
- Chang, T.M.S. (1988). Methods Enzym. 137: 444–457.
- Chag, T.M.S. (1977). Biomedical Application of Immobilized Enzymes and Proteins, Vol I and II, Plenum Press: , NY.
- Chang, T.M.S. (1966). Trans. Am. Soc. Artif. Inter. Organs 12: 13–19.
- Bourget, L., Chang, T.M.S. (1985). FEBS Lettter 180: 5–8.
- Parakash, S., Chang, T.M.S. (1998). Biomat. Artif. Cells Imm. Biotech. 26: 215–224.
- Chang, T.M.S., Lister, C. (1988). Biomater. Artif. Cells Artif. Organs 16(5): 915–926.
- Chang, T.M.S. (1985). Methods Enzymol. 112: 195–203.
- Chang, T.M.S. (1979). Artif. Organs 3: 284–287.
- Gu, K.F., Chang, T.M.S. (1990). Biotech. Bioeng. 36: 263–268.
- Cang, T.M.S. (1999). Annual N.Y. Acad. Sci. 875: 71–84.
- Hamarat, Baysal, Ş., Uslan, A.H. (2000). Artif. Cells, Blood Subs. Immob. Biotech. 28(3): 263–271.
- Hamarat, Baysal, Ş., Uslan, A.H. (2001). Artif. Cells, Blood Subs. Immob. Biotech. 29(5): 405–412.
- Hamarat, Baysal, Ş., Uslan, A.H. (2002). Artif. Cells, Blood Subs. Immob. Biotech. 30(1): 71–77.
- Abuchowski, A., Van Es, T., Palczuk, N.C., Davis, F. (1977). J. Biol. Chem., 252(11): 3578–3581.
- Abuchowski, A., McCoy, J.R., Palczuk, N.C., Van Es, T., Davis, F. (1977). J. Biol. Chem. 252(11): 3582–3586.
- Nijs, M., Gelbeke, M., Azarkan, M., Brygier, J., Guermant, C., Volant, D.B., Musu, T., Paul, C., Looze, Y. (1994). Appl. Biochem. Biotech. 49(1): 75–91.
- Philips, W.T., Kipper, R.W. (1999). J. Phar. Exp. Theur. 288: 665–670.
- Hartmann, J.X., Galla, J.D., Emma, D.A., Kao, K.N., Gamborg, O.L. (1976). Can. J. Genet. Cytol. 18(3): 503–512.
- Hui, S.W., Isac, T., Boni, L.T., Sen, A. (1985). J. Membr. Biol. 84: 137–146.
- Mac Donald, R.I. (1985). Biochemistry 24(15): 4058–4066.
- Beckman, J.S., Minor, R.L., White, C.W., Repine, J.E., Rosen, G.M., Freeman, B.A. (1988). J. Biol. Chem. 263: 6884–6892.
- Beauchamp, C.O., Gonias, S.L., Menapace, D.P., Pizzo, S.V. (1983). Anal. Biochem. 131: 25–33.
- Inada, Y., Matsushima, A., Hiroto, M., Nishimura, H., Kodera, Y. (1994). Methods Enzymol. 242: 65–90.
- Hamarat, Ş., Uslan, A.H. (1996). Artif. Cells, Blood Subs. Immob. Biotech. 24(3): 273–283.
- Fields, R. (1972). Methods Enzymol. 25: 464–468.
- Hamarat Baysal, Ş., Yasa, I., Uslan, A.H. (2002). Prep. Biochem. Biotechnol. 32(3): 277–285.
- Kruse, C.A., Freehauf, C.L., Patel, K.R., Baldeschwieler, J.D. (1987). Biotechnol. Appl. Biochem. 9: 123–40.