Abstract
This article reports a CE (capillary electrophoresis) method for the determination of Levodopa (L-DP) in rabbit brain tissue. L-DP was separated and determined on a fused quartz capillary (75 µm ID, 75 cm length, 50 cm effective length, Beckman Co.) with a mobile phase of phosphate buffer (pH = 2.5, 20 mmol/L THAM, 3% carboxymethyl-β-cyclodextrin), sample pressure 0.6 kPa × 6 s, separation voltage 20 KV, detected at 280 nm and temperature 25°C. The calibration curve was linear (r = 0.9999, n = 6) within the range of 0.5–16 µg/ml for L-DP. The recovery ratio was 87.87%. The mean relative standard deviation (RSD) was 2.73% (n = 5). This method is convenient, rapid, accurate, and brings about good recovery; it can be used for content determination of levodopa in rabbit brain tissue.
Keywords:
INTRODUCTION
Levodopa-2,6-Dimethyl-β-cyclodextrin (DM-β-CD) gel was prepared for nasal administration. We expect that levodopa-DM-β-CD has better fat-soluble properties than levodopa, is easy to permeate through the nasal mucous membrane and the olfactory nerve cell in the nasal cavity, gets to the brain tissue and brings into play its therapeutic action, and lessens its side-effects by reducing the dopamine content in peripheral blood [Citation[1-3]].
MATERIALS
Instruments: Beckman P/ACE5000 Capillary Electrophoresis, Beckman UV Detector, fused quartz capillary (75 µm ID, 75 cm length, 50 cm effective length, Beckman Co.).
Reagents: L-DP, National Institute for the Control of Pharmaceutical & Biological Products; L-DP Tablets, Kunming Biological Products Corporation; Borax, Beijing Xinguang Chemical Reagent Plant; Sodium Hydroxide, Beijing Hongxing Chemical Plant; double distilled water.
CE conditions: Mobile phase is phosphate buffer (pH = 2.5, 20 mmol/L THAM, 3% carboxymethyl-β-cyclodextrin), sample pressure 0.6 kPa × 6 s, separation voltage 20 KV, detected at 280 nm and temperature 25°C.
METHODS AND RESULTS
1. Animal Experiments
The rabbits (weight 3.5 kg,♀) were separated into 2 groups (6 rabbits in every group) randomly; they were the control group (L-DP tablets oral administration) and the test group (L-DP gel nasal administration). The rabbits of test group were administrated with 1 g L-DP gel (about 20 mg L-DP) through nasal cavity by syringe matching hose; the rabbits (fasted for 12 hours before administration) of control group were administrated with L-DP tablets through gavage. The rabbits were killed by decapitation at the time of 1 hour after administration, and the rabbit heads put into liquid nitrogen immediately [Citation[4]].
2. Preparation and Processing of Homogenate of Rabbit Brain Tissue
We dissected the cerebral hemisphere, homogenated the brain tissue and diluted hydrochloric acid (weight ratio 1:3) with homogenizer. We measured 2 ml homogenate precisely into centrifuge tube, then put into the same volume protein precipitator, mixed for 2 minutes, centrifuge treating it for 15 minutes 4000 rpm, supernatant fluid was filtered with 0.45 µm membrane, 10 µl subsequent filtrate was the injected sample [Citation[5]].
3. CE Conditions
The above-mentioned samples were assayed by capillary electrophoresis, with electrolytic conditions of phosphate buffer (pH = 2.5, 20 mmol/L THAM, 3% carboxymethyl-β-cyclodextrin), sample pressure 0.6 kPa × 6 s, separation voltage 20 KV, detection wavelength 280 nm and temperature 25°C. The phosphate buffer was filtered with 0.45 µm membrane and degassed by ultrasonic. The quartz capillary was rinsed with sodium hydroxide solution and super pure water for 3 minutes, phosphate buffer for 5–6 minutes before next sample was assayed [Citation[6], Citation[7]].
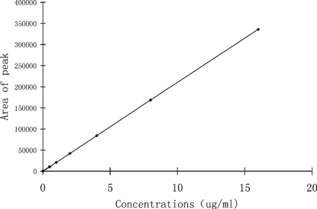
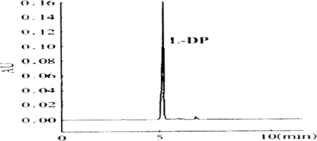
4. CE Behavior of L-DP and Standard Curve
16.0 mg L-DP was weighed precisely, dissolved with dilute hydrochloric acid, and we prepared a standard solution whose concentration are 0.5 µg/ml, 1.0 µg/ml, 2.0 µg/ml, 4.0 µg/ml, 8.0 µg/ml and 16.0 µg/ml, then determined their absorption spectrum. We could get the linear relation from peak area (Y) and L-DP concentration (X): is the standard curve of L-DP. is the CE behavior of L-DP.
5. Recovery and Repetition Tests of the Determination Method
We put 0.5 ml 20, 80, 320 µg/ml L-DP standard solutions into 1 ml blank control brain tissue homogenate, respectively, processed them according to above mentioned method, then assayed the L-DP concentrations by CE as the measured concentration. The rate of recovery is the ratio between the measured concentration and the virtual concentration, 87.87%. is the result of the recovery test.
Table 1. Results of recovery tests
We injected the 20, 80, 320 µg/ml L-DP standard solution 5 times, respectively. The RSD of the repetition test was 3.25%, 2.55%, 2.40%, respectively. is the result of the repetition test.
Table 2. Results of repetition tests (n = 5)
6. Assay of L-DP in Brain Tissue
We dissected the freezing cerebral hemisphere, homogenated the brain tissue and diluted hydrochloric acid (weight ratio 1:3) with homogenizer. We measured 2 ml homogenate precisely into the centrifuge tube, then put into the same volume protein precipitator, mixed for 2 minutes, centrifuge treating for 15 minutes 4000 rpm. The supernatant fluid was filtered with 0.45 µm membrane, and 10 µl subsequent filtrate was then injected into CE.
shows the results of the L-DP content in the brain tissue.
Table 3. Assay of L-DP in brain tissue (n = 6)
DISCUSSIONS
Tmax (time of the maximal concentration of blood) of L-DP is 1.2 hours, its t1/2 is 1.6 hours, L-DP can be metabolized to dopamine in liver rapidly. Therefore we killed the rabbits at the time of 1 hour after administration, and could determinate the maximal content of L-DP, avoiding more L-DP metabolized to dopamine [Citation[8]].
The rabbits were killed by decapitation, put into liquid nitrogen without dissection of cerebral hemisphere; the brain tissue would stop its metabolism immediately, therefore the measured content of L-DP could indicate the exact L-DP condition in the brain tissue at the time [Citation[9]].
A 3% (w/v) carboxymethyl-β-cyclodextrin of phosphate buffer has minimal interference on the determination of L-DP, and can separate drug from the protein enough on the condition of low voltage [Citation[10]].
From the results of assay of L-DP in brain tissues, compared with the tablets group, the nasal gel group had 5–9 folds increasing of the content of L-DP in brain tissue; the latter had significant healing effect over the former. The reason is possibly that L-DP can permeate the olfactory nerve partly to the brain tissue, and the gel has approximately zero level sustained release in vitro.
This work was supported by the 2003 Award Foundation of Shandong Province for Excellent Young Scientist.
REFERENCES
- Weiner, W.J. (2006). Myotonia congenita—a cause of muscle weakness and stiffness. Nat Clin. Pract. Neurol. 70: 393–399.
- Guigoni, C., Dovero, S., Aubert, I. (2005). Levodopa-induced dyskinesia in MPTP-treated macaques is not dependent on the extent and pattern of nigrostrial lesioning. Eur J Neurosci. 22: 283–287.
- Ikram-ul-Haq, Ali S., Qadeer, M.A. (2002). Biosynthesis of L-DOPA by Aspergillus oryzae. Bioresour Technol. 85: 25–29.
- Johirul, M., Shiddiky, A., Kim, R.E. (2005). Microchip capillary electrophoresis with a cellulose-DNA-modified screen-printed electrode for the analysis of neurotransmitters. Electrophoresis 26: 3043–3052.
- Wang, J., Zhou, Y., Wang, A.F. (2005). Determination of levodopa methyl ester and its metabolites in rat serum by CZE with amperometric detection. Anal Bioanal Chem. 382: 198–203.
- Blanco, M., Valverde, I. (2003). Chiral and non chiral determination of Dopa by capillary electrophoresis. J Pharm Biomed Anal. 31: 431–438.
- Lunte, S.M., O'Shea, T.J. (1994). Pharmaceutical and biomedical applications of capillary electrophoresis/electrochemistry. Electrophoresis 15: 79–86.
- Napolitano, A., Del, Dotto, P., Petrozzi, L. (1999). Pharmacokinetics and pharmacodynamics of L-Dopa after acute and 6-week tolcapone administration in patients with Parkinson's disease. Clin. Neuropharmacol 22: 24–29.
- Zhiquan, Z., Yong, S., Qiqing, Z., , et al. (2005). Assay of CCNU in tumor by RP-HPLC. Artificial Cells, Blood Substitutes, and Immobilization Biotechnology 33: 371–375.
- Chen, X., Xie, J., Li, C. (2004). Investigation of the factors that induce analyte peak splitting in capillary electrophoresis. J Sep Sc. 27: 1005–1010.