Abstract
Isolation and purification of bovine hemoglobin (HbBv) was carried out after reaction of whole blood with carbon monoxide. Washing/centrifugation steps were used to eliminate leukocytes, platelets, and plasma proteins. Hypotonic media and ultrasound radiation were used to lyse red blood cells. Lyse by ultrasound was shown to lead to solutions at the highest concentrations in HbBv, and the least concentrations in major phospholipids contaminants. Additional purification procedures were performed to remove membrane proteins and phospholipids. In the first case, proteins were denatured by thermal treatment, and filtered. To eliminate phospholipids, liquid chromatography was used with strong anion exchangers. Purity of HbBv was evaluated by normal phase high performance liquid chromatography (HPLC), electrophoresis, and size-exclusion HPLC.
1. INTRODUCTION
A significant number of research groups have been working on the development of oxygen carriers, so-called blood substitutes. These products are designed to replace the respiratory function of hemoglobin in cases of severe blood loss. They would offer clinical advantages over the transfusion of red blood cells, including a long shelf life, minimal risk of transmitting infectious diseases (they could be sterilized), and no need of previous typing and cross-matching [Citation[1-8]].
Currently, there are two major classes of oxygen carriers in phase III clinical trials, perfluorocarbon emulsions and hemoglobin-based oxygen carriers, which present different paradigms in relation to efficacy, safety, and production. In the case of hemoglobin-based oxygen carriers, the availability of pure hemoglobin is a key factor in the preparation of the final products. The low supply of human hemoglobin from outdated red blood cells consists in the most critical factor to its use in commercial products [Citation[9], Citation[10]]. By contrast, bovine blood is abundant, and may be collected in slaughterhouses from controlled healthy animals. Moreover, in the presence of physiological concentrations of chloride ions, bovine hemoglobin have an oxygen affinity similar to that of human hemoglobin completely saturated by its allosteric effector 2,3-diphosphoglycerate [Citation[2]].
Hemoglobin can transport oxygen outside the red blood cell. It is composed of two identical α and two identical β globin chains, each bound to a heme group. This structure is stabilized by hydrogen bondings, van der Waals forces, intra-and intermolecular salt bonds. In solution, tetrameric hemoglobin is in unfavorable equilibrium with its αβ dimers, which are easily filtered through the glomerulus. As a result, hemoglobin has a short half-life in circulation, and leads to tubule obstruction and renal failure [Citation[11]]. To prevent tetramer dissociation, hemoglobin has been chemically modified or encapsulated [Citation[12-14]]. Prior to these procedures, residual stroma composed of lipids and membrane proteins, water-soluble proteins other than hemoglobin, and possible pathogenic viruses should be removed [Citation[15], Citation[16]].
The preparation of stroma-free hemoglobin has been described in the literature, but few papers give detailed information on methods and quantitative evaluation on purity. Crystallization of human hemoglobin from sodium phosphate solution has been used for some years. Because of the long time required (4 days), crystallization has been replaced by chromatographic methods. Proteins other than hemoglobin, present in hemolysates, were separated by absorption on DEAE-cellulose (DEAE-52) at pH 7.5, using 0.01 mol/L sodium phosphate buffer, but no attempt was addressed to remove phosphoslipids [Citation[17]]. In order to minimize hemoglobin auto-oxidation, saturation of hemoglobin solutions with carbon monoxide (CO) was suggested. This procedure was followed as the first step to obtain concentrated solutions from outdated human red blood cells [Citation[18]]. Organic solvents were used for hemolysis and removal of stromata. Heating to 60–62°C led to denaturation of water-soluble proteins other than hemoglobin, which were removed by centrifugation [Citation[18]]. A more complex methodology was described for large-scale production of hemoglobin from outdated human red blood cells [Citation[19]]. For the extraction of phospholipids from red blood cells or aqueous solutions of hemoglobin, styrene-divinylbenzene commercial disks were used. Phosphatidylethanolamine, phosphatidylinositol, phosphatidylcholine, and sphingomyelin were recovered at 92% yield in average, whereas the recovery yield of phosphatidylserine was 65% [Citation[20]]. More recently, bovine hemoglobin (HbBv) was purified by a two-step process [Citation[21]]. In the first, ion-exchange chromatography was used to remove other proteins from hemolysates. In the second step, lipids from the cell membrane were eliminated by hydrophobic interaction chromatography. Optimization of the methodology was achieved by adding poly(ethylene glycol) to the phosphate buffer at pH 6.8 [Citation[21]].
In a previous work by our group, proteins other than hemoglobin were eliminated from lysed outdated human red blood cells, previously treated by CO gas, by thermal treatment at 60°C followed by filtration. Ion-exchange chromatography was used to remove residual phospholipids from small volumes of hemolysates. High-performance liquid chromatography and electrophoresis were performed to evaluate purity [Citation[22]]. In the present work, bovine hemoglobin (HbBv) was isolated and purified from whole bovine blood. Using available techniques to evaluate purity, HbBv solutions were obtained without the presence of phospholipids and proteins other than hemoglobin.
2. MATERIALS AND METHODS
2.1. Materials
Bovine blood was collected in an anticoagulant citrate/dextrose solution and maintained at 5°C until purification procedures. The dye-binding reagent used for albumin quantification (2.5 mM bromcresol in 0.82 M lactic acid at pH 4 containing 30 mL/L of Tween 80), the stock solution of bovine serum albumin at 0.4 g/L stabilized with sodium azide, Drabkin's reagent and hemiglobincyanide (HiCN) were purchased from Doles Reagentes (Goiânia, Brazil). Phospholipid standards phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidylcholine (PC) and sphingomyelin (SM), bovine serum albumin and MW-SDS-70L Kit for Electrophoresis were purchased from Sigma Chemical Co. (St. Louis, MO, USA) and used as received. Acetonitrile and methyl alcohol (HPLC grade) were provided by Vetec Química Fina Ltda. (Rio de Janeiro, Brazil).
Anion exchangers, AG MP-1 from BioRad Labs (Bromley, England) and Q Sepharose Fast Flow (Q-SFF) from Pharmacia Biotech (Wikströms, Sweden) were used after purification and conditioning. The AG MP-1 resin was washed with ethyl alcohol and with deionized water, treated with 0.1 N NaOH for 15 min and neutralized by successive washings, and finally treated with 0.1 N HCl for 15 min and neutralized by extensive washings.
All other reagents and solvents (PA grade) were supplied by Vetec Química Fina Ltda. (Rio de Janeiro, RJ, Brazil) and used as received.
2.2. Isolation of Hemoglobin
Bovine blood (200 g) was submitted to carbonylation reaction with CO gas under mild shaking for 180 s, to convert oxyhemoglobin (HbO2) to carbonylhemoglobin (HbCO) [Citation[18]], and centrifuged at 1000 g at 25°C for 20 min in a Hermle Centrifuge model Z 383 K (National Labnet Company Inc., New Jersey, USA) to eliminate leukocytes, platelets and some plasma proteins other than hemoglobin. The supernatant was discarded. The resulting suspension was washed with an equal weight of isotonic saline solution (0.9% NaCl, w/v), and centrifuged at 1000 × g for 20 min. The washing/centrifugation procedure was repeated three other times.
To verify the efficiency of the washing/centrifugation process, each supernatant was analyzed at 626 nm in a Thermolyne Turner spectrophotometer, model SP-870 (Dubuque, IA, USA) according to the bromcresol green method at pH 4 [Citation[23]], using a calibration curve prepared with dilute solutions of bovine serum albumin.
Hemolysis was performed in water, in 1 mM NaCl, and in 10 mM Tris/HCl buffer at pH 7.4, using 1:1 and 1:2 (w/w) ratios of bovine red blood cells concentrate/hypotonic solution at 8°C for 24 h. Hemolysis was also carried out by sonication at 8°C for 5 min with a 750 W Cole Parmer Processor (Vernon Hills, IL, USA), equipped with a standard pin of 13 mm diameter at 40% amplitude.
After hemolysis, the suspension of lysed cells was submitted to heating at 60°C for 1 h in a water bath under stirring in the dark, and centrifuged at 2000 × g for 40 min. The bottom layer was discarded. The resulting hemoglobin solution was filtered through 0.22 µm Millipore membranes.
2.3. Purification of Hemoglobin by Ion Exchange Chromatography
2.3.1. Small Volumes
A Flash 12i chromatography system, purchased from Biotage, Division of Dyax Corporation (Charlottesville, VA, USA) was used. Polyethylene columns of 75 mm × 12 mm were prepared by allowing 8.5 mL of the anion exchangers AG MP-1 or Q-SFF to settle under gravity. Mixed columns were also prepared by packing equal amounts of both resins successively. Prior to use, each column was equilibrated at room temperature and 1.0 Pa with 10 mM Tris/HCl pH 7.4 for approximately 30 min. Hemoglobin lysates (1 mL) were eluted with the same buffer at rates that varied (in the range 0.05–0.68 mL/min). Fractions of 1 mL were collected and analyzed by spectrophotometry to determine hemoglobin concentration.
2.3.2. Liquid Column Chromatography
Higher volumes of lysates (100 mL) were purified by liquid column chromatography. A glass column 55 mm in diameter and 200 mm in height was used with 23 mL of the anion exchangers AG MP-1 or Q-SFF, which were settled under gravity. Mixed columns were also prepared by packing equal amounts of both resins successively. After equilibration with 10 mM Tris/HCl pH 7.4 at room temperature for 12 h, hemoglobin lysates (100 mL) were eluted with the same buffer. Fractions of 5 mL were collected and analyzed by spectrophotometry to determine hemoglobin concentration.
2.4. Determination of Hemoglobin Concentration
Hemoglobin concentrations were determined by the hemiglobincyanide (HiCN) method [Citation[24]]. Hemoglobin lysates and purified hemoglobin solutions (20 µL) were mixed with 5 mL of 1:100 diluted Drabkin's reagent, homogeneized for 3 min and analyzed by absorption spectrophotometry at 540 nm. In each case, the hemoglobin concentration was determined in relation to a calibration curve, prepared with diluted solutions of standard HiCN.
2.5. Characterization of Isolated and Purified Bovine Hemoglobins
2.5.1. Extraction of Phospholipids
Phospholipids were extracted from the hemolysates and from hemoglobin solutions after purification by anion exchange chromatography, according to the literature [Citation[25]]. To 20 mL of a solution prepared by diluting 2 g of hemoglobin lysate with 25 mL of deionized water, 50 mL methyl alcohol and 25 mL methylene chloride were added. After stirring for 10 min, 25 mL of methylene chloride and 25 mL of 2 M KCl were added. The resulting mixture was stirred for 10 min and transferred to a decantation funnel. The lower layer was collected and dried at 40°C under low pressure. In the case of purified hemoglobin solutions, 1 g was taken from the two most concentrated fractions, to which 10 mL of deionized water were added, and the procedure described above was followed. The residue obtained by drying the lower layer was redissolved in 1 mL chloroform, filtered in poly(trifluorethylene) 0.22 µm membranes and used to analyze the presence of phospholipids by high performance liquid chromatography.
2.5.2. High Performance Liquid Chromatography (HPLC) of Residual Phospholipids
Normal phase HPLC was carried out with a Pharmacia LKB-HPLC pump model 2248 from Pharmacia-Biotech (Uppsala, Sweden), equipped with a HP 3396 Series II integrator from Hewlett Packard (Palo Alto, CA, USA). Separations were performed on a stainless-steel column (250 mm × 4.6 mm i.d.), packed with porous silica 100 Å in diameter, from Phenomenex, (Montgomeryville, PA, USA) at 30°C and a constant flow rate of 1.0 mL/min. Solutions of the standard phospholipids phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidylcholine (PC) and sphingomyelin (SM) were prepared in chloroform at 1.5 g/L. A stock solution of mixed standard phospholipids was prepared at 144 µg/mL PS, 10 µg/mL PE, 120 µg/mL PC, and 300 µg/mL SM concentrations, and used to quantify residual phospholipids from hemolysates and purified hemoglobin solutions. The samples were applied with a Hamilton syringe via a 20 µL Rheodyne 7125 injector (Cotati, CA, USA) and eluted with acetonitrile/methyl alcohol/phosphoric acid in 900:95:5 volume ratio [Citation[18], Citation[26]]. The same solvent mixture was used as mobile phase for the analysis of phospholipids extracted from hemoglobin experimental samples. The elution was monitored with a UV-Vis Shimadzu model SPD-10AV from Shimadzu Scientific Instruments (Columbia, MD, USA), set at 210 nm.
2.5.3. Electrophoresis
Sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out in a single-sided vertical Owl Scientific Inc. system, model P81 (Woburn, MA, USA), equipped with a Electrophoresis Power Supply E 835 from Consort nv (Turnhout, Belgium), at 2 W. Gels were stained in Comassie blue G-250 and destained in acetic acid/ethyl alcohol aqueous solution. The experiments were performed according to the procedure of Laemmli. Samples at 1 g/L concentration were prepared by heat denaturation at 100°C for 5 min in a buffer containing 1.0 mL 1 M Tris/HCl pH 6.8, 4 mL deionized water, 1.6 mL SDS at 10% (w/v), 0.8 mL glycerol at 87% (v/v), and 0.2 mL bromphenol blue at 0.05% (w/v). Hemoglobin and MW-SDS-70L solutions (10 µL) were applied to each lane of the gel and processed at 30 mA, 120 V, for 120–180 min. MW-SDS-70L markers are composed of bovine α-lactalbumin (MW ∼ 14,200), bovine trypsinogen (MW ∼ 24,000), bovine carbonic anhydrase (MW ∼ 29,000), glyceraldehyde-3-phosphate (MW ∼ 36,000), and bovine albumin (MW ∼ 66,000).
2.5.4. Size Exclusion HPLC of Purified Hemoglobin
Normal phase HPLC was carried out with the same equipment described in item 2.6.2, on a Biosep Sec S 3000 stainless-steel column (300 mm × 7.0 mm i.d.), packed with 5 mm porous silica 100 Å in diameter, from Phenomenex (Montgomeryville, PA, USA) at room temperature, and a constant flow rate of 0.7 mL/min. The hemoglobin sample was dissolved in 0.1 M Tris/HCl pH 7.4 with 0.2 M MgCl2 at 0.1% concentration. The same solvent mixture was used as mobile phase for the analysis. Elution was monitored with the same UV-Vis equipment, set at 280 nm.
3. RESULTS AND DISCUSSION
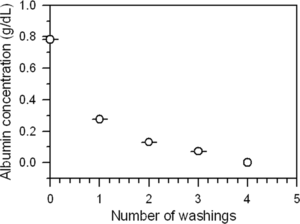
Carbonylation of whole bovine blood was performed as the first step of the purification methodology. This procedure was followed to take advantage of the heat-stability of carbonylhemoglobin [Citation[27]], and to facilitate the removal of other proteins by heat-induced denaturation and filtration in a later stage. After centrifugation and elimination of the supernatant, the suspension consisting of red blood cells (RBC) was washed with 0.9 M NaCl, and centrifuged four times. Every cycle of washing/centrifugation was accompanied by quantification of albumin (in the supernatant), by spectrophotometry at 626 nm. shows the decreasing concentration of albumin, from approximately 0.8 g/dL to undetectable levels.
As described in the experimental section, hemolysis was performed in hypotonic media and by ultrasound radiation. Water, 1 mM NaCl, and 10 mM Tris/HCl buffer pH 7.4, at 1:1 and 1:2 (w/w) ratios of RBC/hypotonic solution were used. In all cases, hemolysates were free from blood cells, as visualized by optical microscopy. As expected, hemolysis by sonication gave rise to the most concentrated hemoglobin solution (23.09 ± 3.60 g/dL), compared to hypotonic media in 1:1 ratio, which led to hemoglobin solutions in the range 14.24 ± 0.002 g/L to 15.81 ± 0.005 g/L. Since prolonged standing in a diluted solution is known to favor hemoglobin oxidation [Citation[28]], this method of hemolysis seems adequate mainly when stock solutions are produced to be stored for further uses.
Heat treatment at 60°C for 1 h was carried out to denature proteins other than hemoglobin. Centrifugation and filtration were used to remove cellular debris and denatured protein contaminants. Anion exchange chromatography was chosen to eliminate residual phospholipids.
Ion exchangers are the most widely used types of stationary phases for separation and purification of proteins. The relatively mild binding and elution conditions allow high recovery with intact biological activity. For small volumes, the Flash 12i chromatography system was used with AG MP-1, Q-SFF and two layers of each resin as stationary medium. Both AG MP-1 and Q-SFF resins are strong anion exchangers. In all cases, elution was carried out with 10 mM Tris/HCl pH 7.4. The objective was to adsorb lipids from the cell membrane and enable hemoglobin to flow through the exchange resins. Since HbBv is a globular protein 55 Å in diameter, and the pore dimensions of macroporous resins are larger than 100 Å, HbBv can partially pass through the pores under the applied pressure. Nevertheless, the red color of HbBv could be seen during the operation from outside of the cartridge, as well as outside of the glass column, and no significant retention of the protein was observed.
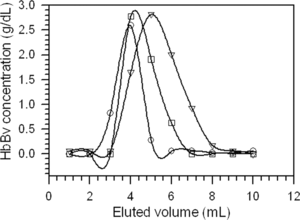
At pH 7.4, HbBv eluted at lower volumes on AG MP-1 stationary phase, and the phospholipids were retained on the top of each column. Chromatograms for hemoglobin eluates that had been lysed in water, in 1 mM NaCl, in 10 mM Tris/HCl pH 7.4, and by ultrasound radiation were obtained. As an example, shows the chromatograms of HbBv, previously hemolysed in water at 1:2 (w/w) ratio of RBC/water, on AG MP-1, Q-SFF, and on double layers of both resins at pH 7.4 using Tris/HCl buffer as the mobile phase. Only one peak was obtained with a reasonable width.
Figure 2 Average elution curves for the hemoglobin solution previously hemolysed in water at 1:2 ratio (w/w) of RBC/water; purified with AG MP-1 (○), Q-SFF (▿), and AG MP-1/Q-SFF layers (□). Points represent average values from analyses in triplicate.
Higher volumes of purified HbBv were obtained by liquid column chromatography. In this case, ultrasound radiation was the method chosen to lyse RBC. shows concentrations of HbBv determined for the most concentrated eluates. Differences in concentrations seem to rely on the physical properties of the resins, whereas AG MP-1 is formed by solid beads that favor eluent and eluate phase separation, Q-SFF resin is a gel, in which the eluent is easily mixed with the eluate.
Table 1. HbBv concentrations determined for the most concentrated eluates previously lysed by sonication, after purification by liquid column chromatography on AG MP-1, Q-SFF, and AG MP-1/Q-SFF layers
Normal phase HPLC with UV detection has been used to quantify the main phospholipids from the red cell membrane [Citation[18], Citation[22], Citation[26]]. To access HbBv purity in relation to the presence of residual phospholipids, this technique was used with organic extracts, before and after anion exchange chromatography. shows HPLC chromatograms of phospholipids markers, and phospholipids extracted from isolated hemoglobin solutions obtained by hemolysis in hypotonic media at 1:1 ratio of RBC/hypotonic medium, and by ultrasound. The lowest limit of sensitivity of the HPLC method for the detection of PS and PE was 0.01 µg/dL. For the detection of PC and SM, the lowest limits of sensitivity were 0.03 µg/dL, and 0.20 µg/dL, respectively. For the standards solutions, elution times of 4.16, 5.30, 10.00, and 15.20 min were observed for PS, PE, PC, and SM, respectively. SM appears as a double peak because consists of diastereomers [Citation[29]].
Figure 3 Normal phase HPLC chromatograms for phospholipids from a solution of commercially available markers (a); a water hemolysate (1:1 ratio of RBC/water) (b); a saline hemolysate (1:1 ratio of RBC/1 mM NaCl) (c); a buffer hemolysate (1:1 ratio of RBC/10 mM Tris/HCl pH 7.4) (d); an ultrasound hemolysate (e).
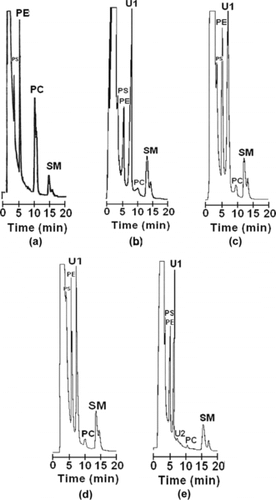
As reported previously for human hemolysates, two other peaks deserve attention. These peaks, denoted as U1 and U2, were attributed to diacylglyceride products resulting from degradation of phospholipids [Citation[26]]. The first peak was detected around 6.57 min for every hemolysate. Peak U2 was detected at 7.94 min for the sample hemolysed by ultrasound. shows an estimation of phospholipids concentrations detected by HPLC for hemolysates in water, saline, and buffer solutions, and hemolysates produced by sonication. Hemolysis by sonication led to the lowest concentrations of the main phospholipids, as observed before for human RBC [Citation[22]]. As observed in , phospholipids composition in hemolysates follows the same order as in bovine blood [Citation[30]], SM being the most abundant.
Table 2. Phospholipids concentrations in hemolysatesFootnotea, determined by normal phase HPLC
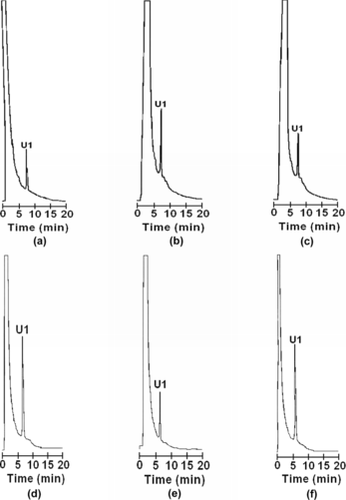
Additional purification of hemolysates on anion exchangers eliminated residual PS, PE, PC, and SM, according to HPLC chromatograms. No peak was observed at the elution times corresponding to these phospholipids. For example, in , HPLC chromatograms of organic extracts from water (1:1 ratio RBC/water) and ultrasound hemolysates, which had been additionally purified by liquid column chromatography on AG MP-1, Q-FF and AG MP-1/Q-FF resins, revealed only one peak at the elution time of U1.
Figure 4 Normal phase HPLC chromatograms of phospholipids from a water hemolysate submitted to additional purification by liquid chromatography on AG MP-1 (a); Q-SFF (b); AG MP-1/Q-SFF resins (c); an ultrasound hemolysate submitted to additional purification by liquid column chromatography on AG MP-1 (d); Q-SFF (e); AG MP-1/Q-SFF layers (f).
After storage for 60 days at − 18°C, organic extracts from ultrasound hemolysate samples, before and after additional purification, were analyzed by normal phase HPLC (). In the case of the hemolysate, as shown in , and in comparison with the results shown in , the concentrations of PS, and SM decreased, whereas the concentration of PE remained constant. As for PC, after storage, its concentration decreased to the point of not being detected. As for the diacylglyceride products U1 and U2, a decrease in the intensity of the U1 peak was observed, whereas U2 became a major peak (a). Peak U1 was completely eliminated in the chromatogram, after elution of the hemolysate on the Q-SFF resin (b). These results corroborate the hypothesis of degradation of stromal phospholipids with storage [Citation[26]].
Figure 5 Normal phase HPLC chromatograms of phospholipids from an ultrasound hemolysate (a), and an ultrasound hemolysate additionally purified on the Q-FF resin (b), after storage for 60 days at − 18°C.
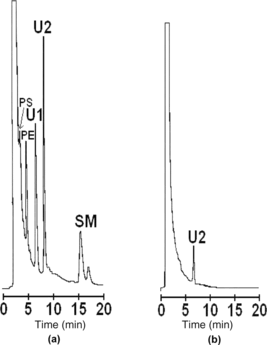
Table 3. Phospholipids concentrations in the ultrasound hemolysate after being stored for 60 days at − 18°C
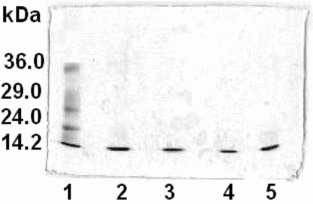
Analyses by SDS-PAGE electrophoresis were carried out for hemolysates and purified HbBv solutions. In , SDS-PAGE analyses for the hemolysate obtained by sonication (lane 2), and corresponding HbBv samples purified on AG MP-1 (lane 3), Q-SFF (lane 4) and AG MP-1/Q-SFF (lane 5) resins were compared with the solution of mixed standards prepared with Sigma MW-SDS-70L markers (lane 1). The hemolysate and the purified samples migrated as a single band at approximately 14 kDa, which reveals the presence of no protein contaminants.
Figure 6 SDS-PAGE electrophoresis analyses for the hemolysate obtained by sonication (lane 2), and corresponding HbBv samples purified by liquid column chromatography on AG MP-1 (lane 3), Q-SFF (lane 4), and AG MP-1/Q-SFF layers (lane 5), in comparison with the standard protein solution (lane 1).
The presence of only one peak eluted at 13.5 min from size-exclusion HPLC () indicates recovery of the hemoglobin tetramer and elimination of other proteins, such as α-, and β-spectrin, with higher molar masses.
CONCLUSION
Compared with hypotonic media, sonication can be indicated as a method for lysing red blood cells. The advantages of sonication are related to the recovery of bovine hemoglobin solutions at higher concentrations, with a lower concentration of the major phospholipids from the cell membrane. Thermal treatment of hemolysates was efficient to denature other proteins than hemoglobin, previously transformed into carbonylhemoglobin by reaction with carbon monoxide gas. Denaturing polyacrylamide gel electrophoresis revealed the presence of hemoglobin alone. Liquid chromatography on strong anion exchangers carried out with 10 mM Tris/HCl pH 7.4 at room temperature removed residual phospholipids, as shown by UV-monitored high performance liquid chromatography of organic extracts. Using sonication as the hemolysis method, the entire process takes 15 h, and produces purified bovine hemoglobin at 16.7 g/dL concentration.
The authors thank CNPq/MS (Proc. 50.5598/2004-3), CNPq (Proc. 47.5320/2004-2), and FAPERJ (Proc. E-26/151.969/2004) for financial support.
REFERENCES
- Chang, T.M.S. (2000). Red blood cell substitutes. Baillière's Clin. Haematol. 13: 651–667.
- Riess, J.G. (2001). Oxygen carriers (“blood substitutes”) – raison d' être, chemistry, and some physiology. Chem. Rev. 101: 2797–2919.
- Moore, E.E. (2003). Blood substitutes: The future is now. J. Am. Coll. Surg. 196: 1–17.
- Chang, T.M.S. (2004). Future generations of red blood substitutes. J. Int. Med. 253: 527–535.
- Winslow, R.M. (2003). Current status of blood substitute research: Towards a new paradigm. J. Int. Med. 253: 508–517.
- Cohn, S.M. (2004). Alternatives to blood in the 21st century. Crit. Care. 8: S15–S17.
- Anbari, K.K., Garino, J.P., Mackenzie, C.F. (2004). Hemoglobin substitutes. Eur. Spine J. 13 (Suppl 1): S76–S82.
- Stowell, C.P. (2005). What happened to blood substitutes? Qúest il arrivé aux substituts du sang? Tranf. Clin. Biol. 12: 374–379.
- Goodnough, L.T., Scott, M.G., Monk, T.G. (1998). Oxygen carriers as blood substitutes. Clin. Orthop. Rel. Res. 357: 89–100.
- Cohn, S.M. (2000). Blood substitutes in surgery. Surgery 127: 599–602.
- Palaparthy, R., Wang, H., Gulati, A. (2000). Current aspects in pharmacology of modified hemoglobins. Adv. Drug Delivery Rev. 40: 185–198.
- Chang, T.M.S. (1999). Future prospects for artificial blood. TIBTECH 17: 61–67.
- Chang, T.M.S., D'Agnillo, F., Yu, W.P., Razack, S. (2000). Two future generations of blood substitutes based on polyhemoglobin-SOD catalase and nanoencapsulation. Adv. Drug Delivery Rev. 40: 213–218.
- Chang, T.M.S. (2004). Hemoglobin-based red blood cell substitutes. Artif. Org. 28: 789–794.
- Feola, M., Simoni, J., Canizaro, P.C. (1991). Quality control of hemoglobin solutions. I. The purity of hemoglobin before modification. Artif. Org. 15: 243–248.
- Tsuchida, E., Takeoka, S. (1995). Stabilized hemoglobin vesicles, in Artificial Red Cells, E. Tsuchida, Ed., John Wiley & Sons: Chichester, Chap. 3, pp. 35–64.
- Cheung, L.C., Storm, C.B., Gabriel, B.W., Anderson, W.A. (1984). The preparation of stroma-free hemoglobin by selective DEAE-cellulose absorption. Anal. Biochem. 137: 481– 484.
- Sakai, H., Takeoka, S., Yokohama, H., Seino, Y., Nishide, H., Tsuchida, E. (1993). Purification of concentrated hemoglobin using organic solvent and heat treatment. Protein Exp. Purif. 4: 563–569.
- Winslow, R., Chapman, K.W. (1994). Pilot-scale preparation of hemoglobin solutions. Methods Enzymol. 231: 3–16.
- Horne, T., Holt-Larkin, S. (1997). Solid-phase extraction of phospholipids from hemoglobin solutions using Empore styrene-divinylbenzene disks. J. Chromatogr. B 695: 259–267.
- Lu, X., Zhao, D., Ma, G., Su, Z. (2004). Polyethylene glycol increases purification and recovery, alters retention behavior in flow-through chromatography of hemoglobin. J. Chromatogr. A 1059: 233–237.
- Andrade, C.T., Barros, L.A.M., Lima, M.C.P., Azero, E.G. (2004). Purification and characterization of human hemoglobin: Effect of the hemolysis conditions. Int. J. Biol. Macromol. 34, 233–240.
- Miyada, D.S., Baysinger, V., Notrica, S., Nakamura, R.M. (1972). Albumin quantification by dye binding and salt fractionation techniques. Clin. Chem. 18: 52.
- Zwart, A., van Asseendeltf, O.W., Bull, B.S., England, J.M., Lewis, S.M., Zijlstra, W.G. (1996). Recommendations for reference method for haemoglobinometry in human blood (ICSH standard 1995) and specifications for international haemiglobincyanide standard (4th edition). J. Clin. Pathol. 49: 271–274.
- Bligh, E.G., Dyer, W.J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917.
- Nakai, K., Sekiguchi, S. (1995). Quality control of stroma-free hemoglobin, in Artificial Red Cells, E. Tsuchida, Ed., John Wiley & Sons: Chichester, Chapter 7, pp. 131–149.
- Yang, T., Olsen, K.W. (1988). Effects of crosslinking on the thermal stability of hemoglobins. 2. The stabilization of metmonoxyhemoglobin, cyanometmonoxy-hemoglobin, and carbonmonoxyhemoglobin-S with bis(3,4-dibromosalicyl)fumarate. Arch. Biochem. Biophys. 261: 283–290.
- Williams, R.C., Tsay, K.-Y. (1973). Convenient chromatographic method for preparation of human hemoglobin. Anal. Biochem. 54: 137–145.
- Ramstedt, B., Slotte, J.P. (2000). Separation and purification of sphingomyelin diastereomers by high-performance liquid chromatography. Anal. Biochem. 282: 245–249.
- Rehman, S.U. (1991). Rapid isocratic method for the separation and quantification of major phospholipid classes by high-performance liquid chromatography. J. Chromatogr. 567: 29–37.