Abstract
Polyhemoglobin (polyHb) is currently being assessed in phase III trials under various formulations. At present, none contain clotting factors or platelet substitutes to aid in hemostasis. We have prepared a novel blood substitute that is an oxygen carrier with platelet-like activity. This is formed by crosslinking fibrinogen to hemoglobin to form polyhemoglobin-fibrinogen (polyHb-Fg). This was studied and compared to polyHb for its effect on coagulation both in vitro and in vivo. In the in vitro experiments, PolyHb-Fg showed similar clotting times as whole blood, whereas polyHb showed significantly higher clotting times. This result was confirmed in in vivo experiments using an exchange transfusion rat-model. Using PolyHb, exchange transfusion of 80% or more increased the normal clotting time (1–2 mins) to > 10 mins. Partial clots formed with PolyHb did not adhere to the tubing wall. With PolyHb-Fg, a normal clotting time is maintained, even with 98% exchange transfusion.
INTRODUCTION
Polyhemoglobin (polyHb) has been developed as a blood substitute for a number of years [Citation[1-3]]. It is currently approved in South Africa for use in surgeries. PolyHb is a blood substitute that is sterilizable, with a more than one-year shelf life at room temperature, and eliminates the need for cross-matching and typing [Citation[2-5]]. Though the blood supply has never been safer, there will continue to be new and emerging risks, like West Nile virus and variant Creutzfeldt-Jakob disease, which are cause for concern [Citation[6]]. PolyHb offers multiple benefits compared to blood by offering a safe and limitless alternative. Current applications are grouped into three areas: perioperative applications, acute hemorrhagic shock, and regional perfusion [Citation[7]].
As an oxygen carrier, polyHb is one of the most advanced in the area. Blood, however, is a multifunctional fluid [Citation[8]]. As a blood substitute, polyHb is limited by its lack of platelets and/or coagulation properties. In situations of high blood volume loss, dilutional coagulopathy could occur when large amounts of polyHb are administered [Citation[9]]. PolyHb would only be able to act as a bridge to whole blood transfusion as the platelets and coagulation factor concentrations in the polyHb-transfused recipient may not be sufficient for primary hemostasis. There has been development in platelet substitutes to combat thrombocytopenia, but with limited success [Citation[10], Citation[11]].
The aim of this study was not to design a treatment for thrombocytopenia, but to develop a polyHb that can be used for severe hemodilution so that it can deliver oxygen to tissues while being able to reduce the potential for excessive bleeding. This study describes the development of a novel blood substitute, polyhemoglobin-fibrinogen (polyHb-Fg), which is capable of having similar clotting times as whole blood. PolyHb and polyHb-Fg were compared by measuring their effects on whole blood clotting time in vitro and in vivo.
MATERIALS AND METHODS
Sample Preparation
Glutaraldehyde (glut), 25% aqueous solution and bovine fibrinogen were purchased from Sigma Company. Lysine (lys) was purchased from Fisher Scientific. All other reagents were of analytical grade.
Stroma-free hemoglobin (SFHb) was prepared as previously described [Citation[8]]. Briefly, whole bovine heparinized blood (from the McGill Animal Resource Centre) was centrifuged and washed with saline three times to separate the plasma proteins, white blood cells and platelets from the red blood cells. The red blood cell solution was then lysed with a sodium phosphate buffer (15 mOsmal, pH 7.4) in order to release the hemoglobin. The stroma was separated from the Hb by two toluene extractions. The final Hb solution was then dialysed (14000 MWCO, Spectropor) against saline before further use (Baxter).
PolyHb was prepared as previously described [Citation[8]], with modifications. Briefly, 10 mL of SFHb was mixed on an orbital shaker at 170 RPM with a 1.3 M lysine solution in a molar ratio of 10:1 lysine to hemoglobin at 5°C. Degassed, 25% aqueous glutaraldehyde was added in four equal aliquots over a period of 15 minutes in glutaraldehyde to hemoglobin molar ratio of 20:1.
PolyHb-Fg was prepared in a similar way as polyHb. A fibrinogen solution of 40 mg dissolved in 4 mL of Ringer's lactate was added 4 hours after polymerization began. After 24 hours of polymerization, for both polyHb and polyHb-Fg, the reaction was stopped by quenching with 2.0 M lysine solution in a molar ratio of 200:1 lys to Hb. The solutions were then dialysed against a Ringer's lactate solution overnight.
In Vitro Clotting Time Experiments
Blood was collected from male Sprague Dawley rats, 180–350 g (Charles River), via cardiac puncture. 5 mL syringes (Becton Dickenson) containing no anticoagulant were used with 20 G needles (Becton Dickenson). The rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (65 mg/kg) and sacrificed once the experiment was complete via lethal injection and/or cervical dislocation.
Glass tubes were prepared with 250 µL and 400 µL of blood substitute and labeled. Two hundred and fifty microliter aliquots of fresh blood were added to the 250 µL aliquots of blood substitute. One hundred microliter aliquots of fresh blood to the 400 µL aliquots of blood substitute. The timing was started when the fresh blood was added. The tubes were left untouched for one minute, after which the tubes were tilted 80° and back to check for clot formation. Clotting time was defined as the point at which the blood no longer flows from its position when inverted [Citation[12]]. If the sample had not yet clotted, tubes were tilted in a similar manner every 10s thereafter. If 2 minutes had gone by without clot formation, the tubes were tilted every 30s. Once a clot had formed, the time was noted as well as a description of the clot. If a clotting time was not reached after 10 minutes, the time was recorded as “ > 10 min.” Measurements were performed in triplicate.
Determination of Hemoglobin Concentration
Hemoglobin (Hb) concentration was measured by spectrophotometric analysis with “Total Hemoglobin Kit” from Sigma-Aldrich.
Screening for Molecular Weight
MicrosepTM centrifugal devices (Pall) were used to determine a general molecular weight distribution. Samples were diluted 10-fold with saline and placed in the 1000 K MWCO device. Samples were spun at 5000 g and 4°C for 8–50 minutes, until the level in each compartment of the centrifugal device remained stable. The portion that passed through the filter was put into a 300 K MWCO device and spun at similar conditions. The volumes and Hb concentrations of each portion were used to calculate the molecular weight distribution of the sample.
Molecular Weight Distribution and Determination of Free Fibrinogen
Samples of fibrinogen (1 mL of 7 mg/mL) and polyHb-Fg (1 mL of 33X dilution) were analyzed via size-exclusion chromatography. A Sephacryl 300 HR column (1.6 cm × 70 cm, Vtotal = 105 mL) was prepared and equilibrated with 0.1 M Tris-HCl (pH 7.5). Samples were passed through the column at a rate of 12 mL/hour. The elution profiles were monitored at 280 nm.
In Vivo Clotting Time Experiments
Male Wistar rats, 250–300 g (Charles River), were anesthetized with an intraperitoneal injection of sodium pentobarbital (65 mg/kg). The left femoral artery was isolated and artery branches were ligated so that a 1.5 cm segment was exposed. The femoral artery was then ligated at the distal end, with a clamp used to prevent blood leakage. The femoral vein on the right side was cannulated with a catheter filled with 0.9% isotonic saline (Baxter) flowing at an extremely slow rate to prevent clotting. Two blood samples were collected from the artery into capillary tubes for hematocrit measurement. A 0.5 mL sample was collected from the artery segment into a glass culture tube and the clotting time was measured as described earlier. The blood substitute solution, pre-warmed to 37°C and 0.22 µm filtered, was pumped through the right vein catheter at a rate of 0.5 mL/min. Blood volume was controlled by monitoring the volume of blood substitute infused into the animal and allowing an equal amount of blood out by releasing the clamp on the clamped segment of the artery. At 5 minute intervals, blood samples were collected from the artery and clotting time was measured. If the artery became clogged, forceps were gently rubbed along the artery in order to remove the clot. There were six animals for each polyHb and polyHb-Fg group.
Statistical Analysis
Clotting time data was analyzed using the Wilcoxon rank sum test. All other data was analyzed with an unpaired student t-test. Differences are defined as statistically significant if p < 0.05. All figures show error bars of one standard error.
RESULTS
Molecular Weight Screening
A general molecular weight distribution was determined. The results are shown in .
Table 1. Molecular weight distributions
Molecular Weight Distribution: PolyHb-Fg and Free Fibrinogen
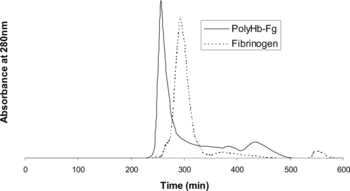
Fibrinogen and polyHb-Fg samples were analyzed on a size-exclusion column to determine the amount of free fibrinogen that remained after polymerization of the polyHb-Fg (). The fibrinogen peak occurs around 300 minutes after sample loading whereas the polyHb-Fg sample has a peak value of approximately 260 minutes. This shows that fibrinogen has been bound to polyHb-Fg solution after polymerization.
In Vitro Clotting Time Experiments
There were significant differences between the polyHb and polyHb-Fg samples (). With polyHb, the clots that formed did not adhere to the glass tubes and no clotting time could be assessed. On the other hand, all of the clots that formed for polyHb-Fg stuck to the walls of the glass tube and could be quantified with a clotting time (p < 0.01). shows the concentration dependence of the clotting time for polyHb-Fg. The polyHb samples still had not clotted completely at similar times as the polyHb-Fg samples according to the definition of the end point for clot formation [Citation[12]]. The polyHb tends to form partial clots that do not adhere to the glass tube.
Figure 2 Clotting times comparison. 10:1 lys, 20:1 glut with 40 mg of fibrinogen dissolved in 4 mL Ringer's lactate added at T = 4 h. All samples unfiltered, [Hb] ∼ 7.2 g/dL. p < 0.01. Figure shows average±SEM.
![Figure 2 Clotting times comparison. 10:1 lys, 20:1 glut with 40 mg of fibrinogen dissolved in 4 mL Ringer's lactate added at T = 4 h. All samples unfiltered, [Hb] ∼ 7.2 g/dL. p < 0.01. Figure shows average±SEM.](/cms/asset/65129abf-f4e4-4081-904d-e57f85e2090f/ianb19_a_258474_f0002_b.gif)
Table 2. Clotting times with fibrinogen added at T = 4 h. Clotting time measurements for 250 µL of blood substitute to which 250 µL of fresh blood is added
It should be noted that these in-vitro results do not necessary reflect what would happen in-vivo. For in-vivo studies we have to take into consideration the presence of different fluid compartments and continuous source of platelets and clotting factors. Thus, the following in-vivo study was carried out.
In Vivo Clotting Time Experiments
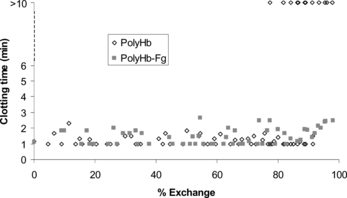
The results are shown in . After filtration, the average hemoglobin concentration for either polyHb or polyHb-Fg was approximately 5 g/dL. PolyHb displayed similar clotting times as polyHb-Fg for exchange percentages up to approximately 80%. Beyond this for PolyHb clots that formed did not always stick to the sides of the tubes and would slide freely. Beyond 93% exchange, no clots stuck for PolyHb. In contrast, the clotting times for polyHb-Fg remained normal up to 98% when there was a slight increase in the clotting time. When using PolyHb-Fg, the arteries had to be cleared more often than when using polyHb.
DISCUSSION
PolyHb has been successfully used as a blood substitute in clinical trials involving perioperative uses [Citation[2], Citation[3]]. In cases of extreme hemorrhagic shock, large amounts of infused polyHb may result in extreme hemodilution requiring the supplement of platelets or coagulation factors. In this study, we have shown that polyHb-Fg was able to maintain similar clotting times as whole blood whereas polyHb could not, both in vitro and in vivo. While polyHb was not able to form normal clots, it did still have some ability to form incomplete clots that were not able to adhere to the sides of the tubes. If these incomplete clots are not able to adhere, the possibilities for embolism increase. In our in vitro studies, blood hemodiluted with polyHb-Fg consistently had similar clotting times as normal blood where the clots firmly adhered to the tube walls. In addition to polyhemoglobin, fibrinogen can also be similarly cross-linked to any type of hemoglobin-based oxygen carriers including conjugated hemoglobin, recombinant hemoglobin, and nonencapsulated hemoglobin.
Professor T. M. S. Chang acknowledges the grant support from the Canadian Institute of Health Research and the MSSS-FRSQ Research Team on Blood Substitutes in Transfusion Medicine. N. S. W. Wong acknowledges her scholarship from the Natural Sciences and Engineering Research Council (NSERC).
REFERENCES
- Chang, T.M.S. (1971). Stabilization of enzyme by microencapsulation with a concentrated protein solution or by crosslinking with glutaraldehyde. Biochem Biophys. Res. Com. 44: 1531–1533.
- Gould, S.A. , et al. (2002). The life-sustaining capacity of human polymerized Hb when red cells might be unavailable. J. Am. Coll. Surg. 195: 445–452.
- Pearce, L.B., Gawryl, M.S., Rentko, V.T., Moon-Massat, P.F., Rausch C.W. (2006). HBOC-201 (Hb Glutamer-250 (bovine), hemopure): Clinical studies. in Blood Substitutes, R. Winslow, Ed., Academic Press: San Diego, pp. 437–450.
- Kjellstrom, B.T. (2003). Blood substitutes: Where do we stand today? Journal of Internal Medicine 253: 495–497.
- Chang, T.M.S. (2006). Nanobiotechnology-based blood substitutes. Trends in Biotechnology 24: 72–377.
- Busch, M.P., , et al. (2003). Current and emerging infectious risks of blood transfusions. JAMA 289(8): 959–961.
- Moore, E.E. (2003). Blood substitutes: The future is now. Journal of American College of Surgeons 196(1): 1–17.
- Chang, T.M.S. (2007). Artificial Cells: Biotechnology, Nanotechnology, Blood Substitutes, Regenerative Medicine, Bioencapsulation, Cell/Stem Cell Therapy, World Scientific Publisher: Singapore.
- Leytin, V., , et al. (2003). Hemolink, an o-raffinose cross-linked haemoglobin-based oxygen carrier, does not affect activation and function of human platelets in whole blood in vitro. British Journal of Haematology 120: 535–541.
- Lee, D.H., Blajchman, M.A. (2000). Platelet substitutes and novel platelet products. Expert Opinion on Investigational Drugs 9(3): 457–469.
- Lapierre, V., Herve, P. (1999). Perspectives. La Presse Medicale 28(4): 1336–1340.
- Lee, R.I., White, P.D. (1913). A clinical study of the coagulation time of blood. The American Journal of Medical Sciences 145: 495–503.