Abstract
In this study, immunogenicity of human hemoglobin (hHb), bovine hemoglobin (bHb), porcine hemoglobin (pHb) and their glutaradehyde polymerized derivatives (hPolyHb, bPolyHb and bPolyHb, respectively) were compared. The nature of the dominant antigen determinants of the chemically polymerized proteins was studied. Glutaraldehyde chemical reaction enhanced the immunogenicity of the hemoglobin derivatives. In mice, the extent of the enhancement was largely comparable among hPolyHb, bPolyHb and pPolyHb. Using the methods of semi-quantitative western blotting and quantitative protein array, it was found that most of the polycloncal antibodies raised in rodents against glutaraldehyde polymerized hemoglobin derivatives of human, bovine or porcine species only weakly or did not cross-react with the hemoglobin derivatives of the other two species, indicating that hPolyHb, bPolyHb and bPolyHb vary significantly in their dominant antigen determinants, despite very high degree of identity in their primary amino acid sequences and high similarity in their three dimensional structures.
INTRODUCTION
Hemoglobin-based therapeutics have a wide rang of potential clinical applications, especially for use as oxygen carriers in prevention and treatment of disorders or pathological conditions with deficiency of tissue oxygen delivery, such as sever blood loss, hemorrhagic shock, trauma, and cardiac conditions [Citation[1]]. For the last 20 years or so, hemoglobin-based oxygen carriers (HBOCs) have been vigorously pursued for therapeutic development using hemoglobin from different sources and produced with different methods [Citation[2]]. Human and bovine hemoglobin have been the most widely used raw material for manufacturing of HBOCs. However, due to the limited supply of human Hb and the possible threat of human blood-transmitted diseases such as hepatitis and HIV and cross-species transmission of mad cow diseases, porcine Hb has also been added to the portfolio of HBOC raw material [Citation[3]]. Among different types of HBOCs, glutaraldehyde polymerized products have gained most advanced progress in commercial development. Hemopure of Biopure Co., USA, a glutaraldehyde polymerized bovine Hb, has obtained market approval in South Africa and proceeded at the last stage of application for a biological license from the U.S. FDA [Citation[1]]. PolyHeme of Northfield Laboratories, USA, a glutaraldehyde polymerized human Hb, is at the final stage of US multicenter phase III clinical trials [Citation[1], Citation[4]]. Lifegen Ltd., China, has developed a product of glutaraldehyde polymerized porcine Hb, which is now in a pre-clinical development stage [Citation[3]].
One of the major safety concerns with regard to HBOCs, especially the ones made from Hb of animal origins, is their possible effect on the immune system or possibility of causing immunological disorders [Citation[5]]. Immunogenicity of hemoglobin [Citation[6], Citation[7]] and chemically polymerized derivatives [Citation[8-10]] has been studied in different laboratories. It has been found that polymerization may enhance immunogenicity of hemoglobin [Citation[7-9]]. Glutaraldehyde polymerization increases immunogenicity of human, bovine and porcine hemoglobin [Citation[10-12]].
This study is to further investigate the immunogenicity of hemoglobin from human, bovine or porcine origin and their related glutaraldehyde polymerized derivatives namely, glutaraldehyde polymerized human hemoglobin (hPolyHb), glutaraldehyde polymerized bovine hemoglobin (bPolyHb) and glutaraldehyde polymerized porcine hemoglobin (pPolyHb), in order to gain insight about the nature of their dominant antigen determinants, which may have important applications in our understanding of the immunological nature of individual hemoglobin products and in the interpretation of experimental and clinical findings with regard to impacts of individual HBOC product on immunological functions of the body.
MATERIAL AND METHODS
Material and Animals
All chemicals, such as NaCl, NaOH, trehalose, sodium dodecyl sulfate (SDS) and (3-glycidoxypropyl)trimethoxy silane (GST), were all purchased from Sigma, USA, otherwise stated specifically. Gold Seal microscope glass slides were from Electron Microscopy Sciences, USA. Hybond—ECL membrane was from Amersham Biosciences, Germany. Horseradish peroxidase (HRP)-conjugated polyclonal antibodies against mouse, rat or rabbit Ig G were purchased from DinGuo Biotechnology Ltd., China. BALB/c mice, Sprague-Dawley rats or New Zealand rabbits were purchased from the Animal House of the Medical School of Xi'an Jiaotong University, China. The animals were maintained and used in the experiments under the caring environment in accordance with the requirements of the Northwest University, Xi'an, China.
SDS-PAGE and Silver Staining
Protein analysis by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was carried out on a mini-gel electrophoresis apparatus (Electrophoresis Cell, Model DYCZ-24D, Beijing Liuyi Instruments, China) using the Laemmli's system described earlier [Citation[13]]. Protein samples were boiled for 3–5 min in the presence of 2% w/v SDS and 100 mM 2-mercaptoethanol prior to being loaded to the gel of 10% or 20%. Proteins separated on SDS-PAGE were visualized by a silver staining method [Citation[14]].
Western Blotting
The western blotting was performed largely on the basis previously described [Citation[13]]. Briefly, proteins resolved on SDS-PAGE gel were transferred to a nitrocellulose membrane. After blocking with 1% milk powder in phosphate buffer saline and binding with an appropriate primary mouse polyclonal anti-hemoglobin antibodies with an appropriate dilution, horseradish peroxidase (HRP)-conjugated secondary antibodies (rabbit polyclonal antibodies against mouse Ig G) incubated with the membrane for 1 h at room temperature. The protein bands on the membrane was then visualized on a Kodak XBT-1 film by adding ECL western blotting substrate (Pierce, USA) to the surface of the membrane.
Purification of Hemoglobin and Polymerization of Hemoglobin
Purification of hemoglobin from blood of various species and polymerization of the purified hemoglobin with glutaraldehyde as the cross-linker was performed as the methods described before [Citation[3]]. Briefly, hemoglobin from the porcine, bovine or human blood was purified through a series of steps, with the final purified hemoglobin had at least 99.9% purity judged by size exclusion chromatography (SEC) and SDS-PAGE analysis. The highly pure hemoglobin then underwent a controlled polymerization by mixing a certain amount of glutaraldehyde with hemoglobin sample. The degree of the polymerization was controlled in such a condition so that resultant polymerized products had a similar SEC profile to ensure that all products had almost the same apparent molecular weight distribution.
Size Exclusion Chromatography (SEC) Analysis
Analysis of purity of purified hemoglobin and molecular distribution of polymerized hemoglobin was performed by fast liquid chromatography using a Superdex 200 10/300 GL column (Amersham Biosciences) operated on the ÄKTAexplorer 100 Air system (Amersham Biosciences). An appropriate amount of a protein sample was filtered through a membrane with pore size of 0.2 µm and then loaded onto the column. The column was then eluted with 50 mM phosphate, pH 7.2 at a flow rate of 0.5 ml/min. The eluted proteins were monitored at both 280 nm and 415 nm.
Animal Immunizing/Boosting and Anti-Serum Preparation
Female BALB/c mice (18±2 g), Sprague-Dawley rat (200±10 g) or New Zealand rabbits (1.8±0.2 kg) were used in the experiments. Before the immunization, a blood sample was drawn from each animal. Serum was isolated from the blood and stored in small aliquots as pre-immune control serum. Solution of appropriate hemoglobin or related polymerized hemoglobin was diluted with 0.9% NaCl to 1 mg/ml. A certain amount of hemoglobin protein (50 µg/mouse, 100 µg/rat or 500 µg/rabbit) was subscutanously injected into each animal in the absence or presence of complete Freund's adjuvant (the volume ratio of the protein sample and adjuvant as 1:1). For subsequent boosting in which adjuvant was included, incomplete Freund's adjuvant was used in place of the complete Freund's adjuvant in the sample preparation and injected through the same rout. Blood samples were collected 12 to 14 days after each of the boosting injections. Serum samples were then made and stored in aliquots at –20°C until use.
Immunoassay
An indirect ELISA method was used to semi-quantitatively measure the serum porcine hemoglobin-specific antibodies [Citation[12]]. Serum samples with a series of dilution were tested. The result of the assay of a serum sample was expressed in a p/n ratio, where p was the mean of OD450 readings of three parallels of x fold-diluted sample of the tested serum of an animal and where n was the mean of OD450 readings of three parallels of the same x fold-diluted sample of the pre-immune serum of the same animal. Tested samples with p/n ratio value larger than 2 were considered to be positive.
Antibody Purification
Antibodies in serum were purified using methods of ammonia sulfate precipitation and anion exchange chromatography as described below. Saturated sulfate ammonia solution was mixed well with an equal volume of serum in test tube. After standing still at 4°C for 2 h, the tube was spun 5000 rpm for 20 min at 4°C. The precipitate was re-dissolved in 15 mM phosphate buffer (pH 6.3), called PB buffer. The sample was then dialyzed against PB buffer at 4°C for 18–20 h with the dialyzing buffer changed every 6–10 h. The dialyzed sample was applied to an anion exchange chromatographic column, which had been pre-washed and balanced with PB buffer. The flow-through from the column was collected and mixed with saturated sulfate ammonia solution at 2:3 volume ratio of flow-through sample and saturated sulfate ammonia solution. After standing still at 4°C for 2 h, the precipitate was obtained by centrifugation and then re-dissolved in phosphate buffered saline solution (PBS). After dialyzed against PBS, the purified antibody sample was tested for protein concentration with Bradford method [Citation[14]]. Aliquots of the samples were stored at –20°C until use.
Antibody Labeling
Serum antibodies were labeled with the monofunctional N-hydroxysuccinimide NHS-ester of the dye Cy3 (Amersham Bioscience, Freiburg, Germany) as recommended by the manufacturer.
Protein Arrays
A protein array method was used in a study of antibody-antigen binding reaction modified from a published method and briefly described below [Citation[15]].
GPTS Surface Derivatisation of Glass Slides
Glass slides were washed with ethanol and then etched by immersion in 10% NaOH at room temperature for 1 h. Immersed in 10% NaOH, the glass slides were cleaned by sonification for 15 min, rinsed four times in water and then washed twice in ethanol. The surface of the cleaned glass slides were treated with 2.5% GPTS dissolved in 10 mM acetic acid in ethanol for 1 h. After silanisation, GPTS-treated slides were washed thoroughly with ethanol, dried at room temperature and stored in a dried container at room temperature until use.
Fabrication of Hemoglobin Protein Arrays
Polymerized hemoglobin samples were diluted in spotting buffer (100 mM NaHCO3, 300 mM NaCl, 0.5% trehalose, pH 8.4) to appropriated concentrations. Spotting of antibodies was carried out with SmartArrayerTM-48 (CapitalBio Co., Beijing, China). The spotted slides were kept at 4°C overnight and then blocked by PBS containing 5% fat-depleted milk powder for 4 h at room temperature. The slides was rinsed four times with PBS containing 0.05% Tween-20 (PBS-T) and then washed twice with PBS. The slides were centrifuged at 500 rpm for 5 min to dry and used immediately.
Incubation of the Hemoglobin Protein Arrays with Cy3-Labeled Antibodies
Solution of Cy3-labeled antibodies (35 µg/ml) against appropriate polymerized hemoglobin was added to the surface of the hemoglobin protein arrays. The area, where protein arrays were located, was then covered with a coverlid to avoid evaporation. The glass slides were incubated at room temperature under nearly 100% humidity for 1 h. After several washes with PBS, the glass slides were centrifuged to dry and stored at 4°C before scanning.
Scanning and Evaluation
Fluorescence signals were detected on a GenePix 4000 A unit and analyzed with the GenePix software package (Axon Instruments, Union City, CA, USA).
Hemoglobin Sequence Alignment Analysis
ClustalW software operated online from EMBL-EBL (European Bioinformatics Institute) was used to perform multiple alignment analysis of the sequences of hemoglobin of various origins, which were obtained from a protein sequence database (Swiss-Prot) accessed via the website of the National Center for Biotechnology Information (NCBI).
RESULTS
Production of Glutaraldehyde Cross-Linked/Polymerized Hemoglobin
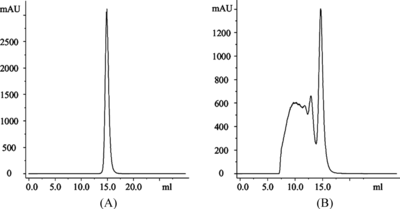
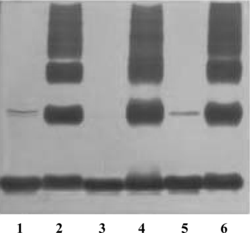
In order to conduct comparative studies of immunogenicity of hemoglobin from different sources, ultra-pure hemoglobin is required. In this study, hHb, bHb or pHb was highly purified to a purity over 99.9% determined by SDS-PAGE and SEC analysis. Our studies demonstrated that the strength of immunogenicity of glutaraldehyde cross-linked/polymerized porcine hemoglobin significantly varies with the size of the molecules (data to be published). Therefore, this study used the polymerized derivatives of hemoglobin, hPolyHb, bPolyHb and pPolyHb, which had comparable molecular distribution of their components, with molecular weight ranging from 64 kD to more than 1,000 kD with average molecular weight about 500 kD. As an example, shows the SEC profile of purified pHb and pPolyHb, respectively. The results of SDS-PAGE analysis of the purity of these hemoglobin and status of chemical cross-linking of the related glutaraldehyde-mediated polymerized hemoglobin are shown in .
The Effect of Glutaradehyde Cross-Linking/Modification on the Immunogenicity of Hemoglobin from Human, Bovine and Porcine Origins
Our study showed that natural unmodified porcine, bovine and human hemoglobin had very weak immunogenicity in terms of their capability of eliciting a specific antibody response in experimental animals. For example, i.p injections of human, bovine or porcine hemoglobin in the absence of an adjuvant in mice, serum samples, tested at 1:50 dilution, mostly had no detectable anti-hemoglobin antibody response, even after three boost injections (). In the presence of an adjuvant, these proteins elicited significant IgG production responses (data not shown). However, glutaraldehyde polymerized hemoglobin of human, bovine or porcine origin exhibited increased antibody-stimulating effects in experimental animals. As shown in , even after the first boosting injection in mice with hPolyHb, bPolyHb or pPolyHb in the absence of any adjuvant, the serum specific antibody response became evident with specific antibody titers ranging from 1:100 to 1:1,000 (). After second boosting injection, the specific antibody titers in the serum could reach over 1:20,000.
Table 1. Comparison of immunogenicity of different hemoglobin and their glutaraldehyde polymerized derivatives tested in mice
However, hPolyHb and bPolyHb seemed to have stronger IgG stimulating effect than pPolyHb in mice. After second boosting injection, the serum titers of specific antibodies in mice stimulated by hPolyHb and bPolyHb mostly reached 1:20,000 or higher, while pPolyHb only elicited IgG production with a serum titers less than 1:20,000.
Similar results were also observed in rabbits and rats. However, the strength of antibody response varied significantly among these rodent animals. After repeated ip administration of a polymerized hemoglobin, mice and rats gave the strongest and weakest IgG responses, respectively. The differences in the extent of the responses between mice and rats were as high as more than 10-fold (data not shown).
The Nature of the Dominant Antigen Determinants in Glutaraldehyde Polymerized Hemoglobin of Different Origins
Next, efforts were made to investigate the nature of the dominant antigen determinants, resulting in the enhanced immunogenicity of the glutaraldehyde polymerized hemoglobin of human, bovine and porcine origins.
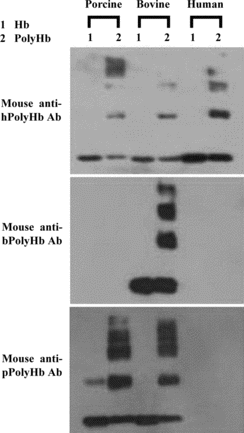
In order to study the nature of the dominant antigen determinants, cross-species-binding reactions between hPolyHb, bPolyHb or pPolyHb and specific mouse, rabbit or rat antibodies against one of the PolyHbs were studied. Based on a semi-quantitative study using SDS-PAGE/western blot method, most of the mouse polyclonal antibodies against hPolyHb, bPolyHb or pPolyHb only slightly or even did not cross-react with the PolyHb of other two species concerned. As shown in , although mouse antibodies against hPolyHb did cross-react to bPolyHb and pPolyHb, its binding to hPolyHb was significantly stronger. Mouse antibodies against bPolyHb strongly reacted with bPolyHb, but did not bind hPolyHb and pPolyHb. Mouse antibodies against pPolyHb strongly reacted with pPolyHb, relatively weakly bound bPolyH, and had no detectable cross-binding with hPolyHb.
Figure 3 Western blotting analysis of cross-species binding reactions between a hemoglobin or its polymerized derivative and mouse polyclonal antibodies against a particular type of PolyHb.
Quantitative studies using a protein array method further demonstrated that the cross species-binding reactions between hPolyHb, bPolyHb or pPolyHb and specific rabbit or rat antibodies against one of the PolyHbs only occasionally occurred. As shown in , rabbit antibodies against hPolyHb, bPolyHb or pPolyHb had very low cross-reacting rates (only 7.4% or less) with a PolyHb from the other two species concerned, except rabbit antibodies against hPolyHb, which cross-bound with bPolyHb at a rate about 24.8%. Furthermore, rat antibodies against hPolyHb or pPolyHb did not display any detectable cross species-binding activity at all.
Table 2. Cross-species binding reaction (%) between glutaraldehyde polymerized hemoglobin and a particular ant-PolyHb polyclonal antibodies from rabbits or rats, as tested by a protein array method
Apparently, the above cross species antibody-antigen binding data indicate that the dominant antigen determinants in hPolyHb, bPolyHb and pPolyHb significantly vary with each other.
DISCUSSION
It is clear that hPolyHb, bPolyHb and pPolyHb have enhanced immunogenicity compared to their corresponding unmodified counterparts (). It is very useful and important to understand whether these chemically modified hemoglobin proteins share the same dominant antigen determinants for their enhanced immunogenicity and, if they do, to what extent. There are two types of possibilities for the enhanced immunogenicity of these proteins. First, there would be a possibility that the enhanced immunogenicity of chemically modified hemoglobin may be due to unique neo-antigen resulted from chemical modification of the proteins. Chemical modification of proteins has been shown to be able to induce neo-antigens in the resultant protein derivatives, such as glutaraldehyde-modified albumin [Citation[16-18]] and glutaraldehyde-treated Ig G [Citation[19]]. Second, the enhanced immunogenicity may also be due to enhanced immunological response towards native antigen determinants of the modified hemoglobin proteins. Any approach to investigate the nature of dominant antigen determinants in hPolyHb, bPolyHb and pPolyHb has to be able to reveal both types of antigen determinants.
Hemoglobin is one of the most conserved proteins in nature in terms of primary amino acid sequence, three-dimensional structures as well as oxygen carrying function. Identity in primary amino acid sequences between hHb, bHb or pHb is between 81% and 88%, with the overall sequence identity among all these three hemoglobin being 80.3% and 76.2% for α subunit and β subunit, respectively (). However, when sequences of the subunits of these hemoglobins are aligned with those of the ones from evolutionarily more distant species, such as mice, rats or rabbits, the identity of the overall primary amino acid sequences drops significantly. Especially aligned with those of rat Hb, it drops about 8% and 13% in α subunit and β subunit, respectively (). Furthermore, it is perceivable that if chemical modification could introduce neo-antigens in the resultant derivatives of human, bovine or porcine hemoglobin, these neo-antigens would be also immunologically stimulatory in rodents. Therefore, we took the advantages of such evolutionary differences to reveal the dominant antigen determinants either existed in native protein structures of human, bovine and porcine hemoglobin or resulted from chemical modification of these proteins by cross-species antibody/antigen binding reactions using specific antibodies raised in mice, rats or rabbits.
Table 3. Amino acid sequence identity score among hemoglobin from different origins using ClustalW software for multiple alignment analysis
Our data in this study showed that each of hPolyHb, bPolyHb and pPolyHb mainly had its own unique dominant antigen determinants detectable using specific rodent antibodies (, ). Cross-species antibody/antigen binding rarely occurred using specific rat or rabbit antibodies (). Although such cross-species binding reactions did occur with some types of the specific mouse antibodies, most of these cross-binding reactions were relatively weak than the binding between a PolyHb and its cognate antibodies ().
Our further studies have demonstrated that the enhanced immunogenicity in hPolyHb, bPolyHb or pPolyHb is not due to introduction of neo-antigens in the chemically modified proteins but due to increased efficiency of cell surface presentation of Hb antigen peptides of these modified proteins by antigen presentation cells, such as dendritic cells and macrophages (data to be published). Therefore, the differences in the dominant antigen determinants in hPolyHb, bPolyHb and pPolyHb reflect the differences in the primary amino acid sequences of these proteins. It is apparent that after evolution of hemoglobin genes branched off from the rodent genes and separately evolved toward human, bovine and porcine hemoglobin genes, changes in the genes of hemoglobin subunits have resulted in introduction of different new antigen determinants in each of these proteins.
Since the mouse antibodies detected more cross-species antibody-antigen reactions () than the antibodies raised from rats and rabbits (), it would be expected that the mouse Hb genes are evolutionally more distant from human Hb genes than the rat Hb or rabbit Hb genes from an immunogenicity point of view.
The finding in this study that hHb, bHb and pHb have different dominant antigen determinants is very important. Although hHb, bHb and pHb share extensive identity in their amino acid sequence and have very similar three-dimensional structures [Citation[20-22]], immunological properties of one of the PolyHbs may not be a direct reference for those of the other PolyHbs. In fact, our other studies have found that even though chemical modification of hemoglobin may not introduce neo-antigens, it may alter the immunogencity of the modified hemoglobin by converting non-immunogenic antigen determinants to immunogenic ones, which may vary with the nature of the hemoglobin and the modifying chemical reagents used. The results of our further studies to reveal the possible origins of the enhanced immunogecity by glutaraldehyde polymerization of hemoglobin molecules will be presented in a separate article. Therefore, understanding of immunogenic properties of a HBOC should be based on studies of the product itself. Cautions must be exercised when prediction about immunological properties of a HBOC product is based on information of other HBOC products, since they could have totally different immunogenic natures.
The study results reported in this article were based on research by Xiaoli Zhu, Wei Chu, and Tongwen Wang who have contributed equally to this study.
REFERENCES
- Stollings, J.L., Oyen, L.J. (2006). Oxygen therapeutics: Oxygen delivery without blood. Pharmacotherapy 26(10): 1453–1464.
- Stowell, C.P. (2005). What happened to blood substitutes? Transfus. Clin. Biol. 12(5): 374–379.
- Wu, B.P., Wang, F., Lou, C., Chen, C., Dan, N. Studies of porcine hemoglobin and glutaraldehyde-polymerized porcine hemoglobin as hemoglobin-based oxygen carriers. To be published.
- Moore, E.E., Cheng, A.M., Moore, H.B., Masuno, T., Johnson, J.L. (2006). Hemoglobin-based oxygen carriers in trauma care: Scientific rationale for the US multicenter prehosptial trial. World J. Surg. 30(7): 1247–1257.
- Dong, F., Hall, C.H., Golech, S.A., Philbin, N.B., Rice, J.P., Gurney, J., Arnaud, F.G., Hammett, M., Ma, X., Flournoy, W.S., Hong, J., Kaplan, L.J., Pearce, L.B., McGwin, G., Ahlers, S., McCarron, R., Freilich, D. (2006). Immune effects of resuscitation with HBOC-201, a hemoglobin-based oxygen carrier, in swine with moderately severe hemorrhagic shock from controlled hemorrhage. Shock 25(1): 50–55.
- Bradley, H.C., Sansum, W.D. (1914). Some anaphylactic reactions. J. Biol. Chem. 18: 497–506.
- Heidelberger, M., Landsteiner, K. (1923). On the antigenic properties of hemoglobin. J. Exp. Med. 38: 561–571.
- Hertzman, C.M., Keipert, P.E., Chang, T.M. (1986). Serum antibody titers in rats receiving repeated small subcutaneous injections of hemoglobin or polyhemoglobin: A preliminary report. Int. J. Artif. Organs 9(3): 179–182.
- Chang, T.M., Lister, C., Nishiya, T., Varma R. (1992). Immunological effects of hemoglobin, encapsulated hemoglobin, polyhemoglobin and conjugated hemoglobin using different immunization schedules. Biomater. Artif. Cells Immobilization Biotechnol. 20(2–4): 611–618.
- Bleeker, W.K., Zappeij, L.M., den Boer, P.J., Agterberg, J.A., Rigter, G.M., Bakker, J.C. (1995). Evaluation of the immunogenicity of polymerized hemoglobin solutions in a rabbit model. Artif. Cells Blood Substit. Immobil. Biotechnol. 23(4): 461–468.
- Hamilton, R.G., Kelly, N., Gawryl, M.S., Rentko, V.T. (2001). Absence of immunopathology associated with repeated IV administration of bovine Hb-based oxygen carrier in dogs. Transfusion 41(2): 219–225.
- Zhu, X.L., Luo, C., Wang, T.W., Wu, B.P., Wang, F., Chen, C., Dan, N. Immunological studies of porcine hemoglobin and its glutaraldehyde-polymerized/modified derivatives: Immunogenicity and basic immunological properties. To be published.
- Dan, N., Middleton, R.B., Lehrman, M.A. (1996). Hamster UDP-N-acetylglucosamine:dolichol-P N-acetylglucosamine-1-P transferase has multiple transmembrane spans and a critical cytosolic loop. J. Biol. Chem. 271(48): 30717–30724.
- Harris, E.L.V. (1989). Protein Purification Methods: A Practical Approach. London: LRL Press, pp. 24–45.
- Kusnezow, W., Jacob, A., Walijew, A., Diehl, F., Hoheisel, J.D. (2003). Antibody microarrays: An evaluation of production parameters. Proteomics 3(3): 254–264.
- Onica, D., Lenkei, R., Ghetie, V. (1978). Immunogenicity of glutaraldehyde-treated homologous albumin in rabbits. Immunochemistry 15(9): 687–693.
- Onica, D., Dobre, M.A., Lenkei, R. (1978). Immunogenicity of glutaraldehyde treated homologous monomeric albumin in rabbits. Immunochemistry 15(12): 941–944.
- Calugaru, A., Zamfir, G., Onica, D. (1983). Antibodies from patients with liver diseases and from normal human or animal sera against glutaraldehyde-polymerized albumins: Lack of species specificity. Experientia. 39(10): 1139–1141.
- Onica, D., Mota, G., Calugaru, A., Manciulea, M., Dima, S. (1983). Immunogenicity and effector functions of glutaraldehyde-treated rabbit and mouse immunoglobulin G. Mol. Immunol. 20(7): 709–718.
- Perutz, M.F., Fermi, G., Poyart, C., Pagnier, J., Kister, J. (1993). A novel allosteric mechanism in haemoglobin. Structure of bovine deoxyhaemoglobin, absence of specific chloride-binding sites and origin of the chloride-linked Bohr effect in bovine and human haemoglobin. J. Mol. Biol. 233(3): 536–545.
- Katz, D.S., White, S.P., Huang, W., Kumar, R., Christianson, D.W. (1994). Structure determination of aquomet porcine hemoglobin at 2.8 A resolution. J. Mol. Biol. 244(5): 541–553.
- Lu, T.H., Panneerselvam, K., Liaw, Y.C., Kan, P., Lee, C.J. (2000). Structure determination of porcine haemoglobin. Acta. Crystallogr. D. Biol. Crystallogr. 56: 304–312.