Abstract
The production of a fully functional bioartificial liver assist device (BLAD) would greatly enhance available treatment options for patients suffering from acute liver failure. Currently, inadequate oxygen provision to hepatocytes seeded within hollow fiber bioreactors hampers development of a viable hollow fiber-based BLAD. Experimentally, oxygen provision to primary rat hepatocytes cultured within hollow fiber bioreactors was measured, it was observed that supplementation with an oxygen carrier (bovine red blood cells at ∼ 2% human hematocrit) did not significantly improve oxygenation compared to the absence of an oxygen carrier. Therefore, an oxygen transport model of an individual hollow fiber within the bioreactor was developed and simulated (up to ∼ 10% human hematocrit) to more fully examine the effect of oxygen carrier supplementation on oxygenation within the bioreactor. The modeling analysis, supported via the experimental results, was utilized to predict optimal bioreactor operating conditions for the delivery of in vivo-like oxygen gradients to cultured hepatocytes in clinically relevant settings.
INTRODUCTION
Acute liver failure (ALF) is characterized by the rapid and temporary necrosis of the liver in patients with no previous liver condition, affecting approximately 2,000 people each year in the United States alone. A unique aspect of ALF is that in ∼ 25% of cases, the patient's liver retains the ability to regenerate to pre-ALF functional capacity after the initial trauma. Therefore, a device that could temporarily sustain an ALF patient would be useful in providing the patient with enough time to realize the potential of native liver regeneration, or to bridge the patient towards orthotopic liver transplantation.
Oxygen provision to cultured hepatocytes is one of the major design issues that must be overcome in developing a viable BLAD [Citation[1], Citation[2]]. The amount of oxygen delivered to hepatocytes (liver cells) as well as the specific range of oxygen tensions experienced by hepatocytes in culture is important for proper function, and the realization of different hepatocyte phenotypes (along with extracellular matrix and hormonal variations). The variation in hepatocyte phenotype with respect to gradients in oxygen tension is referred to as zonation, and results in the development of three major oxygenation zones that are present in the liver sinusoid, which are the periportal (60–70 mHg), pericentral (35–60 mHg), and perivenous (25–35 mHg) regions. A good review of specific liver functions that take place in each of these zones is presented in the literature [Citation[3]]. Briefly, highly oxygen-dependent functions take place in the periportal zone, and these functions taper into the pericentral zone, where perivenous activities are first observed. Hepatocytes function in this manner allowing the liver to maintain homeostasis for several key metabolites in the body. Therefore, the three hepatic zones must be present for a BLAD to replace all of the liver's various functions. Additionally, we aim to maintain the average pO2 experienced by cultured hepatocytes within the normal human liver sinusoid range ∼ 41–47 mmHg [Citation[4-6]] Provision of both the desired oxygen spectrum (25–70 mmHg) and in-vivo-like average oxygen concentrations to cultured primary hepatocytes is the main focus of this work.
We have chosen to utilize a hollow fiber bioreactor as the initial BLAD design. Previous studies have indicated that, due to the multifunctional nature of the liver, a fully artificial organ (like a dialyzer or an iron lung) is not sufficient for adequate patient support. Therefore, an artificial device housing hepatocytes is necessary to replace the wide range of liver functions. Hollow fiber bioreactors are an attractive option for this application, since they provide a high surface area to volume ratio, facilitate cell attachment, and their biomimetic three-dimensional design allows for cell-cell communication that is essential for hepatocyte viability and function [Citation[7]]. There are a variety of cell types that could potentially be utilized within such a device. In this study, primary rat hepatocytes were utilized due to their ability to retain high levels of differentiated function [Citation[8], Citation[9]]. Primary hepatocytes do not generally proliferate in vitro, and this may be problematic for long-term BLAD usage. We have previously cultured C3A hepatoma cells within a hollow fiber bioreactor, and suggested that they may represent a more appropriate source of hepatocyte-like cells for long-term BLAD usage [Citation[10]].
An oxygen transport model was developed to allow simulation of the oxygen tension experienced by cultured hepatocytes within the hollow fiber bioreactor. Based on the Krogh tissue model, where a single cylindrical vessel is surrounded by an annulus of tissue that represents a single subunit within a “bed of capillaries” [Citation[11]], this model contains another annulus between the flowing lumen and cell space to account for the hollow fiber membrane within the hollow fiber device. Additional improvements to this model include the manner in which reversible hemoglobin-oxygen binding/release kinetics is taken into account [Citation[10]], Michaelis-Menten oxygen consumption kinetics [Citation[12], Citation[13]], a fractional cell volume factor, and a variable oxygen diffusivity coefficient within the cell space.
In this study, bovine red blood cells (bRBCs) were employed as the oxygen carrier within the experimental portion of this work, due to similar physical properties they share with human RBCs. Operating parameters derived from the experimental system were utilized in computer simulations of oxygen transport in an individual hollow fiber to facilitate the optimized development of a hollow fiber bioreactor that provides the correct spectrum of oxygenation to cultured hepatocytes. Oxygen transport simulations, supported via data generated from the experimental system, predicted optimized bioreactor operating parameters for the given BLAD.
MATERIALS AND METHODS
Hollow Fiber Bioreactor System
The CellMax® QuadTM (Spectrum Laboratories, Rancho Dominguez, CA) hollow fiber bioreactor system was utilized in this study, and consisted of a 250 mL media reservoir bottle (containing between 125 to 250 mL of culture media), ∼ 2 meters of silicone tubing, a peristaltic pump, and inline dissolved oxygen probes (DO-166 Lazar Research Laboratories, Los Angeles, CA) attached to the bioreactor entrance and exit (see ). Two separate hollow fiber bioreactor systems were run during the study. The experimental system was supplemented with bRBCs in the circulating media stream, while the control system lacked bRBCs in the circulating media stream. A small vortexer (12-812, Fisher Scientific, Hampton, NH) was implemented as a means of periodically, gently agitating the reservoir bottles to prevent sedimentation of bRBCs within the media bottle. A peristaltic pump circulated the media from the media bottle through the bioreactor at three different flow rates in order to obtain hepatocyte oxygen consumption data at differing media flow rates. Finally, the entire bioreactor was maintained within a Haraeus incubator (Kendro Laboratory Products, Hanau, Germany) at 37°C and 5% CO2.
Figure 1 Schematic of the experimental hepatic hollow fiber bioreactor, along with a cross-sectional view of a single representative hollow fiber. Solid rectangles within the enlarged fiber represent the modeled space.
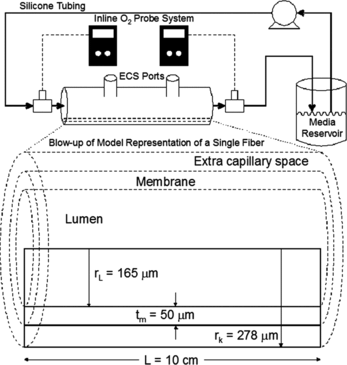
The hollow fiber bioreactors (400-012, Spectrum Labs.) used in this study consisted of a 12 mL extra capillary space (ECS, the volume outside the hollow fibers, where the hepatocytes were cultured) volume, and hollow fibers with an outer surface area of 1500 cm2 and a 95% particle size cut off of 0.3 µm. The small particle size cut off of the hollow fiber membrane prevents bRBCs from entering the ECS.
The oxygen probes attached to the inlet and exit ports of the bioreactor provided a simple method for measuring the oxygen lost from the circulating media stream and consequently transported to the hepatocytes within the ECS of the bioreactor (hereafter referred to as the global bioreactor oxygen consumption rate, OCR). Prior to use within the system, the oxygen probes were calibrated with a two point calibration protocol consisting of first exposing the probes to a saturated sodium sulfite solution (zero initialization point), and subsequently to an oxygen saturated distilled water solution (high initialization point). After calibration, the probes were sterilized in 70% ethanol. The ethanol was then washed from the probes using sterile culture media, and the probes were finally inserted into the bioreactor system. After the achievement of steady state (∼ 1 hour), the flow rate was varied and the system allowed to re-establish equilibrium. The dissolved oxygen concentration values were recorded, and this process was repeated for the other experimental system.
Hepatocyte Isolation and Culture Media
Primary rat hepatocytes were utilized within this study. Hepatocytes were isolated at Wayne State University using the method of Seglen as modified by Dunn [Citation[18], Citation[19]]. Rats were anesthetized, and a two-stage collagenase perfusion of the liver was performed via the portal vein. Dissociated hepatocytes were collected in 50 mL centrifuge tubes and washed 3 times in Hanks balanced buffer solution. Cell counts were performed with a hemacytometer on each of the centrifuge tubes and viability was assessed via the exclusion of the trypan blue stain. Finally, approximately 150 million viable cells were inoculated into the ECS of each of the hollow fiber bioreactors. The cells consequently occupied about an eighth of the ECS volume.
The complete cell culture medium utilized in the experimental study was composed of 90% Dulbecco's modified Eagle's medium (D6429, Sigma, St. Louis, MO), 10% fetal bovine serum (F6178, Sigma), 1000 Units/ml of penicillin and streptomycin, 0.5 Units/ml of insulin, 100 µg/ml of glucagon, 100 µg/ml of epidermal growth factor, and 750 µg/ml of hydrocortisone (Sigma). The medium supplying the experimental system was additionally supplemented with sterile washed bRBCs (943, Quad Five, Ryegate, MT) at about 100 million cells/ml, while the control system was not supplemented with an oxygen carrier. Finally, the medium inoculated into the ECS along with the hepatocytes did not contain an oxygen carrier, but was additionally supplemented with collagen extracted from rat tails at a concentration of 1 mg/ml.
Throughout the 10-day study, the media reservoir bottle was replaced daily with fresh complete medium. After removing the media from the system, aliquots were taken for analysis of various biomarkers, which were intended to be indicative of the overall health of the cell culture. Briefly, glucose consumption was measured via a simple glucose strip test (Home Diagnostics Inc., Fort Lauderdale, FL) and urea production was quantified using a kit based on the diacetylmonoxime method (0580, Stanbio Laboratory, Boerne, TX). The results of these assays are not presented within this article, but were used to indicate that the cultures were behaving as expected.
COMPUTER MODELING
A single axisymmetric hollow fiber with a representative extra capillary space (ECS) was simulated in the oxygen transport model, which was developed elsewhere [Citation[10], Citation[19]]. Briefly, the two-dimensional modeled space is presented at the bottom of where the upper rectangle represents the lumen containing flowing media, the middle rectangle represents the hollow fiber membrane, and the lower rectangle represents the ECS containing cultured hepatocytes. The hollow fiber bioreactor system in the experimental study was the basis for the dimensions used within the developed oxygen transport simulations. The geometry of an individual hollow fiber contained within the bioreactor, along with measured flow rates, dissolved oxygen concentrations, and bRBC concentrations, were used as input parameters in each of the oxygen transport simulations. A finite element software package (Comsol Multiphysics, Burlington, MA) was used to predict the oxygen transport profile within the hollow fiber bioreactor.
Model Components
Within the lumen, Poiseuille flow is assumed to be present along the entire length of the modeled hollow fiber. Calculations indicate that there are no significant entrance effects on the velocity and bRBC concentration profiles, since the profiles develop within the encapsulated end of the hollow fibers [Citation[20]]. The addition of bRBCs to the circulating media can potentially disrupt the flow pattern and cause a bRBC concentration profile and an altered velocity profile to develop. However, at the low concentrations of bRBCs used within the experimental studies, the altered profiles require a lumen length considerably longer than that of the actual bioreactor in this study, and consequently bRBC supplementation was not expected to significantly alter the flow profile [Citation[21]]
Each of the three regions (lumen, membrane, and ECS) of the model contained a separate convection-diffusion equation for transport of oxygen that had to be solved.where
is the diffusivity of oxygen (as defined for each region: Dl, Dm and DECS), pO2 is the partial pressure of oxygen, and R represents the consumption (or production) of oxygen. The diffusivity of oxygen in water was utilized to describe the oxygen diffusivity within the lumen and the membrane, since the membrane is considered to be fully hydrated
. The Renkin equation supports the fully hydrated assumption, indicating an almost identical oxygen diffusivity in the membrane as in water [Citation[22]]. Additionally, the membrane utilized has been shown to be highly porous with low tortuosity [Citation[21]]. The diffusivity of oxygen in the ECS, Equation 2, will be affected by the amount of cells present, since the diffusivity of oxygen though a hepatocyte is different than that of water. Therefore to determine the effective diffusivity of oxygen through the ECS we introduced a new parameter, the fractional volume of the ECS space that is filled with hepatocytes (ε), which was implemented in conjunction with both the diffusivity of oxygen in water (
) and through hepatocytes (DHep) as shown in Equation 2.
Within the lumen, for the control case of no media stream bRBC supplementation, the reaction term (R) in Equation 1 is not present. However, for the experimental case (with media stream bRBC supplementation), the reaction term described oxygen binding/release to/from the bRBCs (discussed later). As the membrane within the hollow fiber did not consume or produce oxygen within either the control or experimental systems, the reaction term (R) was neglected for oxygen transport through the membrane. Within the ECS, the consumption of oxygen by hepatocytes has been shown to be most accurately represented by Michaelis-Menten kinetics [Citation[2], Citation[23]], Equation 3. In addition to the oxygen diffusivity, Equation 3 indicates that the consumption of oxygen is also influenced by the volume fraction of hepatocytes within the ECS (ε).where Vmax and Km are the Michaelis-Menten parameters. With these equations, the control case of no bRBCs present in the system can now be solved numerically.
In the model, we also take into consideration the fact that the membrane pores are too small for the bRBCs to pass through. Hence, the bRBCs were assumed to be confined solely within the lumen. Since the cross-membrane pressure drop was expected to remain small to negligible, we thus neglected the flow of media through the membrane, thereby removing the convection term in Equation 1. At the experimental flow rates studied, the flow of media through the membrane and ECS was negligible. Due to this fact, oxygen was transported solely by only diffusion within these two regions. With these equations in hand, the control case of no bRBC media stream supplementation can be solved numerically.
The transport of oxygenated hemoglobin, which is encapsulated within the bRBCs, was calculated via a second convection-diffusion equation, Equation 4, which was coupled to Equation 1.where DRBC is the diffusivity of bRBCs within the lumen, S is the fractional saturation of hemoglobin monomers with oxygen, HbT is the total concentration of hemoglobin, and
is the reaction rate law describing the rate of binding/release of oxygen to/from hemoglobin. The diffusivity of bRBCs within the lumen (Equation 4) is calculated from the Stokes-Einstein equation [Citation[24]]. The reaction rate (signifying the binding or release of oxygen to/from hemoglobin) in Equation 4 is equal in magnitude and opposite in sign (
) to the reaction rate within the convection-diffusion equation of dissolved oxygen within the lumen, Equation 1. This rate of oxygen consumption or production was accounted for via the variable reaction rate model proposed by Moll [Citation[25]]. Similar calculation methods have been successfully used in recent studies [Citation[26], Citation[27]], and are briefly described below.
The variable reaction rate model considers the reaction of oxygen with hemoglobin as a single step reaction.where k+ is the forward reaction rate constant and k− is the reverse reaction rate constant. This yields the reaction rate law that describes the rate of formation of oxy-hemoglobin contained within bRBCs (Equation 6).
The reverse reaction rate constant is kept constant, and the forward reaction rate constant is considered a variable related to bRBC's oxygen-hemoglobin equilibrium curve (OHEC), which yields Equation 7.where Seq is the equilibrium fractional saturation of hemoglobin with oxygen as a function of
as determined by the bRBC's OHEC. Combining Equations 6 and 7 yields Equation 8, which allows the reaction rate of hemoglobin and oxygen to be calculated at any point within the hollow fiber.
Within Equation 8, Seq is the equilibrium saturation value of the fractional saturation of hemoglobin with oxygen as defined by the Adair equation (Equation 9).where a1−4 are the Adair constants that define the OHEC utilized in the oxygen transport model. The resultant set of coupled convection-diffusion equations can be solved for the case of bRBC supplementation.
Characteristic Parameters
The set of parameters, their values, and literature sources are presented in . In addition to these parameters, simulations were conducted over a range of umax and pO2 values to match the experimentally measured data. The diffusivity of oxygen within water and through hepatocytes was reported in the literature [Citation[23], Citation[28]]. The diffusivity of oxygen within the ECS was found by utilizing literature values of the oxygen diffusivity through hepatocytes [Citation[29]], as well as the oxygen diffusivity through water (determined via Equation 2). The Vmax value of 5.87 µM/s is within the range of values reported from several literature sources: 10 µM/s [Citation[28]], 5 µM/s [Citation[13]], and 4.45, 7.4 and 4.8 µM/s [Citation[2]], utilizing a per unit volume oxygen consumption rate. This value was derived from a previously determined zeroth-order kinetics oxygen consumption rate of 48 amol/(cell-s) for rat hepatocytes in a three-dimensional gel [Citation[9]]. The conversion assumes that ∼ 150 million cells were inoculated in each of the ECS of the two hollow fiber bioreactors. This cell number yields about 12.5 million hepatocytes per cm3, which is close to the 10 million per cm3 cell density reported by Nyberg [Citation[9]]. The Km of 3 mmHg is the median value that has been reported for hepatocytes, which ranges between 0.5 and 5.6 mmHg [Citation[23], Citation[28]]. Additionally, this Km value has been previously used within modeling studies reported by the authors that were found to agree with experimentally collected data [Citation[10], Citation[21]]. The void volume within the ECS was calculated by estimating two parameters, namely the total volume of hepatocytes that were inoculated into the hollow fiber bioreactor and the volume of the ECS. Each of the bioreactors was inoculated with ∼ 150 million cells within the ECS. Given an average cell diameter of ∼ 25 µm [Citation[30]], it was determined that the inoculated hepatocytes filled between an eighth to a tenth of the total ECS volume. This consequently necessitated the inclusion of the fractional hepatocyte volume factor (ε) in the oxygen transport model, in order to account for the voids in the ECS. The concentration of circulating bRBCs was kept constant throughout the experiments at approximately 2% of the normal human in vivo hematocrit, measured via a hemacytometer. The OHEC of bovine RBCs was measured with a Hemox Analyzer (TCS Scientific Corp., New Hope, PA), and the Adair parameters were regressed via a nonlinear six parameter model as described elsewhere [Citation[31]].
Table 1. Parameters of the hollow fiber bioreactor utilized in oxygen transport simulations
RESULTS AND DISCUSSION
As previously mentioned, hepatocytes were inoculated into the ECS of the two hollow fiber bioreactor systems and cultured for three weeks. For these experiments, the hepatocyte oxygen consumption parameters (Vmax and Km) were estimated. These parameters were subsequently used in oxygen transport simulations. Using the oxygen transport model, the most ideal bioreactor operating conditions for maintaining tissue-like hepatocyte densities were determined. The predicted operating conditions are expected to be useful for the development of future BLADs as they are based on a more practical set of clinical requirements, such as providing an in vivo-like oxygen spectrum to cultured hepatocytes.
Experimental Data
Utilizing the number of cells within each hollow fiber bioreactor, the oxygen consumption rate was calculated to be very close to that previously reported for primary rat hepatocytes within a three-dimensional bioreactor. The range of oxygen consumption rates measured spanned a range of 65 to 87 amol/cell/s, and was within the range of values previously reported, 48–89 amol/cell/s [Citation[9], Citation[32], Citation[33]]. The seeding densities of three-dimensional gels within previous experiments were also very similar (5–10 Mcells/cm3) to that employed in these studies (∼ 12.5 Mcells/cm3).
The oxygen transport model employed in our study showed good agreement with measured experimental data (). The experimental results showed only a marginal increase in oxygenation to cultured hepatocytes with media supplementation of bRBCs. This was likely due to the fact that under the experimental culture conditions, the hepatocytes were not oxygen limited, which was a result of the relatively low density of inoculated hepatocytes. Only at the lowest flow rate was a significant increase in oxygenation between the control case and the bRBC supplemented case observed. The model presented in this work was able to capture the observed phenomena, providing additional evidence for the validity of the model.
Figure 2 Comparison of experimentally measured oxygen consumption rates across the entire hollow fiber bioreactor along with values calculated by the oxygen transport model under identical conditions. ![]()
![Figure 2 Comparison of experimentally measured oxygen consumption rates across the entire hollow fiber bioreactor along with values calculated by the oxygen transport model under identical conditions. Display full size – Plain media, Display full size– RBC supplemented media, and Display full size– Oxygen transport simulations for both cases. Oxygen consumption rates were measured for the following sets of Q [mL/min] and inlet [mmHg]: A – 4.72/79, B – 8.35/85, C – 12.18/84, D – 4.72/77, E – 8.35/76, and F − 12.18/71.](/cms/asset/a51883f4-01b5-4c18-9801-c4d8bcf0d90d/ianb19_a_258479_f0002_b.gif)
ECS Zonation
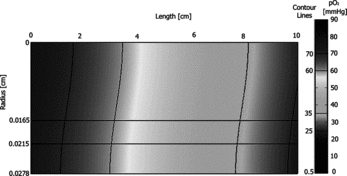
Oxygen transport simulations were used to calculate the oxygen concentration profiles for each set of model parameters, and the resulting two-dimensional oxygen concentration distributions (for case A from ) are presented as shows the oxygen distribution within a single representative hollow fiber element. Contour lines have been added to delineate each of the hepatic oxygenation zones. The upper rectangle represents the flowing lumen, which contains bRBCs in the specific cases noted, the middle rectangle represents the semipermeable hollow fiber membrane that excludes bRBCs and limits radial flow of the media, and the lower rectangle represents the ECS which contains a continuum of hepatocytes. The two-dimensional cross-sections provide information on the distribution of oxygen concentration within the hepatocyte containing ECS. It is clear that these bioreactor operating conditions result in favorable oxygenation to cultured hepatocytes as the oxygen distribution is shown to be close to the desired goal of replicating all three oxygenation zones in the ECS. The resulting simulation data was cylindrically integrated over appropriate ranges of oxygen tension to provide the percent of the ECS volume within each of the hepatic oxygenation zones as: 18% periportal, 47% pericentral, and 20% perivenous. For this particular case, 85% of the ECS is within the desired oxygen spectrum, with 13% experiencing hyperoxic oxygenation and 2% experiencing hypoxic oxygenation. Additionally, the entire ECS was integrated to yield an average ECS pO2 of ∼ 50 mmHg. Similar values were found for the other experimental cases shown in .
Figure 3 Oxygen concentration profile predicted by the oxygen transport model at one of the experimental conditions; umax = 0.18 cm/s, , and no RBC supplementation – case A from Figure 2.
and indicate that the control case of no RBC supplementation at the lowest flow rate studied provided an almost ideal oxygenation environment to the cultured hepatocytes. However, as previously mentioned, only ∼ 10% of the ECS volume was filled with hepatocytes during the experimental study. Utilizing this low hepatocyte density, an enormous number (∼ 250) of hollow fiber bioreactors operating in parallel would be required in order to reach the desired minimum of 20% of the in vivo hepatocyte mass necessary to sustain an ALF patient (10–40% of the liver mass has been reported to be essential for maintaining proper liver function [Citation[7], Citation[34-36]]). For this reason, the model was also utilized to predict operating conditions with more feasible cell numbers, flow rates, and bRBC concentrations. Ideally, the hollow fiber bioreactor would be operated with hepatocytes at tissue-like densities in the ECS volume. This would provide enough cells to cover the surface of all of the hollow fibers within the bioreactor. However, the increased hepatocyte density within the hollow fiber bioreactor would likely lead to the formation of significant hypoxic zones in the ECS volume, and even anoxic zones in some cases. This problem can be alleviated by increasing the oxygen carrier concentration within the circulating media stream. Utilizing the oxygen transport model, operating conditions that incorporate an increased concentration of bRBCs can be shown to provide in-vivo-like oxygenation to the ECS of the hollow fibers. These operating conditions will be discussed in the next section.
Simulated Hollow Fiber Bioreactor Operating Conditions
Comparison of experimentally measured oxygenation data to oxygen transport simulations provided supporting evidence for the validity of the oxygen transport model. Therefore, bioreactor operating conditions (presented in ) likely to be more relevant for ALF patient support within a clinical setting were explored using the model. First, the hepatocyte cell mass within the hollow fiber bioreactor was increased to fill the entire ECS (ε = 1). Filling the entire ECS volume may not be experimentally attainable; however, an ideally operating bioreactor would have a tissue-like density of hepatocytes. This reduces the number of hollow fiber bioreactors needed to sustain a patient to ∼ 25, which could be further reduced by utilizing bioreactors with larger ECS volumes. The flow rate through each of the bioreactors was calculated using a flow rate similar to that utilized for hemodialysis (∼ 300 mL/min) split up into each of the bioreactors, yielding an individual bioreactor flow rate of 12.18 mL/min. Additionally, two lower flow rates (4.72 and 8.35 mL/min) were examined, as it is desirable to remove as little blood from the patient as possible and because an increased flow rate can lead to the development of Starling flow, which reduces hepatocyte metabolism due to increased shear forces on the cells [Citation[37], Citation[38]]. Generally, venous blood is fed into the BLAD, due in large measure to the accessibility of veins and the low venous blood pressure compared to arterial pressure. However, the average mixed venous dissolved oxygen tension is ∼ 40 mmHg [Citation[39]], which is too low to replicate all three oxygenation zones needed for proper zonation to develop. Therefore, the ALF patient's venous blood would have to be oxygenated prior to entering the hollow fiber bioreactor. The inlet oxygen tension was examined over a range of values, to determine oxygen tensions that replicate all three oxygenation zones. It was determined that the average mixed arterial dissolved oxygen tension, 90 mmHg, was best suited as the inlet oxygen concentration entering the bioreactor. Finally, the bRBC concentration within the hollow fiber bioreactor system was varied over a range of values. It was determined that the full human in vivo hematocrit was not necessary to provide the desired oxygenation spectrum to cultured hepatocytes within the hollow fiber bioreactor. Increased RBC concentrations would be expected to lead to fouling of the membrane via bRBC hemolysis; therefore, for future clinically relevant BLAD designs, the concentration of human RBCs that pass through the bioreactor should be minimized by partial plasmapheresis.
Table 2. Clinically-relevant parameters of the hollow fiber bioreactor employed in oxygen transport simulations
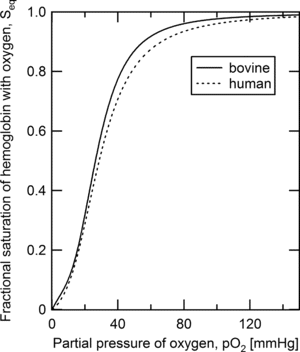
The Adair constants defining the oxygen-hemoglobin equilibrium curve (OHEC) for bovine hemoglobin were utilized in the initial modeling study presented above. However, in clinical usage this type of device would be operating with human RBCs present in the lumen, and thus the model was updated with Adair parameters, which are presented in , that describe the equilibrium binding of oxygen to hemoglobin contained within human RBCs. As the OHEC utilized for bovine and human RBCs are very similar (), the model is expected to be equally valid with human RBCs as the oxygen carrier within the lumen.
Figure 4 Oxygen-hemoglobin equilibrium binding curves for bovine and human red blood cells predicted from the Adair constants presented in Tables and .
These new simulation parameters provided insight into possible clinical applications of this type of BLAD. The oxygenation zones within the hollow fiber bioreactor were calculated for the control case of no RBCs (complete plasmapheresis) and 10% of the normal human RBC concentration (partial plasmaphersis). indicates the fraction of the ECS volume that is within each of the previously specified hepatic oxygenation zones under these conditions. In addition to the three desired hepatic oxygenation zones, hyperoxic (pO2 > 70 mmHg) and hypoxic (pO2 < 25 mmHg) zones were also observed. Furthermore, a white line is included to display the portion of the hypoxic region that is anoxic (< 0.5 mmHg). For this set of results, it is observed that with no RBCs in the circulating media, there are severe hypoxic and anoxic regions within the hollow fiber bioreactor ECS. The addition of RBCs completely removes the anoxic region, and reduces the hypoxic region in each of the cases examined. Additionally, a near ideal oxygenation environment, as previously described, can be provided to hepatocytes within the hollow fiber bioreactor ECS for case L in .
Figure 5 Hepatic zonation of the ECS in each of the defined oxygenation zones for the predicted bioreactor operating conditions at an inlet dissolved oxygen tension of 90 mmHg at several flow rates: G&J – 4.72 [mL/min], H&K – 8.35 [mL/min], and I&L – 12.18 [mL/min], with either plain media circulating (G, H, & I) or circulating RBC supplemented media at 10% of the human in vivo RBC concentration (J, K, & L).
![Figure 5 Hepatic zonation of the ECS in each of the defined oxygenation zones for the predicted bioreactor operating conditions at an inlet dissolved oxygen tension of 90 mmHg at several flow rates: G&J – 4.72 [mL/min], H&K – 8.35 [mL/min], and I&L – 12.18 [mL/min], with either plain media circulating (G, H, & I) or circulating RBC supplemented media at 10% of the human in vivo RBC concentration (J, K, & L).](/cms/asset/ab1b2e27-cf65-4395-821d-77e04b6a353c/ianb19_a_258479_f0005_b.gif)
In addition to quantifying ECS zonation, the average ECS pO2 was calculated for each of the cases examined in . For the control cases of G, H and I, the average pO2 values were calculated to be 5, 8, and 12 mmHg, respectively. In each of the control cases (those with complete plasmapheresis), the outlet oxygen concentration was reduced to almost 0 mmHg. However, with the addition of 10% of the normal human in vivo RBC concentration to the circulating media feed stream (partial plasmapheresis), a dramatic increase in the amount of oxygen delivered to the hepatocytes is observed. The average pO2 values within the hollow fiber ECS for the experimental systems (with RBCs present) are 25, 34, and 41 mmHg for cases J, K, and L respectively. The zonation of the hollow fiber presented in indicates that case L approaches ideal oxygenation of the cultured hepatocytes. It is fairly obvious that the volume of the periportal zone is smaller compared to the volume of either the pericentral and pervenous zones, but this is an expected result. Oxygen is consumed more rapidly by periportal hepatocytes located in a high pO2 environment, due to the nature of the governing Michealis-Menten kinetics.
The axisymmetric cross-sections of a few representative oxygen concentration profiles are presented in . contains three different simulations: cases I and L from (as cases a and b, respectively), and a case with a higher RBC concentration. Cases a and b can be directly compared to show a significant increase in the dissolved oxygen concentration profile within the ECS. It is observed that the outlet pO2 is essentially 0 mmHg for a, and is raised to ∼ 39 mmHg for b by the inclusion of RBCs within the BLAD feed stream. The volume of ECS within the desired oxygenation region (25–70 mmHg) increased from 21% to 95% by the addition of RBCs. Therefore, supplementation of RBCs to the circulating media stream has a significant impact on both the amount of oxygen, and range of oxygen tensions, available to the hepatocytes within the hollow fiber.
Figure 6 Oxygen concentration profiles predicted by the oxygen transport model at three predicted experimental conditions; umax = 0.465 cm/s and (a) 90mmHg with no RBC supplementation – case I from Figure 5, (b)
90mmHg with 10% supplementation of the human in vivo RBC concentration – case L from Figure 5, and (c)
with 20% supplementation of the human in vivo RBC concentration.
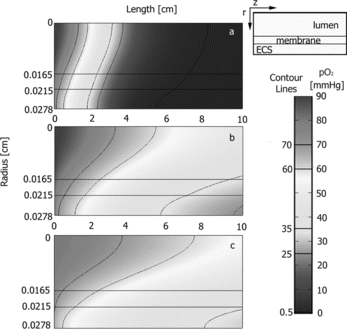
Next, the axisymmetric cross-sectional oxygen concentration profile of b was compared to c within . In c the amount of RBCs present in the circulating media was increased to 20% of the average human in vivo hematocrit, and the inlet dissolved oxygen concentration was decreased to 80 mmHg. By increasing the concentration of RBCs the oxygen carrying capacity of the solution entering the bioreactor is increased and thus the requirements of a high inlet oxygen partial pressure can be relaxed. The bioreactor operating conditions for case b was chosen such that b and c have almost identical overall oxygen consumption rates (OCRs). The inlet and outlet concentrations of dissolved oxygen are considerably different, from 90 to 39 mmHg in case b and from 80 to 48 mmHg in case c; however, it is anticipated that maintaining a similar OCR is more important. A similar average ECS pO2 was observed between b and c, 40 versus 45 mmHg, respectively, and both of these values are close to the design criteria. The oxygen concentration profiles observed are significantly different under the bioreactor operating condition for cases b and c in . In b, it was calculated that 95% of the ECS is within the desired range of oxygen tensions (25–70 mmHg), and in c almost all of the ECS (99.9%) is within the range of oxygen tensions. However, a better distribution of the three hepatic zones is found for case b. The pericentral zone in case c is significantly larger compared to case b, (81% versus 48% of the ECS volume), but the periportal (6% versus 7%) and perivenous (13% versus 40%) are smaller in case c compared to case b. The more even distribution of the oxygen profile, seen in case b, should provide a better distribution of the hepatic phenotypes.
A standard one-at-a-time (OAT) sensitivity analysis was performed on each of the parameters examined as has been previously described in the literature [Citation[10]]. For the range of parameters examined, none of the parameters elicited convergence issues. Further simulations were conducted with higher RBC concentrations, and resulted in either over-oxygenation of the hepatocytes or, at lower inlet dissolved oxygen tensions, the hepatocytes only experienced a small portion of the desired oxygen concentration range. Therefore, within this article, the two most promising possible bioreactor operating conditions were examined. These conditions should allow the hepatocytes to experience an in-vivo-like oxygenation spectrum (). Utilizing the developed oxygen transport model, a set of optimal bioreactor operating conditions could be determined for any experimental setup.
The bioreactor outlet pO2 calculated from the optimal simulation (case b) is 39 mmHg, which approximates the in vivo mixed venous pO2 and thus, when the patient's blood is returned, a significant portion of the oxygen in the blood stream has not been removed. However, since current BLADs remove a patient's venous blood, the feed stream to the hollow fiber bioreactor needs to be oxygenated to ∼ 90 mmHg, for which several methods are available to accomplish this task. One promising method utilizes silicone tubing that is contained in a controlled, elevated oxygen environment [Citation[40], Citation[41]]. This work and previous work has indicated that bioreactor inlet pO2 values of up to 95 mmHg can be attained with a reasonable length (∼ 2 m) of silicone tubing at atmospheric oxygen concentrations. In the future, an oxygen transport analysis of silicone tubing could be employed to calculate the length of silicone tubing and ambient oxygen concentration required to produce the desired blood oxygen level.
CONCLUSIONS
In this study, the oxygen concentration spectrum within a hollow fiber bioreactor housing primary hepatocytes supplemented with an oxygen carrier was examined. An oxygen transport model was developed and validated by experimentally measured data, and was subsequently utilized to predict the best bioreactor operating conditions for a viable BLAD. Previously, the use of hemoglobin-based oxygen carriers that could initially culture hepatocytes in an in vivo-like oxygen environment was investigated. In the work described here, the previously developed model was updated and its use has been extended to examine clinically relevant settings of BLAD implementation, which is expected to aid in the development of commercially viable BLADs. The parameters examined in the study identified bioreactor operating conditions where hepatocytes would experience a biomimetic oxygenation environment. It is believed that a hepatic hollow fiber bioreactor operating in the manner explained could sustain an ALF patient until native liver regeneration can take place, or a suitable donor organ can be found.
REFERENCES
- Simon, L., Karim, M.N. (2001). Identification and control of dissolved oxygen in hybridoma cell culture in a shear sensitive environment. Biotechnology Progress 17(4): 634–642.
- Patzer, J.F. (2004). Oxygen consumption in a hollow fiber bioartificial liver-revisited. Artificial Organs 28(1): 83–98.
- Gebhardt, R. (1992). Metabolic zonation of the liver-regulation and implications for liver-function. Pharmacology & Therapeutics 53(3): 275–354.
- Leary, T.S. , et al. (2002). Measurement of liver tissue oxygenation after orthotopic liver transplantation using a multiparameter sensor – A pilot study. Anaesthesia 57(11): 1128–1133.
- Dardzinski, B.J., Sotak, C.H. (1994). Rapid tissue oxygen-tension mapping using F-19 inversion-recovery echo-planar imaging of perfluoro-15-crown-5-ether. Magnetic Resonance in Medicine 32(1): 88–97.
- Tallgren, M. , et al. (1999). Hepatic and splanchnic oxygenation during liver transplantation. Critical Care Medicine 27(11): 2383–2388.
- Allen, J.W., Hassanein, T. Bhatia, S.N. (2001). Advances in bioartificial liver devices. Hepatology 34(3): 447–455.
- Morsiani, E. , et al. (2001). Long-term expression of highly differentiated functions by isolated porcine hepatocytes perfused in a radial-flow bioreactor. Artificial Organs 25(9): 740–748.
- Nyberg, S.L. , et al. (1994). Primary hepatocytes outperform Hep G2 cells as the source of biotransformation functions in a bioartificial liver. Annals of Surgery 220(1): 59–67.
- Sullivan, J.P., Gordon, J.E., Palmer, A.F. (2006). Simulation of oxygen carrier mediated oxygen transport to C3A hepatoma cells housed within a hollow fiber bioreactor. Biotechnology and Bioengineering 93(2): 306–317.
- Krogh, A. (1919). The number and distribution of capillaries in muscles with calculations of the oxygen pressurehead necessary for supplying the tissue. Journal of Physiology 52: 409–415.
- Buerk, D.G., Saidel, G.M. (1978). A comparison of two nonclassical models for oxygen consumption in brain and liver tissue. Advanced Experimental Medical Biology 94: 225–232.
- Hay, P.D. , et al. (2000). Oxygen transfer in a diffusion-limited hollow fiber bioartificial liver. Artificial Organs 24(4): 278–288.
- Shi, Y., Sardonini, C.A., Goffe, R.A. (1998). The use of oxygen carriers for increasing the production of monoclonal antibodies from hollow fibre bioreactors. Research in Immunology 149(6): 576–587.
- Jones, J.A. (1995). Red-blood-cell substitutes - current status. British Journal of Anaesthesia 74(6): 697–703.
- Faivre, B. , et al. (1998). Hemoglobin autooxidation/oxidation mechanisms and methemoglobin prevention or reduction processes in the bloodstream – Literature review and outline of autooxidation reaction. Artificial Cells Blood Substitutes and Immobilization Biotechnology 26(1): 17–26.
- Everse, J., Hsia, N. (1997). The toxicities of native and modified hemoglobins. Free Radical Biology and Medicine 22(6): 1075–1099.
- Seglen, P. (1976). Preparation of isolated rat liver cells. Methods in Cell Biology 13: 29–83.
- Dunn, J.C.Y., Tompkins, R.G., Yarmush, M.L. (1991). Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnology Progress 7(3): 237–245.
- Kay, J.M., Nedderman, R.M. (1985). Fluid Mechanics and Transfer Processes, Cambridge University Press: Cambridge, UK.
- Sullivan, J.P., Palmer, A.F. (2006). Targeted oxygen delivery within hepatic hollow fiber bioreactors via supplementation of hemoglobin-based oxygen carriers. Biotechnology Progress 22(5): 1374–1387.
- Renkin, E.M. (1954). Filtration, diffusion, and molecular seiving through porous cellulose membranes. Journal of General Physiology 38: 225–243.
- Foy, B.D. , et al. (1994). A device to measure the oxygen-uptake rate of attached cells – importance in bioartificial organ design. Cell Transplantation 3(6): 515–527.
- Bird, R.B., Stewart, W.E., Lightfoot, E.N. (1960). Transport Phenomena, John Wiley and Sons: New York, p. 780.
- Moll, W. (1968). The influence of hemoglobin diffusion on oxygen uptake and release by red cells. Respiratory Physiology 6(1): 1–15.
- Yap, E., Hellums, J. (1987). Use of adiar four-step kinetics in mathematical simulation of oxygen transport in the microcirculation. Advanced Experimental Medical Biology 215: 193.
- Federspiel, W.J., Popel, A.S. (1986). A theoretical-analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvascular Research 32(2): 164–189.
- Hay, P.D., Veitch, A.R., Gaylor, J.D.S. (2001). Oxygen transfer in a convection-enhanced hollow fiber bioartificial liver. Artificial Organs 25(2): 119–130.
- Piret, J.M., Cooney, C.L. (1991). Model of oxygen-transport limitations in hollow fiber bioreactors. Biotechnology and Bioengineering 37(1): 80–92.
- Katayama, S. , et al. (2001). Size-dependent in vivo growth potential of adult rat hepatocytes. American Journal of Pathology 158(1): 97–105.
- Gordon, J., Dare, M., Palmer, A. (2005). Engineering select physical properties of cross-linked red blood cells and a simple a priori estimation of their efficacy as an oxygen delivery vehicle within the context of a hepatic hollow fiber bioreactor. Biotechnology Progress 21: 1700–1707.
- Shatford, R.A. , et al. (1992). Hepatocyte function in a hollow fiber bioreactor – a potential bioartificial liver. The Journal of Surgincal Research 53(6): 549–557.
- Nyberg, S.L. , et al. (1993). Extracorporeal application of a gel-entrapment, bioartificial liver - demonstration of drug-metabolism and other biochemical functions. Cell Transplantation 2(6): 441–452.
- Kamlot, A. , et al. (1996). Review: Artificial liver support systems. Biotechnology and Bioengineering 50(4): 382–391.
- Tsiaoussis, J. , et al. (2001). Which hepatocyte will it be? Hepatocyte choice for bioartificial liver support systems. Liver Transplantation 7(1): 2–10.
- Scotto, J., Opolon, P., Eteve, J. (1973). Liver biopsy and prognosis in acute liver failure. Gut. 14: 927–933.
- Tilles, A.W. , et al. (2001). Effects of oxygenation and flow on the viability and function of rat hepatocytes cocultured in a microchannel flat-plate bioreactor. Biotechnology and Bioengineering 73(5): 379–389.
- Tanaka, Y. , et al. (2006). Evaluation of effects of shear stress on hepatocytes by a microchip based system. Measurement Science Technology 17: 3167–3170.
- Fournier, R.L. (1999). Basic Transport Phenomena in Biomedical Engineering, Taylor & Francis: Philadelphia, p. 90.
- Emery, A.N., Jan, D.C.H., Alrubeai, M. (1995). Oxygenation of intensive cell-culture system. Applied Microbiology and Biotechnology 43(6): 1028–1033.
- Maeda, T. , et al. (2000). Preclinical evaluation of a hollow fiber silicone membrane oxygenator for extracorporeal membrane oxygenator application. ASAIO Journal 46(4): 426–430.
- Freitas, R.A., Jr. (1999). Nanomedicine, Volume I: Basic Capabilities, Landes Bioscience: Georgetown, TX.
- Gibson, Q. (1970). The reaction of oxygen with hemoglobin and the kinetic basis of the effect of salt on binding of oxygen. The Journal of Biological Chemistry 245(13): 3285–3288.
- Popel, A.S. (1989). Theory of oxygen-transport to tissue. Critical Reviews in Biomedical Engineering 17(3): 257–321.
- McCarthy, M.R. (2001). The role of facilitated diffusion in oxygen transport by cell-free hemoglobins: Implications for the design of hemoglobin-based oxygen carriers. Biophysical Chemistry 92(1–2): 117.