Abstract
Objective: By using lingual artery chemoembolization on the basis of detailed basic studies to search an additional way for treatment of certain tongue carcinoma. Methods: Study of lingual artery cast specimens in post-mortem human was processed. Patients with tongue carcinoma were chemoembolized with Carboplatinum microcapsules. Results: Microcapsule embolism located approximately at the fifth to the sixth branches level of deep lingual artery. Effective clinical outcomes complied with the anatomy. Conclusion: Lingual artery chemoembolization with microcapsuled Carboplatinum of 214.0 ± 48.0 μm showed nice efficacy in therapy of mid-tongue carcinoma.
Incidence of tongue cancer is the highest one among oral and maxillofacial cancers. The therapy of microcapsule chemoembolism is a relatively new method to treat malignant tumors, which was performed mainly in hepatic malignant tumors by literature. This technique can effectively interrupt the blood supply of the tumor, increase concentrations of anti-tumor drugs, and prolong the drugs' action time in cancerous lesions while reducing the systemic toxicity Citation[1]. During the last decade, multiple articles have been published about utilizing selective arterial embolism with microcapsules containing anti-tumor drugs to treat malignant tumors Citation[1], Citation[2], Citation[3], Citation[4], Citation[5], Citation[6], Citation[7], Citation[8]. However, to date, no report has been found about utilizing chemoembolization to treat tongue carcinoma except for some other nearby organs Citation[9].We have treated 78 cases of patients suffered from lingual carcinoma since 1993 by lingual artery chemo-embolism with our self-produced ethyl cellulose micro-capsulated Carboplatinum Citation[10], and obtained good clinical efficacy. This study aimed to investigate the correlations among lingual anatomic characteristics, the flow pathway of microcapsules, embolism site, and clinical outcomes; and to develop an alternative treatment approach for certain tongue carcinomas, other than radical surgery and radiotherapy. These modalities, either alone or together, offer the prospect of curing the majority of the patients with certain tongue cancers Citation[11]. The most important is how to perfuse the medicine into the lesion through an appropriate way so that an adequate plasma-drug concentration could be obtained without entering the normal tissue through the blood vessels network.
METHODS
This investigation was approved by the ethics review committees, School of Medicine, Zhe Jiang University, and followed the principles in the Declaration of Helsinki. Informed consent was obtained from the study participants.
Preparation of Human Lingual Artery Cast Specimens
Fifteen percent red and blue acetone solutions were prepared from acrylonitrile butadiene styrene (ABS) resin with moderate red and blue paints for oil paintings, respectively. Fifty-three cadavers had internal jugular veins kept opened, and after the external carotid arteries and lingual arteries were anatomically separated, stable intubations were performed in lingual arteries. The lingual arteries were infused thoroughly to fix vascular endothelial cells with heparin saline and 0.5% pentdialdehyde phosphorate buffer solution (pH = 7.4) successively, in cases where vessels shrink and dilate excessively.
The prepared red and blue ABS acetone solutions were infused into the intubations in both sides until the lingual bodies stiffed, and they were then left to move into solid phase after 48 hours in a warm water bath. Lingual artery cast specimens were finalized by washing with flowing water after 5 to 7 days of tissue removal with 36% hydrochloric acid.
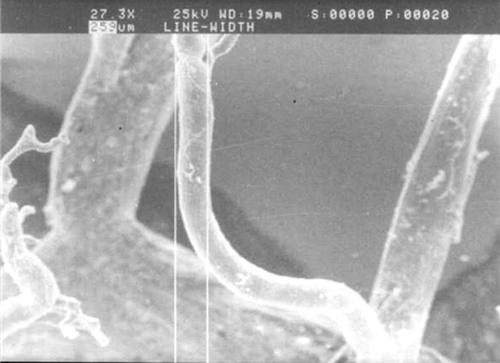
The morphology of lingual artery cast specimens was grossly observed and viewed microscopically with the diameters and angles of the branches measured under a scanning electronic microscope (SEM)Citation[12] ().
Clinical Materials and Embolism Method
Patients
Seventy-eight cases aged from 27 to 65, treated at the 2nd Affiliated Hospital, School of Medicine, Zhe Jiang University since 1993, were enrolled in the study including 43 males and 35 females. The size of carcinoma was from 1 to 6 cm in diameter. Nine cases had lingual carcinoma originating from preexisting leukplakia, while others were all de novo squamous cell carcinomas. The majority of these carcinomas were located at the mid-margin or mid-body of tongue, with 2 cases near the base of tongue and 3 cases at the ventral tongue near the floor of the mouth.
Surgical Procedures
The external carotid and lingual arteries were anatomically identified after neck skin incisions, and then stable intubations in lingual artery were performed, into which self-produced Carboplatinum microcapsules were infused. Twenty-one cases were embolized with catheterization alone directly, while another 57 cases were catheterized and embolized during radical neck lymph nodes dissection. All the mandibles of the 78 cases were kept intact without usual resection in radical surgery.
RESULTS
Lingual Artery Cast Specimens
We observed that the shape of lingual artery cast specimens was similar to the figure of a tongue, and the blood vessels in the two sides of tongue were separated by the fibro diaphragm at the axis of tongue, while connected with each other by the capillary net at the tip of tongue. The deep lingual artery ran ahead along the boundary of medial one-third and median one-third of a half tongue lineally, and then ran in a wavering pattern to the tip of the tongue, keeping close to the mucous membrane of the dorsal tongue, when observed laterally. Branches were distributed into every region of the tongue body, with most branches running vertically to the dorsal tongue; branches to the lateral and medial came next in frequency; and branches to the ventral tongue as the last. The branches differed in length: the longer ones arrived at the sub-mucous membrane after 7 to 8 levels of branches, a few of its lower levels and thinner branches constituted the lingual muscle-vessel nets with the shorter branches, and most of them also ran up to the submucous membrane of the dorsal tongue forming the submucous membrane capillary nets while connecting widely with the submucous membrane-vessel nets of the other side and the base of the tongue. Such anatomic properties revealed the shape of the lingual arterial cast to be like a sheet of fine lace. The mean diameters of the fifth and the sixth levels were measured to be 252.2 ± 99.4 μ m and 162.1 ± 76.0 μ m, respectively, under SEM.
Clinical Outcomes
No patient developed distinct complications after infusion or embolization. Neither were there any treatment-related or immediate post-treatment deaths in the series.
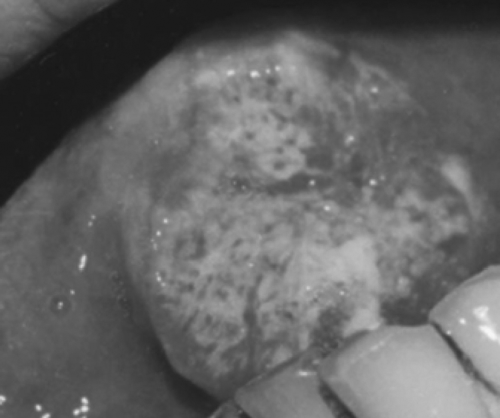
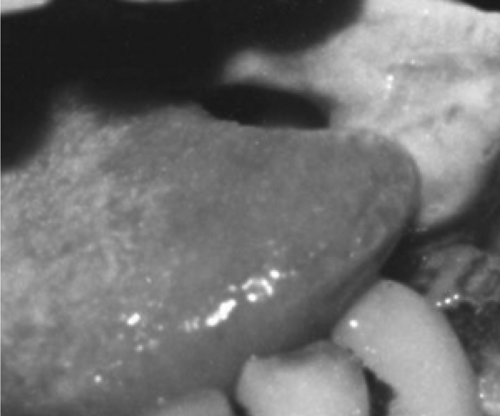
There was congestion and swelling in the embolized site excluding the tip and the base of the tongue on the first day after embolization. The farther the site was away from the tumor, the more significant was the reaction. After three days or so, the area gradually became necrotic with a grey-yellow surface. Patients suffered pain in the area treated. A separation between the necrotic tissue and the normal lingual tissue appeared. The diameter of the necrotic area was smaller than that of the congestion reaction area. It was probably because of the proliferating vasculature around the tumor Citation[13], Citation[14], which was benefited to the microsphere or microcapsule embolization. The necrotic separated area was resected 2 weeks after operation, and four samples of lingual tissue with a diameter of 1 mm were taken from different sites around the resected margin in every patient and prepared for histopathologic evaluation. An expanded resection was performed whenever one of the four sites was found to be positive for carcinoma cells. Fifty-nine cases were given additional resection of lingual body tissue along the necrotic separation margin to attempt more security without recurrence.
The sites, size of lingual carcinoma and embolism results are listed in the table. We defined completely eliminated (CE) when the four sites on the resected margin were all negative for carcinoma histopathologically. Partially eliminated (PE) was defined as when any of the four sites on the margin was positive for carcinoma clinically or histopathologically. Five PE cases were later proved to be positive with carcinoma cell at the dissected margin, and the other 73 cases were negative clinically and histologically ( and ).
TABLE 1 Sites of lingual carcinoma and embolism regions
After 5 years of healing, 69 of the 78 cases (88.5%) were found to be disease-free. After 10 years of healing, 41 cases were still without recurrence. A total of 17 cases (21.8%) suffered from recurrence 2 years after treatment or in the later time. The recurrence–free survival rate at 5 years is higher than 81%, and the recurrence proportion is lower than 32.4% in the published data Citation[15].
DISCUSSION
The blood supply in the tongue is one of the richest parts in the oral-maxillo-facial region. The embolism results of necrosis and separation, as well as the size of the necrosis, relate to the infusion pressure, the quantity of microcapsules infused in a unit time, and the dosage of the embolism. The site and size of the cancer are also relevant. The excessively low infusion pressure or excessively fast infusion velocity will lead to microcapsules embolism in the main stem or large branches instead of arriving at the fifth to the sixth branch levels and result in the unreliable necrosis. So, selecting the dosage as 200 mg to 300 mg of Carboplatinum with the addition of 30–40 ml of meglumine iothalamate suspending liquid, as well as a stable infusion procedure, allows the microcapsules to arrive at a proper vessel level during the lingual artery embolism procedure.
The diameter of microcapsule was controlled to be between 40 μ m to 300 μ m Citation[12], Citation[16], Citation[17]. The morphology of our Carboplatinum microcapsules was regular and approximately globular with an average diameter of 214.0 ± 48.0 μ m Citation[10]. Drug particles were seen scattering among the reticular ethyl cellulose braces under the SEM, with many holes on the surface. The content of Carboplatinum in microcapsule was 51.4%, and the release rate was 62.4% per day by in vitro test Citation[10]. According to the diameters of Carboplatinum microcapsules and the clinical outcomes, we can infer that the position of embolism was between the fifth and sixth levels (252.2 ± 99.4 μ m and 162.1 ± 76.0 μ m, respectively) of branches, which would never result in the substantial necrosis of the whole tongue body, because of the particular vessel structure and the abundant blood supply.
Although one must not draw conclusions from our limited studies to use in all tongue carcinomas, within the limitations of this study it can be concluded that the lingual artery chemoembolization with proper microcapsules possesses promising therapeutic uses for treating patients with carcinoma in mid-margin and the mid-body of tongue. Chemoembolization appears to be effective in eliminating lingual tumors without any other additional painful or deforming therapy, and improves the quality of life in patients. Due to the anatomic properties of lingual arterial branches and the hyperplasic blood flow and vessels around the tumor Citation[18], the microcapsules were easily able to flow to the tumor region, which was the first site after being infused into the lingual artery, and then on to the margin and the dorsum of tongue. The base and tip of the tongue are difficult for blood flow containing microcapsules to produce embolisms because of the additional resistance of blood flow from the connecting capillary net. The dyed sequence of time for different sites using medical blue in tongue confirmed this point. The first site was the tumor and its rounding region; the next one was the margin, back and abdomen of tongue; and the last one was the base and tip of tongue. The quantity of medical blue dyed in each level of tongue also came out in such a sequence.
After embolism infusion, the tumor vessels will be embolized, and the tumor will develop a compromised blood supply, became necrotic, and separate from the designed site Citation[19].
When treating the malignant tumor, there is not yet a perfect single method to completely resolve the whole problem; the sequence-therapy is still a relative proper method. An expanded resection of lingual tissue along the necrosis and separating margin is essential to eradicate the tumor after chemoembolization in cases of large tumor especially when located at the tip and base margin of tongue, except for the small cancer at the mid-margin of tongue, which possesses a nice result of necrosis and separation of nearly a half tongue after embolism.
Loco-regional relapse would have a possibility to occur, resulting in death Citation[18]. It leads us to develop such an alternative or multimodality therapy to increase the life expectancy and to improve the quality of life in patients with complex oral tongue carcinoma. The chemoembolism is a hopeful method worth studying futher.
CONCLUSION
By this method, the malformations for large tissue dissection are avoidable. The detailed study of anatomy, technique and material for lingual arterial chemoembolism lead to its adoption in treating tongue carcinoma. Being well tolerated with few complications, this regimen is worthy of such wider and deeper research as about its possible incomplement and complications Citation[20], Citation[21] in the neck and head field. The average diameter of our globuloid Carboplatinum microcapsules was 214.0 ± 48.0 μm. The position of embolism was between the fifth and sixth levels (252.2 ± 99.4 μm and 162.1 ± 76.0 μm, respectively) of branches, which would never result in the substantial necrosis of the whole tongue body, because of the particular vessel structure and network.
REFERENCES
- Daniel S., Hajarizadeh Homoyan, Mueller Charles R., Fletcher William S., Pommier Rodney F., Woltering Eugene A., et al. Treatment of metastatic carcinoid tumors using multimodality therapy of octreotide acetate, intra-arterial chemotherapy, and hepatic arterial chemoembolization. Am J Surg 1995; 169: 523–528
- Esteban Jose M., Gil J. Therapeutic effect of chemoembolization therapy on hepatocellular carcinoma: evaluation with contrast-enhanced power Doppler sonography and contrast-enhanced harmonic imaging. Acad Radiol 2002; 9: s382–383
- Uraki Junki, Yamakado Koichiro, Nakatsuka Atsuhiro, Takeda Kan. Transcatheter hepatic arterial chemoembolization for hepatocellular carcinoma invading the portal veins: Therapeutic effects and prognostic factors. Eur J Radiol 2004; 51: 12–18
- Plentz Ruben R., Lankisch Tim O., Bastürk Murat, Muller Corrina M., Kirchhoff Timm, Gebel Michael, et al. Prospective analysis of German patients with hepatocellular carcinoma undergoing transcatheter arterial chemoembolization with or without prophylactic antibiotic therapy. J Gastroenterol Hepatol 2005; 20: 1134
- Munro N. P., Woodhams S., Nawrocki J. D., Fletcher M. S., Thomas P. J. The role of transarterial embolization in the treatment of renal cell carcinoma. BJU Int 2003; 92: 240
- Hawis M., Gibbs P., Cebon J., Jones R., Sewell R., Schelleman T., et al. Hepatocellular carcinoma and chemoembolization. Intern Med J 2001; 31: 517–522
- Hayashi P. H., Ludkowski M., Forman L. M., Osgood M., Johnson S., Kugelmas M., et al. Hepatic artery chemoembolization for hepatocellular carcinoma in patients listed for liver transplantation. Am J Transplant 2004; 4: 782–787
- Huang Y.-H., Wu J.-C., Chen S.-C., Chen C.-H., Chiang J.-H., Huo T.-I., et al. Survival benefit of transcatheter arterial chemoembolizaton in patients with hepatocellular carcinoma larger than 10 cm in diameter. Aliment Pharmacol Ther 2006; 23: 129–135
- Kovacs Adorjan F., Turowski Bemd. Chemoembolization of oral and oropharyngeal cancer using a high-dose cisplatin crystal suspension and degradable starch microspheres. Oral Oncol 2002; 38: 87–95
- Genghua Gu, Jianqi Huang, Hong He. Sequential-release of anticancer drugs microcapsulated with ethylcellulose. Chinese Medical Journal 2002; 115: 1730–1732
- Veness M. J. Tongue cancer in younger patients. Aust Radiol 1999; 43: 76–82
- Chunxiao Sun, Mingda Yang, Jianqi Huang, Ming Zhang. The scanning electronic microscope study of human lingual artery cast model. Chin J Stomatol 1997; 32: 190
- Shang Z. J., Li Z. B., Li J. R. VEGF is up-regulated by hypoxic stimulation and related to tumour angiogenesis and severity of disease in oral squamous cell carcinoma: in vitro and in vivo studies. Int J Oral Maxillofac Surg 2006; 35: 533–538
- Uehara M., Sano K., Ikeda H., Sekine J., Irie A., Toyota T., et al. Expression of vascular endothelial growth factor and prognosis of oral squamous cell carcinoma. Oral Oncol 2004; 40: 321–325
- Veness Michael J., Morgan Gary J., Sathiyaseelan Yasoda, Gebski Val. Anterior tongue cancer: Age is not a predictor of outcome and should not alter treatment. ANZ J Surg 2003; 73: 899–905
- Kato T., Nemoto R., Mori H., Takahashi M., Harada M. Arterial chemoembolization with mitomycin C microcapsules in the treatment of primary or secondary carcinoma of the kidney, liver, bone and intrapelvic organs. Cancer 1981; 48: 674–680
- Ross C. J. D., Chang P. L. Development of small alginate microcapsules for recombinant gene product delivery to the rodent brain. J Biomater Sci Polym Ed 2002; 13: 953–962
- Kimura Y., Ariji Y., Gotoh M., Toyoda T., Kato M., Kawamata A., et al. Doppler sonography of the deep lingual artery. Acta Radiol 2001; 42: 306–311
- Jang Myoung Kuk, Lee Han Chu, Kim In Sook, Lim Young Suk, Chung Young, Lee Yung Sang, et al. Role of additional angiography and chemoembolization in patients with hepatocellular carcinoma who achieved complete necrosis following transarterial chemoembolization. J Gastroenterol Hepatol 2004; 19: 1074–1080
- Shim Su Jung, Seong Jinsil, Han Kwang Hyub, Chen Chae Yoon, Suh Chang Ok, Lee Jong Tae, et al. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization inlocally advanced hepatocellular carcinoma. Liver International 2005; 25: 1189–1196
- Toyda H., Fukuda Y., Nakano I., Katano Y., Ebata M., Nagano K. I., et al. Massive bleeding from a gastric erosion after transcatheter arterial chemoembolization for hepatocellular carcinoma in a patient with wild haemophilia A. Haemophilia 2000; 16: 688–692