Abstract
The objective of this study was to develop an engineered rat hyaline cartilage by culturing articular chondrocytes on three-dimensional (3D) macroporous poly(DL-lactic-co-glycolic acid) (PLGA) sponges under chondrogenic induction and microgravity bioreactor conditions. Experimental groups consisted of 3D static and dynamic cultures, while a single cell monolayer (2D) served as the control. The effect of seeding conditions (static vs. dynamic) on cellularization of the scaffolds was investigated. MTT assay was used to evaluate the number of viable cells in each group at different time points. Formation of a hyaline-like cartilage was evaluated for up to 4 weeks in vitro. While 2D culture resulted in cell sheets with very poor matrix production, 3D culture was in the favor of tissue formation. A higher yield of cell attachment and spatially uniform cell distribution was achieved when dynamic seeding technique was used. Dynamic culture promoted cell growth and infiltration throughout the sponge structure and showed the formation of cartilage tissue, while chondrogenesis appeared attenuated more towards the outer region of the constructs in the static culture group. Medium supplemented with TGF-β 1 (5 ng/ml) had a positive impact on proteoglycan production as confirmed by histochemical analyses with Alcian blue and Safranin-O stainings. Formation of hyaline-like tissue was demonstrated by immunohistochemistry performed with antibodies against type II collagen and aggrecan. SEM confirmed higher level of cellularization and cartilage tissue formation in bioreactor cultures induced by TGF-β 1. The data suggest that PLGA sponge inside rotating bioreactor with chondrogenic medium provides an environment that mediates isolated rat chondrocytes to redifferentiate and form hyaline-like rat cartilage, in vitro.
INTRODUCTION
The spontaneous healing capacity of the articular cartilage is very limited, and the surgical techniques generally fail to completely repair this tissue. This avascular tissue with complex properties consists of a sparse number of chondrocytes surrounded by a dense extracellular matrix (ECM) Citation[1], Citation[2]. Approaches based on the tissue engineering concept have been applied to treat damaged articular cartilage using chondrocytes from different sources, and have resulted in the formation of cartilage ranging from hyaline-like to fibrous tissue Citation[3], Citation[4], Citation[5], Citation[6], Citation[7], Citation[8]. Basically, tissue engineering involves the formation of functional new tissues from isolated cells and biodegradable scaffolds by modulating cell signals using related cytokines and assuring proper mechanotransductive conditions Citation[9], Citation[10].
Articular hyaline cartilage is responsible for the smooth gliding motion of the movable joints Citation[1], and this ability comes from the osmotic resistance of the ECM molecules to water flow dispensing high joint loads through absorbing shock and minimizing stress on the subchondral bone Citation[1], Citation[11], Citation[12]. Chondrocytes inside native articular cartilage are embedded in a highly specialized ECM that consists primarily of: (i) proteoglycans, composed of a protein core to which linear sulphated polysaccharides are attached, mostly in the form of chondroitin sulphate and keratin sulphate; (ii) a network of collagen fibers, primarily composed of Collagen type II, trapping the predominant proteoglycan Aggrecan interacting with another glycosaminoglycan, Hyaluronan; and (iii) water Citation[11], Citation[12]. Macroporous scaffolds made up of synthetic polymers and matrices from natural hydrogels have been tested as support material to provide a temporary artificial extracellular environment for the chondrocytes during in vitro chondrogenesis Citation[5], Citation[6], Citation[7], Citation[8], Citation[13], Citation[14].
Studies have approved the use of dynamic culture systems in engineering articular cartilage tissue to constitute the native-like ECM composition Citation[3], Citation[4]. Mechanotransduction seems to be necessary in order to engineer this load-bearing tissue, as well as supplementing with chondrogenic growth factors, such as transforming growth factor-β 1 (TGF-β 1) and L-ascorbic acid Citation[15]. Among the dynamic culture systems, the slow turning lateral vessel (STLV)-type bioreactor provides low shear force, high mass transfer, and simulated microgravity enabling three-dimensional (3D) growth of chondrocytes on scaffolds Citation[15].
The present study was designed to combine these techniques, seeding rat chondrocytes on macroporous poly(DL-lactide-co-glycolide) (PLGA) sponges and culturing inside STLV bioreactor with chondrogenic inducers for a duration of 28 days. Our aim was to investigate the effects of dynamic cell seeding, the use of TGF-β 1 and STLV-bioreactor conditions on articular rat chondrocyte proliferation and redifferentiation into hyaline-like neocartilage.
MATERIALS AND METHODS
Preparation of PLGA Sponge
The polymer used to prepare the sponges was a 85/15 blend (lactate/glycolate) of PLGA (Mn≈ 90,000–126,000; Sigma Chemical Company, St. Louis, MO, USA). Sponges were fabricated by the solvent casting and porogen leaching method Citation[16], Citation[17]. Briefly, 10% (w:v) raw copolymer in dichloromethane was casted into Teflon molds packed with NaCl crystalline particles (sieved to 200-300 μ m). The solvent was allowed to evaporate and the salt was removed by immersing the sponges in distilled luke-warm water. The sponges were then dried in a vacuum oven, cut into cylinder shape (Φ = 6 mm, h = 3 mm). Sponges were sterilized overnight in 70% (v/v) ethanol, washed three times with sterile distilled water and dried. To enhance cell attachment, the sponges were prewetted in Dulbecco's modified Eagle's minimal essential medium (DMEM) containing 20% FCS Citation[17], 100 U/ml penicilin and 100 μ g/ml streptomycin (Pen/Strep) prior to cell seeding experiments.
Isolation and Seeding of Chondrocytes
All protocols involving animals were conducted according to the standards of international regulations. Chondrocytes were isolated from aseptically harvested articular cartilage from the femoral-patellar joints of young Wistar rats weighing 75–100 g. The cartilage pieces were rinsed in sterile PBS containing Pen/Strep (100 U/ml and 100 μ g/ml). The samples were cut into 1–2 mm3 fragments and placed in a spinner flask containing 3 mg/ml collagenase and 0.15 mg/ml hyaluronidase in DMEM supplemented with Pen/Strep. The enzymatic digestion lasted overnight at 37°C. Then, the isolated cells were washed three times with DMEM (cell viability > 90%; confirmed by trypan blue dye exclusion test), and were seeded on sponges by two different techniques: (i) static seeding: 150 μ L cell suspension (containing ∼ 100.000 chondrocytes) was added drop-wise onto each prewetted sponge placed in a well of 6-well plate; kept in the incubator for 10 min and then was gently covered with 6 ml of basal medium; (ii) dynamic seeding: 600 μ l cell suspension (containing ∼ 400.000 chondrocytes) was added into the rotating bioreactor containing 4 sponges inside 6 ml of basal medium. After 4 hours, 18 ml medium was added to the final volume (24 ml/bioreactor). For the monolayer culture, cells were seeded (∼ 50.000 cells/dish) on 35 mm-tissue culture dishes (Falcon Labware), which had type I collagen coating (Vitrogen, Collagen Corp.) with a 1 μ g/cm2 surface concentration. The basal culture medium consisted of DMEM containing 10% FCS, non-essential amino acids, L-glutamine, 50 μ g/ml ascorbic acid, insulin-transferrin-selenite combination (ITS), 100 U/ml penicilin and 100 μ g/ml streptomycin. Cells were incubated at 37°C, 5% CO2 and 95% humidity.
Culture Conditions and Experimental Groups
Two different culture conditions were established: (i) three-dimensional static culture on PLGA sponge; and (ii) three-dimensional dynamic culture on PLGA sponge using the rotating bioreactor. Two-dimensional culture (as single cell monolayer) served as the control. The experimental procedures and groups are shown in and .
TABLE 1 The experimental groups
(i) Chondrocytes-seeded sponges designated for static culture were either (a) kept in the basal medium, or (b) cultured for 3 days in the basal medium and then in TGF-β1 (5 ng/ml) containing chondrogenic induction medium inside the wells of 24-well plates.
(ii) Three-dimensional dynamic culture experiments were performed in a rotating bioreactor (slow turning rotating wall vessel; STLV) (Synthecon Inc., Austin, TX). In this group, both static and dynamic cell seeding methods were tested at the beginning of the cultures; however, dynamic cell seeding was preferred for the rest of the experiments, since this method gave a higher cell seeding efficiency compared with the static seeding method (). The cell-sponge constructs were either (a) kept in the basal medium for the duration of experiments, or (b) cultured for 72 hours in the basal medium and then in the chondrogenic medium. The medium was changed every other day for the duration of the experiments (28 days).
TABLE 2 Effect of seeding technique on cell loading capacity of PLGA sponge
For two-dimensional monolayer control culture, chondrocytes were (a) maintained in the basal medium for the duration of experiments, (b) cultured for 3 days in the basal medium and then exchanged with the TGF-β1 (5 ng/ml) containing chondrogenic induction medium.
MTT Assay
An MTT (3-(4,5-dimethylthiazol-2-yl-2,5-diphenyl tetrazolium bromide)-based assay (Sigma) was used to evaluate the increase in viable cell number inside cell-sponge constructs with mitochondrial dehydrogenase activity. Briefly, 30 μ L of fresh MTT solution (5 mg/mL) was added onto cell-sponge constructs inside 270 μ L medium (without FCS), and were incubated at 37°C and 5% CO2 for 4 h. The MTT taken up by the cells and reduced in the mitochondria to formazan, an insoluble dark blue complex, was visualized under an inverted microscope (Nikon TS 100, Japan). Quantitative data were obtained spectrophotometrically at a wavelength of 570 nm using an ELISA plate reader (Amersham Biotrak II, General Electric, Pittsburgh, PA, USA). The absorbance values were converted to cell number by using a standard curve prepared from six chondrocyte cultures with different cell concentration. Chondrocytes cultured in 35 mm-tissue culture dishes were used as the monolayer control.
Histology and Immunohistochemistry
At set time points, cell-sponge constructs designated for histological or immunohistochemical evaluation were rinsed in 0.1 M phosphate buffer (pH 7.0) and then fixed overnight in 10% neutral buffered formalin at 4°C. After fixation, the samples were dehydrated by immersion in a series of ethanol solutions (60–100%) and xylene solutions in ethanol (50% and 100%), then embedded in paraffin, and sectioned using a microtome. Dishes containing single cell monolayers were fixed in 1% formalin for 5 minutes, and then air-dried.
For the investigation of proteoglycans, sections or dishes were stained with Alcian blue, which colors basophilic structures, like the negatively charged glycosaminoglycans of the articular cartilage; Safranin-O was used to stain the collagens (both from Sigma).
Immunohistochemical staining was carried out using an avidin-biotin complex peroxidase detection system (Lab Vision, Fremont, CA). The primary antibodies used were polyclonal goat anti-rat Collagen type II IgG (1:200) (Santa Cruz Biotech., Santa Cruz, CA) and monoclonal mouse anti-rat Aggrecan IgG (1:200) (Abcam, Cambridge, MA). Biotinylated secondary antibodies were goat anti-mouse IgG (Lab Vision) for Aggrecan, and rabbit anti-goat IgG (Zymed, San Fransisco, CA) for Collagen type II.
Images were acquired with a digital light microscope (Leica DM 4000B, Weitzlar, Germany) equipped with a video camera (Leica DFC 320).
Scanning Electron Microscopy
Cell-sponge constructs were prepared for electron microscopy using routine methods Citation[18]. Samples were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) at 4°C. Then, the samples were dehydrated in a graded ethanol series, mounted on aluminum supports and were sputter-coated with gold using a Sputter Coater (Desk II, Denton Vacuum, Cherry Hill, NJ, USA). A JEOL 100S (Tokyo, Japan) electron microscope was utilized to image samples at a voltage of 10 kV.
Statistical Analysis
Values are reported as means ± standard deviation for a representative example, for the three separately performed experiments. Statistical analysis was carried out using the unpaired Student's t test. A value of P < 0.05 was considered to be statistically significant.
RESULTS
PLGA Sponge
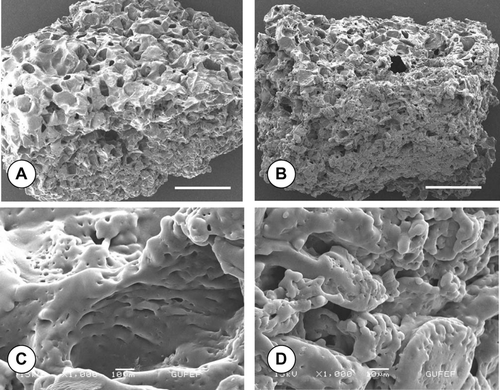
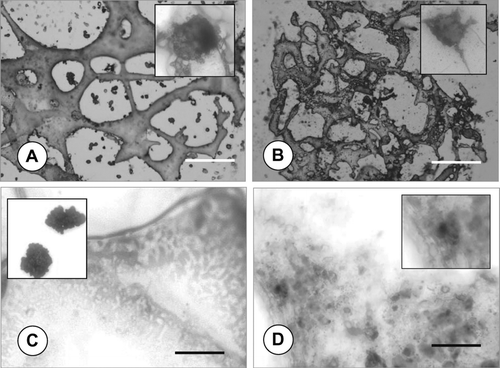
shows the scanning electron microscopy images of the PLGA sponges used in three-dimensional chondrocyte culture experiments. It is evident from the figures that the sponge is composed of macropores (2A, 2B). The positive influence of the bioreactor culture on cell growth and infiltration into sponge pores can easily be observed from the SEM images (compare 2A and 2B). SEM investigations also demonstrated the seeded chondrocytes had completely covered the sponge pore walls and the outer surface of the polymer scaffold, after 28 days of dynamic culture under TGF-β 1-induction. The PLGA sponge had mechanical stability for the duration of experiments, both under static and dynamic culture conditions. Incubation of the sponge with fetal calf serum before cell seeding Citation[17] possibly enhanced attachment of the chondrocytes to the polymer surface.
Figure 2 Representative SEM images of the macroporous PLGA sponges seeded with rat articular chondrocytes retrieved from (A) static, (B) bioreactor cultures after 28 days with TGF-β 1-induction. Infiltration of chondrocytes into sponge pores (C) and outer surface (D) can be visualized in high magnification images of B. Note the decreasing sponge pore size in bioreactor culture (B) compared to the static culture (A). Scale bars, A and B: 100 μ m; C and D: 10 μ m.
Chondrocyte Seeding Technique
demonstrates the effect of seeding technique on cell loading capacity of the PLGA sponge. As can be seen from the table, the dynamic cell seeding technique resulted in higher cell loading (with %83.7 of the introduced at Day 3), and homogenous distribution of chondrocytes inside the sponge pores (). On the other hand, a cell loading of %57.9 could be accomplished by the static seeding at the same time point (). The majority of the chondrocytes maintained their characteristic spherical morphology throughout the 28 day-culture period. The number of cells inside the statically seeded sponge increased and came close to its dynamically seeded counterpart after 2 to 3 weeks of culture period. The rest of the bioreactor culture experiments were conducted with cell-sponge constructs seeded by the dynamic method.
Number of Cells with Mitochondrial Activity
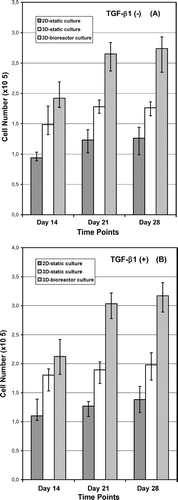
The cell number (MTT viability) data of the chondrocytes cultured on tissue-culture plastic (2D-static), and inside sponges (3D-static and 3D-dynamic) are presented in . The increase in cell number with mitochondrial activity on PLGA sponge evidently enhanced compared with the control 2D-monolayer cultures, at all time points. The number of cells in 3D-sponges increased over 80% when cultured inside STLV bioreactor (compare the cell numbers in experimental groups at Days 14 and 28). Induction of cultures with TGF-β 1 () increased the cell numbers in both experimental groups, and the control group. This was more evident at the first week with about 25% increase (not shown); however, less effective at Days 14 and 28 (with about 10–15% of increase) in both static and dynamic 3D-sponge cultures. Additionally, an apparent increase in cell number by culture time was noted between Days 0 and 14, while this was not the case between Days 21 and 28, probably due to a saturated proliferation when the culture reached confluence ( and ).
Figure 3 Change in the number of chondrocytes cultured on 35 mm-tissue culture dishes (2D-static), and inside sponges (3D-static and 3D-dynamic) under (A) standard, (B) TGF-β 1-induced culture conditions. Cell numbers at Day 0 were: ∼ 25.000 cells/dish and ∼ 50.000 cells/sponge. Absorbance values are presented as normalized means± SD from three independent experiments. (Continued)
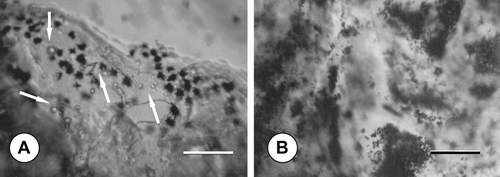
The MTT activity of the chondrocytes was also investigated by inverted microscope. Representative phase-contrast images are presented in ; here the formazan crystals in dark blue colour are clearly visible at the vicinity of the cells inside sponge constructs. Besides, inverted microscopy evaluation showed that the cultures supplemented with TGF-β 1 had larger chondrocyte size, compared with cells cultured in the basal medium enriched with L-ascorbic acid (not shown).
Figure 4 Phase-contrast micrographs demonstrating the dark blue formazan crystals formed from MTT inside sponge pores after 14 days of dynamic culture: in (A) standard, (B) TGF-β 1-containing medium; scale bars: 200 μ m. Note the round-shaped chondrocytes (arrows) at the vicinity of the formazan crystals.
Histochemistry and Immunohistochemistry
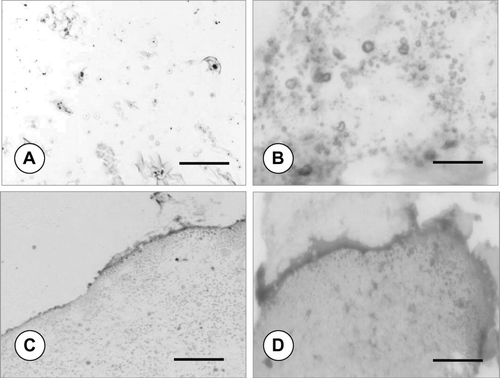
Histochemical stainings revealed a high cell density besides the accumulation of extracellular matrix (ECM) inside the sponge construct pores. The production of proteoglycans could be investigated starting from the first week of culture (not shown). Representative light microscopy images of the proteoglycan stainings at the 28th day of culture (with TGF-β 1) are shown in . Staining with Alcian blue (5A, 5B) demonstrates the existence of matrix production, mainly of glycosaminoglycans. Staining of the collagen content with Safranin-O (5C, 5D) demonstrates the in vitro formation of a hyaline-like cartilage tissue. As can be clearly seen from the figure, the samples retrieved from the bioreactor cultures stained more intensely than the 3D-static sponge cultures both to Alcian blue and Safranin-O (compare 5A with 5B, and 5C with 5D), confirming that the dynamic culture conditions maintained by the STLV bioreactor was in the favor of cartilage tissue formation in vitro. This is evident in and , in which cell aggregates with round-shaped cells are surrounded by the new-formed ECM. Histochemical experiments performed with samples from monolayer cultures and from 3D-cultures not conditioned with TGF-β 1 showed weaker stainings to Alcian blue and Safranin-O, indicating a lesser chondrogenic state (not shown).
Figure 5 Light microscopy images demonstrating proteoglycan stainings. Alcian blue (A, B) and Safranin-O (C, D) stainings of rat articular chondrocytes on PLGA sponge under static (A and C) and bioreactor (B and D) culture conditions with TGF-β 1 at Day 28; scale bars: 200 μ m. Note the strong stainings of the samples retrieved from bioreactor cultures confirming the chondrogenic state.
Anti-Collagen type II and anti-Aggrecan stainings of the cell-sponge constructs cultured under static (6A, 6C) and bioreactor (6B, 6D) culture conditions with TGF-β 1 at Day 28 are given in . Results showed that while 3D-sponge cultures were weakly-stained at Day 7 (not shown), a stronger staining to both antibodies could be observed at the subsequent experimental time points (Days 14 and 28). It was evident that sections taken from the dynamic sponge-chondrocyte cultures were stained more intensely with Anti-Collagen type II and anti-Aggrecan than the static ones (). Additionally, cultures lacking TGF-β 1 demonstrated lower levels of Collagen type II and Aggrecan antibody stainings; however, anti-Collagen type I staining was evident in these cultures (not shown).
Figure 6 Light microscopy images demonstrating immunohistochemical stainings. Anti-rat Collagen type II (A, B) and Anti-rat Aggrecan (C, D) stainings of rat articular chondrocytes on PLGA sponge under static (A and C) and bioreactor (B and D) culture conditions with TGF-β 1 at Day 28; scale bars: 200 μ m.
DISCUSSION
The aim of this study was to determine the effect of STLV-type bioreactor culture and TGF-β 1-induction on the in vitro proliferation, and chondrogenic proteoglycan and protein production of articular rat chondrocytes seeded on macroporous PLGA sponges. Accordingly, sponge/chondrocyte constructs were cultured for 28 days under static and dynamic culture conditions and analyzed for cellularity in terms of MTT activity, and staining of the chondrogenic proteoglycans and/or proteins by histochemistry and immunohistochemistry. Static two-dimensional monolayer cultures served as controls. Increase in the absorbance values recorded at 570 nm wavelength, which demonstrates the conversion of MTT to formazan, was primarily due to increased cellularity. Cellularity of the constructs increased up to 14 days and remained almost constant thereafter. In particular, the largest increase in cellularity was exhibited with constructs cultured under dynamic conditions with additional TGF-β 1-induction.
Phenotypical properties of an articular cartilage can be characterized by the expression of the specific extracellular matrix proteoglycans (i.e. Aggrecan) and structural molecules (i.e. Collagen type II, IX, XI) Citation[1], Citation[5], Citation[19], Citation[20]. After isolation from the articular cartilage tissue and culturing as monolayers, chondrocytes gradually lose their characteristic phenotype Citation[19], Citation[20], as indicated by the formation of a fibroblastic morphology, and decline in ability to synthesize proteoglycans and their specific proteins, instead start to synthesize the prechondrogenic ones, such as Collagen type I and Versican Citation[19], Citation[20]. This immature state continues in 2D-monolayer cultures; however, it can be converted to redifferentiated state by transferring into a three-dimensional culture environment (i.e. scaffold or hydrogel) in a mechanotransductive setting (bioreactor), and by chondrogenic induction (e.g. TGF-β1); thus the reexpression of the chondrogenic phenotype is important before implantation in vivo.
In our study, rat articular chondrocytes cultured on 3D-PLGA sponges were able to express the specific hyaline cartilage molecules, Collagen type-II and Aggrecan, showing the signs of in vitro neocartilage formation. Besides, cultures that received TGF-β 1 showed larger chondrocyte size and more intense staining to antibodies than cultures devoid of TGF-β1 but containing L-ascorbic acid. The chondrogenic medium consisting of TGF-β1 and L-ascorbic acid seems to play an important role in influencing mature cell functions. Hydroxyproline and hydroxylysine are located at the α -chain of collagen molecules, and are made up of the hydroxylation of proline and lysine amino acids, by the help of L-ascorbic acid during collagen synthesis Citation[21]. Transforming growth factor-β1 is a multifunctional cytokine from the TGF-β superfamily, which stimulates chondrocyte growth, chondroprogenitor cell differentiation and the motility of chondrocytes Citation[22], as well as the synthesis and deposition of the extracellular matrix. TGF-β1 is involved in cartilage development during embryogenesis and is expressed during cartilage repair Citation[23], Citation[24].
Shear stress is essential in modulating the mechanical properties of tissue constructs Citation[14]; on the other hand, it may also be the cause of undesired capsule formation surrounding the new forming tissue as well. STLV, a derivative of rotating wall vessel bioreactor first introduced by NASA, exploits the benefit of low shear stress wherein the cells are grown in a microgravity environment Citation[3], Citation[14]. In our study, the use of bioreactor to maintain dynamic culture further increased the growth rate of the seeded cells within the sponges, in agreement with other studies Citation[25], Citation[26].
It seems that the continual rotating action by the STLV-rotational culture system provided more effective transfer of the culture medium to the cells of the construct, and possibly a higher metabolic activity as a consequence. In 3D-culture, the proliferating cells were evenly distributed within the sponge pores, permitting better access to nutrient and gas transport provided by the medium. The positive influence of bioreactor culture on the expression of extracellular matrix components by the cell-sponge constructs was evident, and could be explained by the improved cell proliferation under dynamic conditions, and possibly by the existing mechanotransductive effects. This was proved by the MTT and immunohistochemistry findings.
CONCLUSION
This study demonstrated the possibility of forming articular neo-cartilage tissue in vitro, by culturing articular rat chondrocytes on 3D-sponges under dynamic and chondrogenic culture conditions. The positive influence of STLV-rotational culture system and TGF-β 1-induction on the formation of neo-cartilage was confirmed by the MTT studies. Histochemistry and immunohistochemistry revealed the existence of proteoglycan production and the expression of specific hyaline cartilage proteins of the chondrocyte-sponge constructs. It was concluded that the applied optimal culture conditions reversed the phenotypic modulation (dedifferentiation) of rat chondrocytes after isolation.
The support of the Ankara University Biotechnology Institute (BIBAP 153), and TÜBA (GEBIP 2002-1-10; YME), Ankara, Turkey, is acknowledged.
REFERENCES
- Adolphe M. Biological Regulation of the Chondrocytes, 1st ed. CRC Press, Boca Raton 1992
- Barbero A., Grogan S., Schafer D., Heberer M., Mainil-Varnet P., Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage 2004; 12: 476–484
- Freed L. E., Vunjak-Novakovic G. Tissue culture bioreactors: chondrogenesis as a model system. Principles of Tissue Engineering, R. P. Lanza, R. Langer, W. L. Chick. Academic Press, Austin 1997; 151–165
- Dunkelman N. S., Zimber M. P., LeBaron R. G., Pavelec R., Kwan M., Porchio A. F. Cartilage production by rabbit articular chondroytes on polyglycolic acid scaffolds in a closed bioreactor system. Biotechnol. Bioeng 1995; 46: 299–305
- Risbud M., Ringe J., Bhonde R., Sittinger M. In vitro expression of cartilage-specific markers by chondrocytes on a biocompatible hydrogel: implications for engineering cartilage tissue. Cell Transplant 2001; 10: 755–763
- Guoping C., Takashi S., Takashi U., Naoyuki O., Tetsuya T. Tissue engineering of cartilage using a hybrid scaffold of synthetic polymer and collagen. Tissue Eng 2004; 10: 323–330
- Ochi M., Adachi N., Nobuto H., Yanada S., Ito Y., Agung M. Articular cartilage repair using tissue engineering technique-novel approach with minimally invasive procedure. Artif. Organs 2004; 28: 28–32
- Abe M., Takahashi M., Tokura S., Tamura H., Nagano A. Cartilage-scaffold composites produced by bioresorbable β -chitin sponge with cultured rabbit chondrocytes. Tissue Eng 2004; 10: 585–594
- Lanza R. P., Langer R., Vacanti J. Principles of Tissue Engineering, 2nd ed. Academic Press, London 2000
- Elçin Y. M. Tissue Engineering, Stem Cells and Gene Therapies, 1st ed. AEMB Series: 534, Kluwer Academic-Plenum Publishers, New York 2003
- Elçin Y. M. Stem cells and tissue engineering. Adv. Exp. Med. Biol 2004; 553: 301–316
- Handley C. J., Winter G. M., Ilic M. Z., Ross J. M., Poole C. A., Robinson H. C. Distribution of newly synthesized aggrecan in explant cultures of bovine cartilage with retinoic acid. Matrix Biology 2002; 21: 579–592
- Knudson C. B., Knudson W. Cartilage proteoglycans. Cell Dev. Biol 2001; 12: 69–78
- Frenkel S. R., Di Cesare P. E. Scaffolds for articular cartilage repair. Ann. Biomed. Eng 2004; 32: 26–34
- Freed L. E., Vunjak-Novakovic G. Cultivation of cell-polymer tissue constructs in simulated microgravity. Biotechnol. Bioeng 1995; 46: 306–313
- Mikos A. G., Thorsen A. J., Czerwonka L. A., Bao Y., Langer R. Preparation and characterization of poly (L-lactic acid) foams. Polymer 1994; 35: 1068–1075
- Elçin Y. M., Elçin A. E., Pappas G. D. Functional and morphological characteristics of bovine adrenal chromaffin cells on macroporous poly (DL-lactide-co-glycolide) scaffolds. Tissue Eng 2003; 9(5)1047–1054
- Linn T., Erb D., Schneider D., Kidszun A., Elçin A. E., Bretzel G. B., Elçin Y. M. Polymers for induction of revascularisation in the rat fascial flap: application of vascular endothelial growth factor and pancreatic cell lines. Cell Transplant 2003; 12: 769–778
- Jakob M., Demarteau O., Schafer D., Hintermann B., Dick W., Heberer M., Martin I. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J. Cell. Biochem 2001; 81: 368–377
- Lefebvre V., Peeter-Joris C., Vaes G. Production of collagens, collagenase and collagenase inhibitor during the dedifferentiation of articular chondrocytes by serial subcultures. Biochim. Biophys. Acta 1990; 1051: 266–275
- Clark A. G., Rohrbaugh A. L., Otterness I., Kraus V. B. The effects of ascorbic acid on cartilage metabolism in Guinea pig articular cartilage explants. Matrix Biol 2002; 21: 175–184
- Rosier R. N., O'Keefe R. J., Crabb I. D., Pujas J. E. Transforming growth factor beta: an autocrine regulator of chondrocytes. Connect. Tissue Res 1989; 20: 295–301
- Aaron R. K., Wang S., McCiombor D. Upregulation of basal TGFβ 1 levels by EMF coincident with chondrogenesis-Implications for skeletal repair and tissue engineering. J. Orthop. Res 2002; 20: 233–240
- Reddi A. H. Symbiosis of biotechnology and biomaterials: Applications in tissue engineering of bone and cartilage. J. Cell. Biochem 1994; 56: 192–195
- Vunjak-Novakovic G., Obradovic B., Martin I., Bursac P. M., Langer R., Freed L. E. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol. Prog 1998; 14: 193–202
- Inanç B., Elçin A. E., Elçin Y. M. Osteogenic induction of human periodontal ligament fibroblasts under two and three-dimensional culture conditions. Tissue Eng 2006; 12(2)257–266