Abstract
Transesterification reaction was performed using sunflower oil and short-chain alcohol by immobilized lipases in organic solvents. The fatty acid ester, which is the product of this reaction, can be used as a diesel fuel that does not produce sulfur oxide and minimize the soot particulate. Immobilized porcine pancreatic lipase (PPL) and Candida rugosa lipase (CRL) showed the satisfactory activity in these reactions. Immobilization of lipases was carried out using inorganic absorbance Celit 545 particle as a carrier.
Organic solvent like hexane in reactions was required when methanol and ethanol were used as alcoholic substrate. The reaction could be performed in absence of solvent when 1-propanol and 1-butanol were used as short-chain alcohol. The activities of immobilized lipases were highly increased in comparison with free lipases because its activity sites became more effective. Immobilized enzyme could be repeatedly used without difficult method of separation and the decrease in its activity was not largely observed.
INTRODUCTION
The biodiesel fuel from vegetable oil does not produce sulfur oxide and minimize the soot particulate one-third times in comparison with the existing one from petroleum. Because of these environmental advantages, biodiesel fuel can be expected as a substitute for conventional diesel fuel. At present, biodiesel has been produced chemically using vegetable oil in Europe and the USA. However, the requirement of removal of catalyst and excessive energy requirements are the major drawbacks of such chemical process. Because enzymatic methods may conquer the problems for the reaction, research has been carried out using lipase at the same reactions Citation[1], Citation[2], Citation[3], Citation[4]. Enzymes are generally effective biocatalyst for having substrate specificity, functional group specificity and stereo specificity in aqueous media. Besides, chemical reactions can be also raised directly using lipase in an organic media. The esterification reaction of alcohol and carboxylic acid is difficult in aqueous media, whereas this reaction takes place easily in organic solvent Citation[5], Citation[6], Citation[7], Citation[8].
However, the production of biodiesel fuel by enzymatic method has not been adapted industrially, because of the high cost of enzyme catalyst. In order to use the enzyme catalyst repeatedly, the process of immobilization must be carried out using an appropriate method. In this research, the transesterification reaction for the production of biodiesel fuel was performed from sunflower oil and short-chain alcohol using immobilized lipases obtained by an effective method employing an inorganic porous particle as a carrier in non-aqueous condition.
EXPERIMENTAL
Materials
Lipases from Candida rugosa (CRL) and porcine pancreatic (PPL) were used as enzymes. These enzymes were received from Sigma Chem. Co. (St. Louis, MO). Sunflower oil (contain total saturated fatty acids % 12, oleic acid % 19, linoleic acid % 68, linolenic acid % 1) was obtained from the Olin factory (Edirne, Turkey) and was used as substrate triglyceride. Methanol, ethanol, 1-propanol and 1-butanol was purchased from E. Merck (Darmstadt, Germany) and alcohols were used as short-chain alcohols without being furthermore purified. Celite 545 was purchased from the Aldrich Chemical Co. (Milwaukee, WI). The carrier particle used for immobilization of enzymes was porous Celite 545 that was baked after granulation.
Methods
Immobilization of Enzymes
In an l00 ml beaker, 0.5 g of lipase was dissolved with 50 ml of 0.1 M phosphate buffer solution (pH 7). The aqueous solution obtained was then filtered under reduced pressure. Then, 2.5 g of Celite 545 was mixed with the enzyme filtrate in a flask and shaken continuously for 2 h by a shaker at room temperature. 20 ml of chilled hexane was mixed with the solution and shaken for 1 h. The solution was filtered under reduced pressure and washed with 20 ml chilled hexane. Consequently, the hexane-vaporized residue was obtained, which is immobilized lipase on Celite particle. The immobilized enzyme produced was perfectly dehydrated when stored under vacuum in cooled desiccators until use. The quantity of enzyme immobilized onto the carrier particle was determined by using the Lowry method Citation[9].
Enzymatic Transesterification Reaction
Sunflower oil (20 g, 22.6 mmol) and alcohol were put into an l00 ml three-necked flask fitted with cooler and thermometer. The molar ratio of sunflower oil/alcohol chosen was 1/3 (M/M). Immobilized lipase or free lipase (70 mg) that was the same amount as including enzyme was added into it. Then, it was heated in an oil bath at constant temperature with continuous stirring. The samples were taken with interval to follow reaction situation during reactions. The samples was separated thin layer chromatography as qualitative and the conversion of product was analyzed by gas chromatogram (GC-CAP-4, 6890 mod.) equipped with DB-WAX (30 m-0.32 mm-0.5 μ M) as a column, taking sample at fixed interval as quantitative. Helium was used as a carrier gas; the column temperature was set at 210°C. The injector and detector temperatures were set at 250 and 270°C, respectively. Water content in the reaction medium was analyzed by a Karl Fisher moisture titrator.
RESULTS AND DISCUSSION
Effect of Alcohols and Solvent Types
When methanol was used as a substrate, it did not dissolve well with sunflower oil. Therefore, appropriate organic solvent was necessary to carry out the transesterification reaction. Hexane, 1,4-dioxane, benzene, chloroform and tetrahydrofuran as solvent were used and immobilized CRL and PPL lipases as catalysts at 40°C for 3 hr. The enzymatic activity was the highest with hexane, as in . The activity of immobilized CRL and PPL was fairly low with 1,4 dioxane, benzene, chloroform and tetrahydrofuran. When ethanol was used, the reaction did not take place homogeneously as in methanol. For the practical application of making diesel fuel from the discarded vegetable oil, it is not desirable to use solvent. It is because, after completion of the reaction, the solvent must be removed by distillation, extraction and so on, which need additional energy and effort. The reaction, on the contrary, occurred homogeneously, when propanol or butanol was used as substrate. Therefore, organic solvent is not necessary in these reactions. The enzymatic activity increases with the high amount of hexane, which is shown in as related to the reaction period.
TABLE 1 Yields of fatty acid methyl esters according to solvent kind (reaction conditions in text)
Types of Lipase
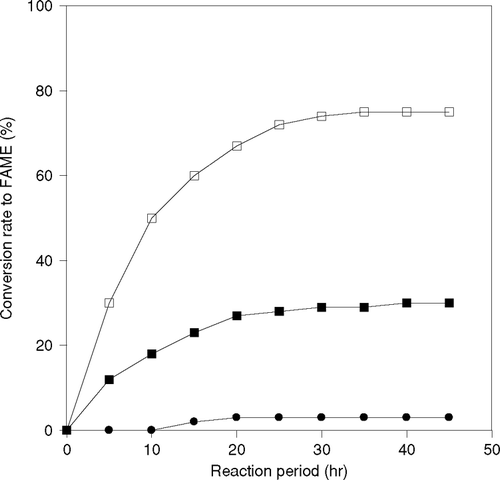
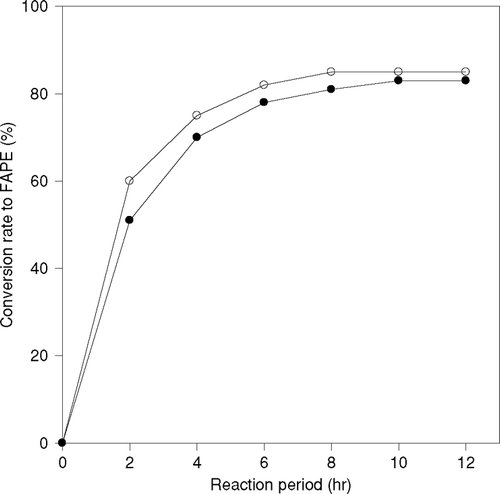
Lipases can be used as a biocatalyst in this transesterification reaction. In this study immobilized CRL and PPL enzymes were adsorbed 80% and 65% of total lipase on support, respectively. The reactions were performed using immobilized CRL and PPL with 1-propanol and 1-butanol as substrate at 50°C. Each immobilized enzyme was added in such an amount that the total amount of lipase in immobilized enzyme analyzed by Lowry method became the same. The conversion to fatty acid propyl or butyl esters is shown in and , respectively. Immobilized CRL showed higher enzymatic activity than immobilized PPL. The reaction was faster with 1-propanol than 1-butanol. The reaction did not proceed significantly in the case of immobilized PPL, as the conversion ratio to fatty acid propyl esters was only 35% and that of butyl ester was 25%, even after 15 h the activity of the immobilized lipase was decreased. Thus, immobilized CRL was selected for further study.
Figure 2 Difference of reaction behavior by the kind of immobilized lipase on production of fatty acid propyl ester (FAPE), (-▪-)PPL, (-□-) CRL.
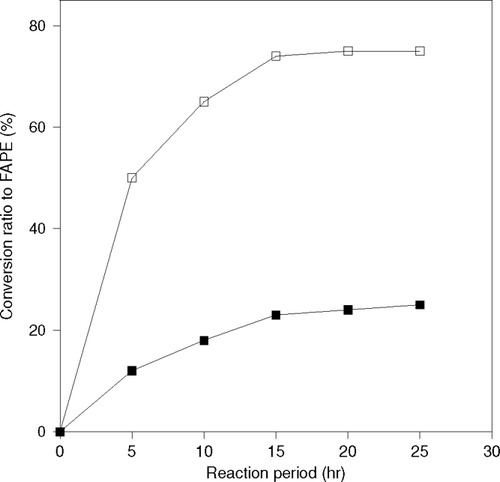
Figure 3 Difference of reaction behavior by the kind of immobilized lipase on production of fatty acid butyl ester (FABE), (-▪-)PPL, (-□-) CRL.
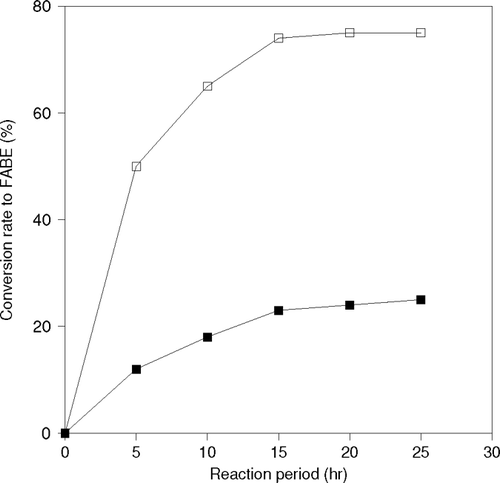
Immobilization of lipase was carried on the surface of Celite, which is a porous particle having the average particle size 0.02–0.1 mm and contains free crystalline silicic acid in our previous research, immobilized PPL using Celit was employed in transesterification reaction of synthesis of stereo specific esters and resolution of racemic alcohols Citation[10]. In our other research synthesis of optically active pentanol esters of isovaleric acid in organic solvent was also employed Citation[11], Citation[12]. It has been seen that the activity of immobilized lipase became remarkably higher than free lipase. In an organic medium, free lipase is aggregated at a considerable degree. Therefore, most of the active site of enzymes are confined inward.
It is explained that when an enzyme is immobilized, its active sites become more effective, as each and every enzymes is dispersed on the surface of the carrier particle. In this research, the transesterification reactions were carried out using free and immobilized CRL with 1-butanol and 1-propanol as alcohol. The results of reactions are shown in . In this work, the immobilized lipase was used in such an amount that the total amount of lipase in immobilized enzyme became the same as that of free lipase. When immobilized enzyme was used, presence of 1-propanol as substrate, the reaction was completed within 12 hr. However, the conversion to product was approximately 65% at 25 hr with free CRL. In the case of 1-butanol also, the rate of reaction was seen as higher with immobilized lipase than the free one.
Figure 4 Effect of immobilization of CRL on production of Fatty acid propyl ester (FAPE) and Fatty acid butyl ester (FABE).
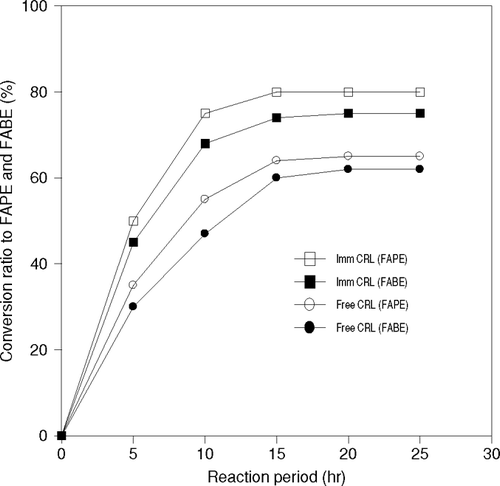
Generally, the activity of immobilized enzyme is decreased in an aqueous system, regardless of the method of immobilization. In contrast, it is noticed that the activity of an immobilized enzyme is higher than the free enzyme in this study.
Effect of Temperature
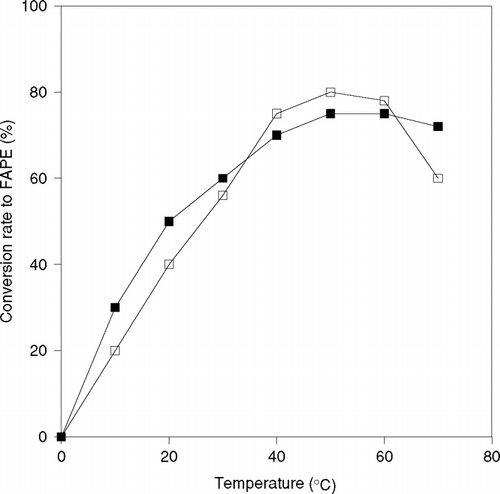
The effect of temperature on this transesterification reaction was examined at the temperature range from 20°C to 70°C with both free and immobilized lipase. The behavior of reaction with 1-propanol for 12 hr using free and immobilized CRL is shown in . The conversion ratio to FAPE was observed highest at 50°C, whereas the activity highly decreased at 70°C in case of free CRL. The rate of reaction seems to be increased at the same temperature when immobilized enzyme was used. In particular, the comparison of conversion ratio at 70°C reveals the fact that thermal stability of enzyme increases because of immobilization. The conversion ratio to FAPE was almost same at 50 and 60°C when immobilized lipase was used.
Effect of Water Content
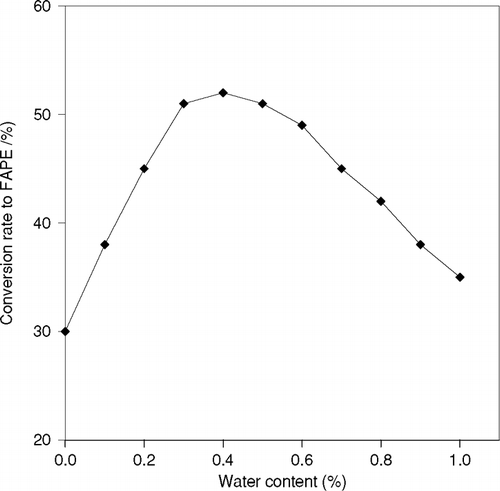
It is well known that the activity of an enzyme in non-aqueous medium is affected by water content in it. In this research, the effect of water content on enzymatic activity was examined adding a small amount of water in the reaction mixture of 1-propanol and sunflower oil as substrates and immobilized CRL. The reaction was performed taking water content ranging from 0 to l wt. % of the total amount of reaction mixture with the constant temperature of 50°C and the duration of 2 hr. Since the conversion ratio of this reaction increases linearly up to 2 hr, the conversion ratio is taken at 2 hr as the comparison of enzymatic activity. The result is shown in . It is observed that the conversion ratio was highest at 0.3 wt.% of water content. The activity of an enzyme at 0.4 wt % is about 20% higher than in absence of water. It is further realized that the enzymatic activity gradually decreased at more than 0.4 wt.% of water content.
Operational Stability of Immobilized CRL
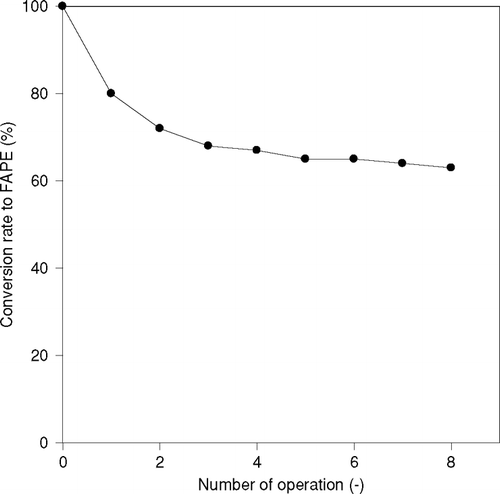
The main advantage of immobilization of an enzyme is that an expensive enzyme can be repeatedly used. It was observed how the reaction behavior changes when an immobilized enzyme is used repeatedly. The change of enzymatic activity as operational stability examined 1-propanol and sunflower oil as substrates by immobilized CRL at 50°C for 1 hr is shown in . The activity of an enzyme was decreased to about a third, when it was used for a second time but the decrease in its activities could be hardly observed in its further use. When an immobilized enzyme was used for the first time, some amount of enzymes was desorbed. The desorbtion of an enzyme could not be observed after further repeated use. Therefore, immobilized enzymes can be used time and again. Immobilized enzyme can be separated by the process of decantation, after completion of the reaction and does not require a special method of separation.
Effect of Pure Substrate as Trilinolein
In order to find the differences in the behavior of the reaction between trilinolein and sunflower oil, transesterification reaction was carried out using 1-propanol as alcohol. The enzymatic activity of immobilized CRL was examined. The results of the reaction of sunflower oil and trilinolein were comparatively studied at 50°C for 2 hour, which is shown in . In both cases, the behavior of reaction was observed to be similar. This situation is not surprising since sunflower oil has % 68 of linoleic acid in fatty acid content of total oil.
CONCLUSIONS
Transesterification reaction was carried out using triglyceride trilinolein (which is one of the main components in some vegetable oil as sunflower oil) and short-chain alcohol by some kind of immobilized lipase. In order to be able to use it repeatedly, the enzyme catalyst must be immobilized by an appropriate method, since the enzymes are generally very expensive. Immobilization of lipase was carried out using porous Celite 545 particle as a carrier. Methanol, ethanol, 1-propanol and 1-butanol were used as alcohol. Immobilized CRL lipase has higher activity than immobilized PPL in these reactions. The activity of immobilized lipase was highly increased in comparison with free lipase. When methanol and ethanol were used as alcohol, the reactions need an appropriate organic solvent like hexane. On the other hand, the reaction could be performed without solvent when 1-propanol and 1-butanol was used. The decrease in activity of the immobilized enzyme was fairly observed even it is repeatedly used. The transesterification reaction was also carried out using trilinolein. The results closely resemble with the reaction of sunflower oil. It is expected that the long-chain fatty acid ester produced by this reaction can be used as a diesel fuel that does not produce sulfur oxide and minimize the soot particulate.
REFERENCES
- Selmi B., Thomas D. Immobilized lipase catalyzed ethanolysis of sunflower oil in a solvent-free medium. JAOCS 1998; 75: 691–695
- Mittelbach M. Lipase catalyzed alcoholysis of sunflower oil. JAOCS 1990; 67: 168–170
- Shimada Y., Watanabe Y., Samukawa T., Sugihara A., Noda H., Fukuda H., Tominaga Y. Conversion of vegetable oil to biodiesel using immobilized Candida antartica lipase. JAOCS 1999; 76: 789–793
- Linko Y. Y., Lamsa M., Huntala A., Rantanen O. Lipase biocatalysis in the production of esters. JAOCS 1995; 72: 1293–1299
- De B. K., Bhattacharryya D. K., Bandhu C. Enzymatic synthesis of fatty alcohol esters by alcoholysis. JAOCS 1999; 76(4)451–453
- Watanabe Y., Shimada Y., Sugihara A., Noda H., Fukuda H., Tominaga Y. Continuous production of biodiesel fuel from vegetable oil using immobilized Candida antartica lipase. JAOCS 2000; 76: 877–892
- Basri M., Heng A. C., Razak C. A., Wan Yunus W. M. Z., Ahmad M., Rahman R. N. A., Ampon K., Saleh A. B. Alcoholysis of palm oil mid-fraction by lipase from Rhizopus rhizopodiformis. JAOCS 1997; 74: 113–116
- Iso M., Chen B., Eguchi M., Kudo T., Shrestha S. Production of biodiesel fuel from triglycerides and alcohol using immobilized lipase. Journal of Molecular Catalysis B: Enzymatic 2001; 16: 53–58
- Lowry O. H., Rosenbrough N. J., Park A. L., Randal R. J. Protein measurement with the phenol reagent. J. Biol. Chem. 1951; 193: 265–275
- Sagioglu A., Telefoncu A. Synthesis of stereo specific esters and resolution of racemic alcohols with immobilized lipases. Indian J. of Chem. 1993; 32B: 85–87
- Sagioglu A., Kiliç A., Telefoncu A. Preparation and properties of lipases immobilized on different supports. Artificial Cells, Blood Substitutes & Biotechnology 2004; 32(4)625–636
- Sagioglu A., Telefoncu A. Immobilization of lipases on different carriers and their use in synthesis of pentyl isovalerates. Preparative Biochemistry & Biotechnology 2004; 34(2)169–178