Abstract
To describe the rule of the Schwann cell proliferation after peripheral nerve injury in detail and to discover the effect of neuroanastomosis, the model of rat sciatic injury was made, with neuroanastomosis on the left side and right side untreated. Then the materials were drawn the 24th hour, 48th hour, 4th day, 7th day, 14th day, 21st day after surgery. Immunohistological staining counted the schwann cells per view with Qw in software of Leica Ltd. The number of Schwann cells increased obviously on the 4th day after surgery and reached the peak in the 7th day. Then it fell down and the neuroanastomosis group changed slower and fibroblast hyperplasia in the untreated group. The axon support is essential for the Schwann cell. The precise rule is help for study on neurotrophic factor.
After peripheral nerves injury, Wallerian degeneration happens in the distal myelins and axons. The proliferation of distal Schwann cells forms a Bunger band, which induces the axons to grow into the distal end. The nerve growth factors secreted by Schwamm cells are essential for regeneration of peripheral nerves Citation[1]. Many studies have demonstrated that the proliferation of Schwann cells is significant for the regeneration of peripheral nerves. This study concentrates on immunohistological observation of quantity and morphological variety of Schwann cells after peripheral nerves injury to describe the rule of Schwann cell proliferation and the effect of epineural suture.
MATERIALS AND METHODS
Experimental Animals and Groups
Forty-eight male adult Sprague-Dawley rats were used, weighing 150∼ 170 g, provided by Peking University People's Hospital. The rats were randomly divided into 6 groups. Each group had 8 rats. Each experimental group (8 rats) was sacrificed respectively at 24 h, 48 h, 4 d, 7 d, 14 d and 21 d postoperatively. After anesthesia by an intraperitoneal injection of ketamine 2 ml/kg, the rat legs were shaved and prepared for operation. The sciatic nerves were exposed and cut off at 5 mm distal to the site where the nerve passed through the gluteus. The left sciatic nerve was fixed to biceps femoris with 1 stitch, while the right one was sutured in normal site with 2 stitchs. The rats were fed separately postoperatively and materials were drawn respectively at the corresponding time.
Method of Drawing Materials
At 24th hour, 48th hour, 4th day, 7th day, 14th day and 21st day postoperatively, a 4 mm segment of each sciatic nerve was drawn. The midpoint of the segment was the suture site. All the materials including 6 normal sciatic nerves from SD rats used for negative control were fixed with 10% formaldehyde for 24 hours, dehydrated with density gradient alcohol, embedded with paraffin wax, and made into 3 mm paraffin section. Then deparaffinage, 3% hydrogen peroxide incubation for 10 min, addition of rabbit antimouse S100 antibody, diluted in 1:150, kept in 4□ for 1 night and then irrigated with PBS solution. Addition of caprine antirabbit second antibody, kept in ambient temperature for 30 min, was irrigated with PBS solution and coloration with DAB. Then it was irrigated with distilled water repeatedly, restained with hematoxylin, dehydrated and mounted with resin.
Image Analysis
All the immunohistological specimens were photographed in magnification of 400 times. Each nerve was photographed and analyzed in 5 different visual fields. Each visual field is 1392 × 1040 pinel and each pinel is 0.1575 um. The images were analyzed with the Software Qwin V3 (Leica Ltd. German). The Schwann cell quantity, average perimeter, area, karyoplasmic ratio, average myelin diameter and thickness were analyzed in each image.
RESULTS
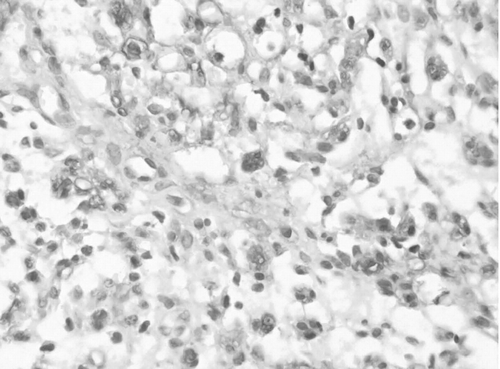
Observation in Microscope ()
The part near the nucleus of Schwann cells and myelins was stained deeply and myelins were stained lightly, while interstitial cells were not stained. The color of Schwann cells became light at the 4th day postoperatively, but S100 of Schwann cells on the suture side expressed increasingly.
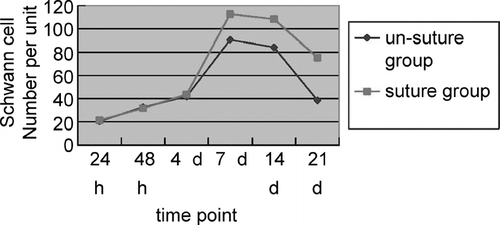
Quantity and Morphological Variety of Non-myelinated Schwann Cells (NMSC) ()
(1) Right side: In the distal end, the Schwann cell quantity increased at the 48th hour postoperatively (p < 0.05) and reached the peak value at the 7th day, while the NMSC quantity increased by 3–4 times and began to decrease at the 21st day. In the proximal end, cells and myelins were stained well; the NMSC quantity reached the peak value at the 7th day and began to decrease at the 14th day. (2) Left side: In the distal end, the Schwann cell quantity increased at the 4th day postoperatively, when S100 expressed decreasingly, and reached the peak value at the 7th day, while the NMSC quantity increased by 3–4 times and decreased obviously at the14th day. The stained cells became scarce at the 21st day and proliferation of many fibrocytes was observed. In the proximal end, the NMSC quantity reached the peak value at the 7th day and began to decrease at the 14th day.
Myelin Morphological Variety
The myelins began swelling at the 24th hour postoperatively and became the most serious at the 7th day. Increasing of nuleuses in the myelin was observed at the 14th day and the surrounding myelins were stained deeply. Swelling of myelins on the right side became light at the 21st day and neoformative myelins with little diameter could be observed, while on the left side myelins broke down and proliferation of many fibrocytes was observed at the 21st day.
DISCUSSION
In 1850, Waller reported that after peripheral nerve injury, the whole distal nerve fiber as well as partial proximal myelin and axon disintegrated, which was called Wallerian's degeneration. The proximal axon pullulated and grew into distal Schwann cells, then the regenerated axon myelinizated and connected the target organ again. Schwann cells played an important role in the process of nerve regeneration, such as phagocytosis, frame provision and secretion of NGFs. After the axon grew into the distal end, Schwann cells connected with the axon through adhesion molecules to keep nerve fiber alive and lead the axon to grow orderly. Consequently it is very important to maintain and promote Schwann cell function in the process of nerve regenenration.
This study is to describe the rule of Schwann cell proliferation after peripheral nerve injury. In the study we find that after peripheral nerve injury Schwann cell quantity in the distal end increases at 48h postoperatively and reaches the peak value at 7th day by 6 to 7 times, which mainly are NMSCs. If undergoing primary perineurium suture, NMSC quantity begins to decrease at the 14th day and becomes about 3 times normal at the 21st day. Antigenicity of Schwann cells decreases in the injury nerve without primary suture and fibrocytes proliferate at the 21st day postoperatively. Schwann cells in adult mammals can be categorized into 2 types by functions: MSCs and NMSCs. Both have specific antigen phenotypes Citation[3], Citation[4], Citation[5]. The study indicates that after peripheral nerve injury, S100 of Schwann cells expresses decreasingly, but if the injury nerve underwent primary perineurium suture, S100 expresses increasingly after Schwann cells obtain axon support again. S100 is a kind of Ca-binding proteins, which is rather conservative in mammals Citation[6]. It is related to many cell functions, such as signal transmission, cell maturation, energy metabolism, and so on. The expression change of S100 indicates the change of cell phenotype and function, for Schwann cells lose support of β -Neureguin secreted by axon after axon disintegrates in Wallerian degenenration. β -Neureguin is significant to Schwann cells. Studies in vitro indicate that exosomatic Schwann cells will necrosis without axon support.
Results manifest that the quantity of Schwann cells in the unit area of injury nerve with primary suture is significantly more than that of the injury nerve untreated in statistics. It reveals that S100 expresses decreasingly, phenotype changes and fibrocytes proliferate, because Schwann cells lose nutrient support of the axon. The interaction between Schwann cells and axon is important to the maintenance of quantity and function of Schwann cells. Primary suture of injury peripheral nerve is beneficial to nerve concrescence, avoidance of nerve fibrosis.
In conclusion, the study indicated that after peripheral nerve injury, the primary suture should be done as soon as possible to promote Schwann cell function to help nerve injury concrescence. It is beneficial to further studies on NGF adjustment and confirmation of key factors in Schwann cell proliferation to obtain the accurate rule of Schwann cell variation after peripheral nerve injury.
This paper was funded by Chinese National 973 Project (No: 2003CB515306), Chinese National 973 Project (NO: 2001CCA01300) and Peking University People's Hospital Foundation.
REFERENCES
- Dezawa M., Adachi-Usami E. Role of Schwann cells in retinal ganglion cell axon regeneration. Prog Retin Eye Res 2000; 19(2)171–204
- Feneley M. R., Fawcett J. W., Keynes R. J. The role of Schwann cells in the regeneration of peripheral nerve axons through muscle basal lamina grafts. Exp Neurol 1991; 114(3)275–285
- Hachisuka H., Mori O., Sakamoto F., Sasai Y., Nomura H. Immunohistological demonstration of S-100 protein in the cutaneous nervous system. Anat Rec 1984; 210(4)639–646
- Zhang Dianying, Baoguo Jiang, Heping Li, et al. The immunohistological observation of peripheral nerve degeneration process. Chinese Clinical Anatomical Magazine 1999; 4: 353–355
- Katsetos C. D., Karkavelas G., Frankfurter A., Vlachos I. N., Vogeley K., Schober R., Wechsler W., Urich H. The stromal Schwann cell during maturation of peripheral neuroblastomas. Immunohistochemical observations with antibodies to the neuronal class III beta-tubulin isotype (beta III) and S-100 protein. Clin Neuropathol 1994; 13(4)171–180
- Zimmer D. B., Cornwall E. H., Landar A., Song W. The S100 protein family: history, function, and expression. Brain Res Bull 1995; 37(4)417–429