Abstract
Hemoglobin-based blood substitute is widely studied. As the starting materials, hemoglobin (Hb) is mainly supplied from outdated human blood and animal sources. But there are many disadvantages. As the alternative source, human placenta hemoglobin (PHb) has its own advantages. We take the lead in being occupied in the research of blood substitutes based on the placenta blood. Purified PHb readily undergoes dissociation to form two dimers that are easily filtered by the kidneys and have high 02 affinity (low P50), failing to transport 02 to tissues. As a first step, to enhance PHb stability and P50, we first adopted DBBF to modify deoxyPHb leading to DBBF-αPHb. According to detection, we knew that the PHb stability was improved, 02 affinity of the PHb solution was reduced, DBBF-αPHb had the appropriate capacity of oxygen-carrying and oxygen-unloading, and DBBF-αPHb kept the biological activity well. On the whole DBBF-αPHb could be considered as a first step toward the synthesis of a potential blood substitute.
INTRODUCTION
Hospitals throughout the world currently rely solely on donated blood for transfusions. But the donation of blood by healthy citizens is seriously inadequate; in addition, blood transfusion has other limitations such as viral infections, difficulty of storage and transportation, and needing cross-matching because of blood group antigens Citation[1]. A more effective alternative to human blood or red blood cells is desirable.
Hemoglobin-based blood substitute is widely studied. As the starting material, Hb is mainly supplied from outdated human blood and animal sources (bovine blood, porcine blood, etc.). But there are many disadvantages Citation[2], Citation[3], Citation[4], Citation[5]: on the one hand, due to Hb from animal sources, some underlying problems must be confronted, such as antibodies causing immune system response in the recipient and animal-borne infectious diseases; on the other hand, because the supply of the donated blood is far beyond that needed, less and less outdated blood could be supplied. Human placenta blood as the alternative source has been proved to have some advantages. First, underlying problems from animal sources can be avoided. Second, in China, the number of new infants each year exceeds 20 million, so, the supply of human placenta blood is plentiful. In addition, the placenta blood with higher 02 affinity can transport more 02 to the hypoxia tissues. The Institute of Tianjin Union Biotechnology Development takes the lead in being occupied in the research of blood substitutes systematically, using placenta blood.
Hb has been evaluated as an ideal blood substitute in surgical procedures Citation[6]. The use of stroma-free Hb as a therapeutic has two main disadvantages. During the Hb extraction from the RBC, its allosteric effector(2,3-DPG) is lost; therefore, purified Hb has low P50, which indicates Hb has a far greater affinity for 02 than normal human blood and makes it an ineffective molecule for delivering 02 to tissues. The second is related to the dissociation of purified Hb tetramers into αβ dimers, which are easily filtered by the kidneys leading to hematuria. In fact, the PHb has higher 02 affinity and lower P50 Citation[7]. So a kind of appropriate intramolecular modifier is needed to reduce the 02 affinity of PHb and stabilize the structure of PHb tetramers, which is the first step toward the synthesis of a potential blood substitute. In fact, intramolecular modified Hb dispersions can display a large range of oxygen affinities, which translates into a wide range of accessible P50 values Citation[8], Citation[9], Citation[10], Citation[11].
The base research of PHb-based blood substitutes is not mature. The structures of PHb and HbA (adult hemoglobin) have some differences that mainly lie in the distinction between β chains and γ chains. In this case, DBBF as a modifier was first adopted to modify deoxyPHb through the a Chains, avoiding the problem above. The results of SDS-PAGE and HPLC were shown that the stability of PHb tetramers was improved and the capability of oxygen-unloading to the tissues was enhanced. The capability of oxygen-carrying and biological activity was tested, which reveals that the basic characters of PHb were reserved well. A further advantage of modifying is that the thermostability of the PHb can increase, making it possible to be pasteurized under deoxygenated conditions to destroy any viruses that may be present.
MATERIALS AND METHODS
Preparation of PHb
After the human placenta blood centrifuged at 2000 rpm for 10 min at 4°C, the plasma and white cells were removed; the packed red cells, washed three times with isotonic NaCl solution, were lysed with five volumes of hypotonic Na2HPO4−NaH2PO4 buffer (pH = 7.2) for 1 h. Before heading, the solution (pH was adjusted to 6.0 by ascorbic acid) was deoxygenated by exposure to vacuum and flushing with nitrogen. The solution was heated for 2 h. Stromas were eliminated by centrifugation and the supematant was filtered with 0.22Jμm membrane.
Reaction of PHb and DBBF
Under an ice bath, the PHb solution, to which Bis-tris had been added to the concentration of 5 mM and inositol hexaphosphate (IHP, pH was adjusted to 7.2 by NaOH), had been added to the concentration of 10 mM, was first deoxygenated by exposure to vacuum and flushing with nitrogen. After 1h, DBBF was added to a final molar excess 1.5–3 DBBF: PHb. Under deoxygenated condition, the reaction proceeded for 2–3 h at 37°C water bath.
Preparation of the DBBF-PHb Adduct
The deoxygenated glycin solution was then added to a final concentration of 0.2 M, to terminate the reaction. After 30 min, the reaction vessel was transferred to an ice bath.
The reaction mixture was finally dialyzed to remove these small molecular substances. All were in deoxygenated form.
Poly- (PLP-PHb) and Animal Experiments
The above purified PHb solution was modified with pyridoxal phosphate (PLP). Cross-linked Hb was prepared by the reaction between glutaraldehyde (GDA) and PHb in phosphate buffer solution (PBS), an excess amount of lysine was added to inactivate the unreacted GDA, the reacted mixture was ultrafiltrated against with PBS by system containing membrane cut off molecular weight below 100 kD to remove uncross-linked hemoglobin and redundant GDA and lysine. Finally, the filtrated solution was performed through GPC column and Poly-PHb was collected according to the molecular weight.
All animals used in the experiments were handled according to national standards. Anesthetized Sprague-Dawley rats weighing 240∼ 260g were used to evaluate the effect of poly-PHb. About 70% of total blood volume was involved in blood replacement. Blood was slowly withdrawn at the rate of 0.5 ml/min, and immediately thereafter the same volume of poly-PHb was infused at the same rate.
Analytical Methods
Spectroscopic Assay. The concentration of PHb was determined by conversion of PHb into cyanmethemoglobin and detecting the absorption at 540nm Citation[12]. The percentage of MetHb was determined by the absorption of 630 nm according to the method of Evelyn Citation[13]. The percentage of recovery was based on grams of PHb recovered, from hemolysate to final product.
SDS-Polyacrylamide Gel Electrophoresis Assay. The result of PHb by DBBF cross-linking was determined by SDS-PAGE. After electrophoresis, proteins were stained with Coomassie Brilliant Blue R-250 and analyzed by Protein Molecular Weight Marker (Fermentas Life Sci.).
HPLC Assay. The HPLC assay was also carried out for determining the percent of total cross-linked PHb and the percent of uncross-linked PHb. The analysis was conducted with an instrument equipped with Superdex™ 75 10/300 GL column (30 cm × 10 mm, Amersham Biosciences) and HPLC system (VARIAN). The mobile phase was 50 mM Tris-HCI buffer at pH 7.2 with 1 M MgC12, flow rate was 0.5 ml/min and the detection wave-length was 280 nm. The 1 M MgC12 mobile phase served to dissociate any uncross-linked tetrameric PHb into pairs of dimers, while cross-linked PHb remained intact. Hence, uncross-linked PHb and intramolecularly cross-linked PHb tetramers elute separately. The data was analyzed by Star Chromatography Workstation (VARIAN).
All-wave Scan. The method was to determine the biological activity. All-wave scan was conducted using CARY50 DV-Visible spectrophotometer (VARIAN). The absorption spectrums of purified PHb before and after modified by DBBF were recorded at 200∼ 800 nm.
Oxygen Equilibrium Measurements. Oxygen equilibrium curves of DBBF-αHb solutions were measured .The oxygen pressure was determined by a Stat PHOX blood gas analyzer Hemox analyzer and oxygen saturation was determined by the oxygen saturation assay Citation[14]. The temperature was maintained at 37°C, Hb solution was adjusted to 7%, and the pH was adjusted to 7.4.
RESULTS AND DISCUSSIONS
Preparation of PHb
The method of purification of PHb was based on the Institute of Tianjin Union Biotechnology Development. Purity of purified PHb was above 99%. MetHb formation was less than 1%. According to per gram PHb, purified PHb could carry 02, which nearly has the same capacity of carrying 02 compared with whole blood. So, purified PHb with well characters was gained.
Reaction of PHb and DBBF
The tetramer of the Hb from animal sources and outdated adult blood is composed of two αβ dimers. The PHb, which has some differences in the structure, is composed of mostly the two αγ dimers and partly the two α β dimers. The intramolecular modifiers extensively used are mostly PLP and αβFPLP. They are adopted to modify the Hb ∼ chains (cleft 2,3-DPG) to reduce the 02 affinity of Hb. So, if they are adopted to cross-link the PHb γ chains, there is the problem of whether the result of modification could reach the desirable degree similar to cross-link the HbA ∼ chains or not. But DBBF cross-linking PHb through the α chains could avoid it.
It is noted that DBBF not only cross deoxyHb through a chains, leading to DBBF-αHb; DBBF could also cross oxyHb through ∼ chains, leading to DBBF-β Hb. But, DBBF-∼ Hb has more 02 affinity than purified Hb, so it is not suitable for blood substitute Citation[14].
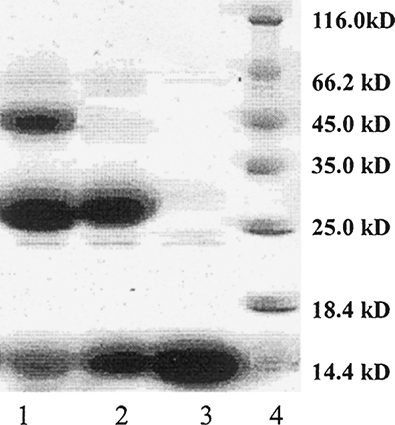
As shown in , under completely deoxygenated conditions, there were two obvious bands, composed of 1,6000, 3,2000, while if not completely deoxygenated conditions there was another obvious band which indicate that therewas not only a chains cross-linked. So we could infer that under completely deoxygenated conditions, DBBF reacts with a high degree of selectivity to modify PHb through the a chains, while, if not well deoxygenated, DBBF could also cross the two ∼ or γ chains. So, in order to avoid DBBF crossing the cleft region, deoxygenated conditions must be strict. Inositol hexaphosphate (IHP) added were used for the same purpose. The method of the reaction above effectively controls the reaction occurring in the cleft 2,3-DPG.
Stability and Degree of Polymerization Assay
Hb, although normally a tetramer, readily undergoes dissociation to form two dimers. The dimers, having a molecular weight of only 32,000, are readily filtered from the circulation by the kidneys.
It is shown from that there was only one obvious band of 16,000 for purified PHb while there was another band of 32,000 for DBBF-αPHb, which indicated that a chains had been effectively cross-linked and revealed that DBBF-αPHb could not completely be broken into monomers like purified PHb because of a chains of PHb cross-linked covalently. In other words, DBBF-αPHb had more stability.
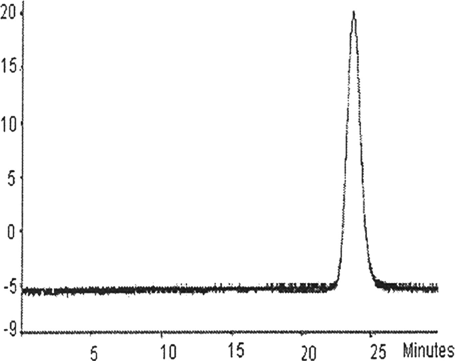
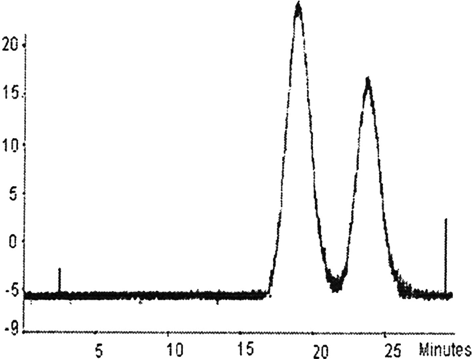
As shown in , the buffer system of HPLC, including 1mol/L MgC12, could completely dissociate unmodified tetrameric hemoglobin to form hemoglobin dimmers. So, from HPLC, degree of polymerization was accurately detected. As is shown in , first reference to about 60% PHb was effectively cross-linked. Therefore, DBBF cross-linked PHb intramolecularly to effectively prevent the process of dissociation from occurring.
Biological Activity
All-wave Scan Assay
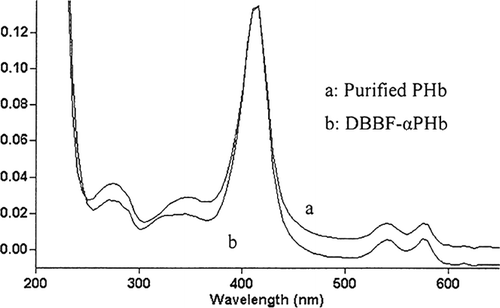
The method to test the changes of PHb before and after modification was all-wave scan. The maximum absorption of DBBF-αPHb was at 415 nrn which was the same as purified PHb (). The absorption spectrums of PHb were virtually unchanged before and after modification. So, we could infer that modification procedure did not have an adverse influence on the structure of PHb that was the premises of biological activity. Additionally, after modification, the PHb solution showed absorptions in 540nm and 576 nm, which were the symbols of carrying 02 Citation[15], therefore, the 02 carrying capacity of DBBF-αPHb might be reserved.
Oxygen Equilibrium Measurements
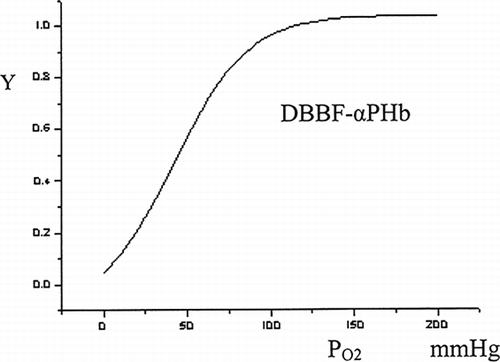
Purified PHb have the capacity of good oxygen-carrying, but have higher 02 affinity (lower P50) than the whole blood, making it an ineffective molecule for delivering 02 to tissues. The intermolecularly polymerized Hb solutions have higher 02 affinity than purified Hb. So, 02 affinity must be reduced by intramolecularly modification. From the oxygen equilibrium curves of DBBF-αHb (), the P50 of the PHb solution through DBBF cross-linking α chains could be advanced above 40 mmHg, which exceeds the whole blood. The capacity of oxygen off-loading can be improved, which is important for oxygen carriers.
As stated above, the intramolecular cross-linkers extensively used are mostly PLP, NFPLP and DBBF. Both PLP and NFPLP can cross-link Hb through ∼ chains. Most of the modifiers can increase the P50 to decrease the 02 affinity of the Hb solution, but there are other differences in biological activity characters. For example, the Hill coefficient of NFPLP-Hb is about 1.9 Citation[16], which indicates the biological cooperation has a small decrease, and the Hill coefficient of DBBF-Hb is between 2.9 and 3.1 Citation[17], keeping good biological cooperation. We believe that there is a correlation between biological activity of modified Hb and the Hb site modified (cleft 2,3-DPG or not). In addition, DBBF modified PHb in the deoxy-form, which was also another advantage to DBBF-αPHb keeping good biological activity. According to the literature Citation[18], the high spin Fe(II) electronic structure and stereochemistry was not obvious influenced by Hb modification in the deoxy-form. This is in agreement with the result that DBBF-αPHb keeps good biological activity.
Poly-(PLP-PHb) and Animal Experiments
In this study, we knew poly-PHb and poly-AHb had no obvious distinction. Poly-PHb had the capacity of oxygen-carrying and oxygen-unloading well, but we found another disadvantageous phenomenon that the rats had hematuria. Although the reacted mixture was ultrafiltrated with PBS by a system containing the membrane cut-off molecular weight below 100 kD to remove hemoglobin with low weight; the hemoglobin solution lacked stability when it was injected into the body. In fact, PLP, which has only one group, CHO, could replace 2,3-DPG to lower oxygen affinity, but it could not improve the stability of the tetramer of the PHb. Hence the dimers without cross-linking were easily broken down in the circulation, though the dimers were not easily broken down in the hemoglobin solution. So, before ultrafiltration, some researchers used some solution to completely dissociate the hemoglobin solution; for example, the MgC12 solution with a high concentration. Using this to deal with Poly-(PLP-PHb), we found the effective part gained was very low. We used DBBF, which has two groups CHO, and DBBF can intramolecularly cross-link the PHb. As stated above, the stability improved and it would provide an advantage to gain the more effective part. Therefore, DBBF could replace PLP to modify PHb.
CONCLUSION
As stated above, human placenta blood as the alternative source has some advantages. The research of blood substitutes based on human placenta hemoglobin is of importance to developing a potential blood substitute with good performance.
DBBF, as one of the modifiers extensively adopted, was first adopted to cross-link deoxyPHb leading to DBBF-αPHb. The influence of deoxygenation is vital to the process of DBBF cross-linking αPHb. According to detection, we knew that the PHb stability was improved, 02 affinity of the PHb solution was reduced, DBBF-αPHb had the appropriate capacity of oxygen-carrying and oxygen-unloading and DBBF-αPHb keep good biological activity.
On the whole, DBBF modifying human placenta hemoglobin though α chains could be considered as a first step toward the synthesis of a potential blood substitute with good performance.
REFERENCES
- Schreiber G. B., Busch M. P., Kleinman S. H., Korelitz J. J. The risk of transfusion-transmitted viral infections. New Eng. J. Med. 1996; 334(26)1685–1690
- Chang T. M. S. Semi-permeable microcapsules. Science 1964; 146: 524–525
- Chang T. M. S. Blood Substitutes: Principle, Methods, Products and Clinical Trails. Karger Publisher, Basel 1997; 1–141
- Chang T. M. S. Red blood cell substitutes. Best Pract. Res. Clin. Haemat. 2000; 13: 651–668
- Winslow R. M. Blood substitutes. Advanced Drug Delivery Reviews 2000; 40: 131–142
- Chang T. M. S. Future prospects for artificial blood. Trends Biotechnol. 1999; 17: 61–67
- Oshtrakh M. I., Semionkin V. A. Study of normal human fetal and adult hemoglobins by Mössbauer spectroscopy. Mol Bioi (Moscow) 1985; 19: 1310
- Winslow R M. Current status of blood substitute research: towards a new paradigm. J. Intern. Med. 2003; 253: 508–517
- Bakker J. C., Berbers G. A. M., Bleeker W. K., Denboer P. J., Biessels P. T. M. Preparation and characterization of cross-linked and polymerized hemoglobin-solutions. Biomater. Artif. Cells. Immobilization Biotechnol. 1992; 20: 233–241
- Lieberthal W., Fuhro R., Purifieddman J. E., Toolan G., et al. O-raffinose cross-linking markedly reduces systemic and renal vasoconstrictor effects of unmodified human hemoglobin. J. Pharmacol. Exp. Ther. 1999; 288: 1278–1287
- Carmichael F. J. L., Ali A. C. Y., Campbell J. A., et al. A phase I study of oxidized raffinose cross-linked human hemoglobin. Crit. Care Med. 2000; 28: 2283–2292
- Drabkin D. The standardization ofhemoglobin measurement. Am. J. Med. Sci. 1949; 217: 710–711
- Evelyn K. A., Malloy H. T. Microdetermination of oxyhemoglobin, methemoglobin, and sulfhemoglobin in a single sample of blood. J. Bio/. Chem. 1938; 126: 655–662
- Zhu Z. Y., Chen Z. H., et al. Clinic Medical Detection. Shanghai Scientific & Technical Publishers, Shanghai 1978; 381–384
- Wang H. Y., Zheng W. J., Bu F. R., et al. Researches on the manufacture of blood substitute's stroma polymerized bovine hemoglobin. Chin. Pharm. J. 2001; 36(6)416–419
- Benesch R., Benesch R. E., Yung S., Edalji R. Biochem. Biophys. Res. Commun. 1915; 63: 1123–1129
- Walder R. Y., Andracki M. E., Walder J. A. Preparation of intramolecularly cross-linked hemoglobins. Methods in Enzymology. 1996; 231: 270–280
- Oshtrakh M. I., Milder O. B., Semionkin V. A., et al. Characterization of the heme iron in pyridoxylated hemoglobin cross-linked by glutaraldehyde using Mossbauer spectroscopy. International Journal of Biological Macromolecules 2000; 28: 51–58