Abstract
A water-in-oil-in-oil double-emulsion solvent/evaporation method was used to prepare vincristine sulfate (VCR) loaded poly(lactide-co-glycolide) microspheres, and then VCR microspheres were mixed with collagen and (or) chitosan swelling solution and lyophilized to form polymeric films. The films were cross-linked by 0.3% glutaraldehyde (GA). Encapsulation efficiency and release kinetics of VCR microspheres were determined, as well as release kinetics and in vitro degradation of the film. The rate of VCR release from the film submerged in PBS (pH 6.8) and the content were measured by high-performance liquid chromatography (HPLC). The physichemical properties of the film, such as surface morphology, mechanical function, and differential scanning calorimetry, were also measured. VCR was released from the film in a prolonged period and the initial burst release of the film was less significant. In the degradation experiment, the film containing chitosan degraded more slowly than that without chitosan. The films comprising collagen and chitosan could achieve the release kinectics of a relatively constant release. It has a promising future.
INTRODUCTION
Cancer chemotherapy is not always effective. The main problems may be in drug delivery to the tumor, drug toxicity to normal tissues, and drug stability in the body. The treatment of malignant tumor with chemotherapeutic drugs requires special consideration due to the location of the neoplasm. Interstitial chemotherapy could minimize the systemic toxicity and achieve the goal of target therapy Citation[1]. Vincristine is a naturally occurring alkaloid. Vincristine sulphate (VCR) is the 1:1 sulphate salt of the alkaloid extracted from Catharanthus roseus. VCR has been used extensively for treatment of various cancers including AIDS-KS Citation[2], Citation[3], Citation[4]. In vivo data has indicated that the longer exposure of VCR above a critical threshold concentration induced more profound cytotoxicity. Hence, controlled release formulations of VCR appears to be a generally attractive goal to pursue Citation[5].
It has been reported that collagen and chitosan both are potentially useful pharmaceutical materials owing to their good biocompatibility and low toxicity. Therefore, they have many applications in the formulations employed in drug delivery Citation[6], Citation[7]. However, the untreated collagen scaffold showed the fast biodegrading rate and the low mechanical strength, which limit its further use. Lie Ma has confirmed that cross-linking of the collagen by GA and addition of chitosan are effective methods to modify the biodegrading rate and to optimize the mechanical property Citation[8].
Antitumor activity can often be enhanced by improvements in the method of drug administration, particularly the methods that focus therapy on the tumor site. Implant film provides an opportunity to deliver high, localized doses of chemotherapy for a prolonged period after tumor resection. In all of these studies, interstitial administration of antitumor agents by film resulted in higher drug activity in the tumors, when compared to more conventional delivery strategies Citation[9]. In this report, the production and characterization of implant films made of collagen, chitosan and PLGA microspheres for the delivery of VCR are described. Sustained release of VCR could decrease the initial burst release and prolong the release period. After removal of the bulk tumor's mass, this implant film will be placed in the resection cavity. Here we report the preparation and characterization of the complex film.
MATERIALS AND METHODS
Materials
Vincristine sulfate (purity > 98%) was purchased from Shanghai Biopharmaceutical. PLGA (MW50,000–800,000, 50:50) was purchased from Sigma. Chitosan (MW200,000; degree of deacetylation is 85%) was purchased from Qingdao Institute of Meteria Medica. Collagen type I was harvested from cattle tendon by using pepsin. All other solvents and chemicals were of analytical grade or HPLC grade and obtained from commercial sources.
Preparation of Microspheres and Film
A water-in-oil-in-oil double-emulsion/solvent evaporation method was used to prepare VCR-loaded PLGA microspheres. Briefly, 500 μ l of solution containing 10 mg VCR was added to 4 ml of dichloromethane/ acetonitrile (1:1 v/v) containing 200 mg PLGA. The mixture was homogenized (F6/10, Fluka, German) at 1,0000 rpm for 1 min to make W/O emulsion. The primary emulsion was then poured into 200 ml liquid paraffin containing 2% Span 80 under stirring, after evaporation. Then, the microspheres were collected by filtration through 0.8 μ m nylon filter paper after evaporation.
PLGA microspheres were mixed with collagen and/or chitosan swelling solution, and lyophilized. The films were cross-linked by 0.3% GA, and lyophilized again. The dried films were then cut into slices (10 mm × 10 mm). The film thickness was adjusted to 1.5 mm, stored in a dry place and kept away from light prior to use Citation[10], Citation[11], Citation[12].
Morphology of Microspheres and Film
Surface morphology of microsphere and film were observed by scanning electron microscopy (PHILIPS XL-30 ESEM, Netherlands). The sample was placed on a brass stub and coated with gold under vacuum. Freeze-dried microspheres were redispersed in distilled water and sized by laser diffractometry by using a Multisizer S (Malvern Instruments, UK).
Determination of Drug Content
Briefly, 10 mg microspheres were dissolved in 1 ml dichloromethane and VCR was extracted using 5 ml of phosphate buffer and methanol. The recovery rate was determined from a control experiment with a mixture of microspheres and a known amount of VCR.
Mechanical Properties and Differential Scanning Calorimeter of the Film
The films were then cut into strips (50.0 mm × 1.0 mm). Determination of the specific surface area of the film and mechanical function of the film were characterized with a tensile testing machine (the clamp interval is l40 mm; stretch rate is 30 mm/min). Tensile strength and percentage of breaking elongation of the film were recorded.
Thermal characterization of film was performed with a 2910 DSC (TA, SWISS). Samples were weighed (3.00 ± 0.50) mg and placed in sealed aluminum pans. The equipment was calibrated with indium. The samples were scanned at 10°C/min from 25 to 300°C. All the determination was performed in triplicate.
In Vitro Degradation and Release of the Film
The degradation characteristics of the film in vitro were evaluated by measuring weight loss. For this purpose, preweighed films were placed in 8 ml phosphate-buffered saline (PBS, 50 mM, pH 6.8) containing 220 U/ml collagenase type I. The temperature of the incubator was maintained at (37 ± 1)°C with continuous agitation of (50 ± 2) rpm. Samples were recovered periodically, lyophilized and then weighed to determine the weight loss.
The microspheres and the film were incubated in 2 ml release medium (PBS) containing 40 U/ml collagenase type I. At each sampling time, the medium was withdrawn after centrifugation at 4,000 rpm for 5 min, and replaced with fresh PBS. The removed medium was analyzed for amount of drug released by HPLC.
Determination of VCR Content by HPLC
VCR content was determined using a UV detector (Gilson, France). The wavelength was set to 296 nm. A YMC C18 4.6 × 15.0 cm column fitted with C 18 pre-column was used for UV detection. The mobile phase consisted of 10 mM sodium phosphate buffer pH 7.0 and methanol (40:60, v:v) with column temperatures set at 40°C. The retention time for VCR detected was (11.1 ± 0.1) min. A calibration curve was constructed to determine VCR concentration in the range from 1.0 to 128 μ g/mL, and the r2 value was at least 0.9999.
Statistical Analysis
Results are reported as mean ± standard deviation. All data were analyzed using ANOVA. A P value of less than 0.5 was considered as evidence of a significant difference.
RESULTS
Characteristics of Microspheres and Film
shows the scanning electron micrographs of the microspheres and the film. The microspheres fabricated with PLGA had smooth surface; no pores or asperities could be found. The encapsulation efficiency of microspheres was (79.0 ± 1.0)%. The mean diameter was (27.97 ± 0.90) μ m. The film is milk white, and has equal thickness. During the release period, the chitosan-free collagen film was loosened to suppleness after 2 weeks. The SEM images show that the porosity of the chitosan-free collagen film became larger, and the microspheres were fewer. The integrated microspheres and porous microspheres were distributed on the surface of chitosan-collagen film or embedded in it after 2 weeks during the release periods. The porosity of the chitosan-collagen film did not increasing significantly (Chitosan 3:2; D2.D1 degradation 2W; E. microspheres).
Mechanical Properties and Thermal Characteristics of the Film
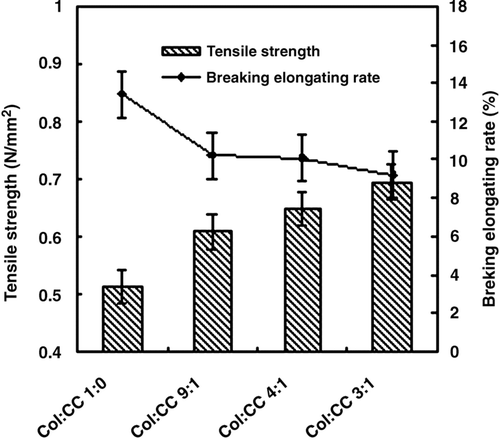
As shown in , the tensile strength of the film was strengthened and the breaking elongating rate of the film decreased with more proportion of chitosan.
DSC thermogram of the collagen film showed some peaks after 2 weeks during the release period; the denaturalization temperature peak of the collagen film is left shift, and the melting range became wider. It suggested that there are more degradation products than ever. This change was not obvious on the thermogram of the collagen-chitosan complex film.
In Vitro Release and Degradation of the Film
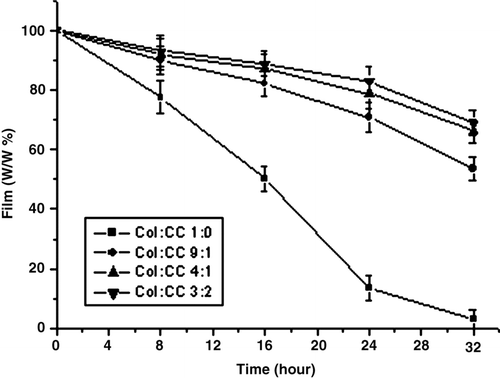
The degradation mechanism of the film in vitro was clarified by mass loss measurements. shows the relationship of the mass of the film with the time. After being immerged in PBS solution (containing 200 U/mg collagenase), the morphology of the film changed disintegrity. At 32 hours, the chitosan-free collagen film was severely degraded into small fragments. But as for the films of collagen-chitosan (9:1, 4:1, 3:2, w/w), the mass loss were (41 ± 2.0)%, (28 ± 1.9)% and (26 ± 2.2)%, respectively. The degradation curve slope reflects the degradation rate. These figures clearly indicate that the degradation rates of the collagen-chitosan film were slower than that of the collagen film.
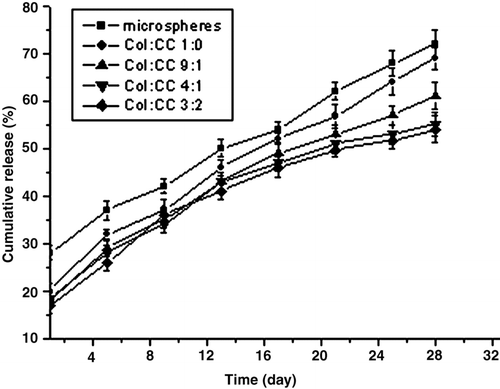
The releases in vitro of VCR from microspheres and film are shown in . VCR was released from the film in a prolonged period and the initial burst release of the film was less significant than the microspheres. The initial release of microspheres was (27.3 ± 1.2)%, but as for the films of collagen and collagen-chitosan (9:1, 4:1, 3:2, w/w), it was (20.4 ± 1.9)%, (20.2 ± 2.1)%, (18.0 ± 1.1)%, (16.3 ± 1.8)%, respectively. The chitosan-free collagen film is loosened and porous after 2 weeks during the release period. The microspheres fell off from the film and were suspended in the medium.
DISCUSSION
The natural polymer of collagen has low antigenicity and is biocompatible, biodegradable, and less toxic than synthesized polymers. Chitosan is a copolymer of glucosamine and acetylglucosamine derived from the natural polymer chitin, which is commercially available. It has been reported that collagen and chitosan are potentially useful pharmaceutical materials owing to their good biocompatibility and low toxicity Citation[13]. Glutaraldehyde crosslinking of collagen and/or chitosan significantly reduce the antigenicity and biodegradation Citation[14].
This implant film can be placed in the tumor resection cavity. To mimic the acidic environment around the tumor in vivo, we select PBS (pH 6.8), containing collagenase type I, as the in vitro release medium. Because of the intermolecular electrostatic attraction, collagen forms the polyelectrolytic coordination compound Citation[7]. Chitosan molecule contains hydroxyl and amino groups; collagen contains carbonyl and amino groups. Therefore, the complex film contains electrostatic attraction and hydrogen bond. Collagen was used as the pharmaceutical material, but its application was limited due to its inherent poor mechanical stability and rapid biodegradation. After addition of chitosan, the resistance of collagenase against degradation was obviously augmented Citation[8]. The complex film can prohibit the collagenase from recognizing the collagen, leading to the decreasing of its degradation rate Citation[14].
The degradation mechanism of the chitosan is complicated. It can be hydrolyzed by lysozyme, free radical, and so on Citation[15]. Some factors can inhibit its degradation. Collagenase type I is a specific enzyme to the degradation of the collagen. The degradation rate of collagen is faster than that of chitosan in vivo. In this study collagenase type I was only selected in the degradation experiment. It should be noted, however, that correlating the rate of drug release measured in general assays with the rate of drug release into tissues is a difficult problem in the development of new drug delivery systems. And there are still many steps and details in the technology of preparing the film, which could affect the biodegradation of microspheres and film and release of drugs.
The film showed a biphasic release patterns, with an initial burst followed by a slower release phase. After an initial burst, a continuous VCR release was observed for 4 weeks. The generally rapid rate of release of VCR was due to both a short diffusion path in microspheres and a much larger surface area for diffusion/dissolution. Since some microspheres are completely located on the surface of the film, a burst was observed in the release curve. As expected, increasing chitosan content caused an impressive decrease in release rate and released amount. This was due to the fact that complex film with increasing chitosan content decreases film degradation rate Citation[14], Citation[15], Citation[16], Citation[17]. The remaining drug was released due to the degradation of the matrix. Morphological studies using SEM demonstrated that the film did not degrade at the same rate.
As reported previously, drug release from biodegradable matrices can occur by one of the following mechanisms or their combination: diffusion controlled, osmotic controlled, and degradation controlled release mechanisms Citation[7]. In this implantable film, the degradation rate of complexed matrix can significantly control the drug release. The drug is released by dissolution from the polymer (PLGA and collagen/chitosan), and then diffusion through the wall. Since the degradation rate of the collagen-chitosan film decreases with the proportion of chitosan increase, we can conclude that the drug release rate from the collagen is faster than that of the collagen-chitosan film.
Incorporation of chitosan into collagen film improved its mechanical property and reduced the biodegradation rate against collagenase. With more proportion of chitosan, the tensile strength of the film was strengthened, and the breaking elongating rate of the film decreased. In the last case (collagen/chitosan, 3:2), the break is brittle, in relation with the glassy behavior of chitosan at room temperature. As for the mechanical properties, the collagen/ chitisan (4:1 w/w) complex film is a proper proportion.
CONCLUSION
In the study, a novel implant film prepared may have application for site-specific chenmotherapy for a variety of malignancies. Additionally, the degradation rate of the collagen-chitosan film was significantly slower than the collagen film. The film (collagen:chitosan 4:1) is proper. These results indicated that collagen-chitosan film may be used as a long-acting implantable drug-delivery-carrier.
This work was supported by the National Research and Development Project of High Technology “863” Project (No. 2002AA326040) and the Doctoral Fund of Ministry of Education of China (No. 20060023050).
REFERENCES
- Vogelhuber W., Spruß T., Bernhardt G., Buschauer A., Göpferich A. Efficacy of BCNU and paclitaxel loaded subcutaneous implants in the interstitial chemotherapy of U-87 MG human glioblastoma xenografts. International Journal of Pharmaceutics 2002; 238: 111–121
- Lobert S., Vulevic B., Correin J. Interaction of vinca alkaloids with tubulin: a comparison of vinblastine, vincristine, and vinoelbine. Biochemistry 1996; 35: 6806–6811
- Conter V., Valsecchi M. G., Silvestri D., Campbell M., Magyarosy E. D. E., Gadner H., Stary J., Benoit Y., Zimmermann M., Reiter A., Riehm H., Masera G., Schrappe M. Pulses of vincristine and dexamethasone in addition to intensive chemotherapy for children with intermediate-risk acute lymphoblastic leukaemia: a multicentre randomised trial. The Lancet 2007; 369: 123–131
- Gidding C. E. M., Kellie S. J., Kamps W. A., de Graaf S. S. N. Vincristine revisited [J]. Critical Reviews in Oncology:Hematology 1999; 29: 267–287
- Lynch J. L., Wade C. L., Zhong C. M., Mikusa J. P., Honore P. Attenuation of mechanical allodynia by clinically utilized drugs in a rat chemotherapy-induced neuropathic pain model. Pain 2004; 110: 56–63
- Tang Z.-X., Shi L.-E., Qian J.-Q. Neutral lipase from aqueous solutions on chitosan nano-particles. Biochemical Engineering Journal 2007; 34: 217–223
- Maeda H., Sano A., Fujioka K. Controlled release of rhBMP-2 from collagen minipellet and the relationship between release profile and ectopic bone formation. International Journal of Pharmaceutics 2004; 275: 109–122
- Ma L., Gao C., Mao Z., Zhou J., Shen J., Hu X., Han C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2004; 24: 4833–4841
- Liu D.-Z., Sheu M.-T., Chen C.-H., Yang Y.-R., Ho H.-O. Release characteristics of lidocaine from local implant of polyanionic and polycationic hydrogels. Journal of Controlled Release 2007; 118: 333–339
- Arpornmaeklong P., Pripatnanont P., Suwatwirote N. Properties of chitosan–collagen sponges and osteogenic differentiation of rat-bone-marrow stromal cells. International Journal of Oral and Maxillofacial Surgery 2008; 37: 357–366
- Sun Y., Xu L., Zhang Q., et al. The controlled release study of vincristine sulfate. Artificial Cells, Blood Substitutes, and Biotechnology 2003; 31: 81–88
- Sun Y., Zhou H., Zhang Q., et al. Improved controlled release study of lomustine. Artificial Cells, Blood Substitutes, and Biotechnology 2000; 28: 173–180
- Thomas C., Sharma P. Biomater Artif Cells Artif Org 1990; 18: 1–24
- Jameela S. R., Misra A., Jayakrishnan A. Crosslinked chitosan microspheres as carriers for prolonged delivery of macromolecular drugs. J Biomater Sci Polym Edn 1994; 6: 621–632
- Yan J., Li X., Liu L., Wang F., Zhu T., Zhang Q. Potential use of collagen-chitosan-hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Artificial Cells, Blood Substitutes, and Biotechnology 2006; 34: 27–39
- Zhang Y., Cheng X., Wang J., Wang Y., Shi B., Huang C., Yang X., Liu T. Novel chitosan/collagen scaffold containing transforming growth factor-β 1 DNA for periodontal tissue engineering. Biochemical and Biophysical Research Communications 2006; 344: 362–369
- Sionkowska A., Wisniewski M., Skopinska J., Kennedy C. J., Wess T. J. Molecular interactions in collagen and chitosan blends. Biomaterials 2004; 25: 795–801