Abstract
Hepatic hollow fiber bioreactors are a promising class of bioartificial liver assist device (BLAD). The development of this type of device is currently hindered by limited oxygen transport to cultured hepatocytes, due to the low solubility of oxygen in aqueous media. In order to increase the oxygen spectrum to cultured hepatocytes housed within a hollow fiber bioreactor, several different engineering strategies were explored in this study. These included: supplementing the circulating media stream of the hollow fiber bioreactor with a hemoglobin-based oxygen carrier (bovine red blood cells) with defined oxygen binding and release kinetics and operating the bioreactor with media flow through the hollow fiber membrane into the extracapillary space (ECS). We hypothesize that these two strategies can be used to improve hepatocyte oxygenation and possibly attain an in vivo-like pO2 spectrum, similar to that observed in vivo in the liver sinusoid. This work is significant, since provision of an in vivo-like pO2 spectrum should create a fully functional BLAD that could potentially bridge thousands of liver failure patients towards native liver regeneration of damaged tissue or, if necessary, orthotopic liver transplantation.
INTRODUCTION
Lack of proper oxygenation to hepatocytes cultured within a hollow fiber bioreactor remains an important problem in the development of a viable BLAD. The oxygen demand of hepatocytes have not been completely fulfilled within the experimental systems utilized in our previous work Citation[1], Citation[2], Citation[3], Citation[4]. Traditionally, hepatic hollow fiber bioreactors were operated with the ECS port valves closed Citation[5], thus restricting media flow primarily to the lumen. This resulted in the development of insignificantly small Starling flows through the hollow fiber membrane and ECS, where diffusion is the dominant mechanism of mass transport to cultured hepatocytes Citation[6]. In this study, oxygen transport to hepatocytes was increased by allowing a fraction of the inlet media stream to permeate through the hollow fiber membrane, thereby flowing through the ECS, and exiting via the two ECS ports (permeate flow). We hypothesize that inclusion of convective transport to hepatocyte cultures will increase oxygen transport, leading to the retention of differentiated functionality. Additionally, a bRBC supplemented hollow fiber bioreactor system was operated and compared with the control and convection enhanced systems.
In the experimental study described in this paper, all three experimental hollow fiber bioreactor systems were concurrently operated. Currently, most hepatic hollow fiber bioreactor systems operate with a stagnant cell space (closed ECS ports) to reduce shear forces on cultured cells to a minimum Citation[7]. The first experimental system described in this paper was operated in this manner as the control system. Also, an experimental hollow fiber bioreactor system, with closed ECS ports and an oxygen carrier (bRBCs) supplemented to the circulating media feed stream, was operated. This second experimental system was operated similarly to previously published bRBC supplemented systems Citation[1], Citation[8]. Finally, a third experimental hollow fiber bioreactor system was operated with the ECS port valves open to allow a fraction of the total inlet media flow to permeate through the hollow fiber membrane and flow through the ECS. This last experimentally operated system was an attempt to increase oxygen transport to the cultured hepatocytes via convection enhanced mass transfer. For all three hollow fiber bioreactor systems, metabolic, synthetic, and detoxification markers were tracked throughout the entire experiment and inline dissolved oxygen concentration measurements were taken at select time points. This study examines oxygen transport to hepatocytes housed within a hollow fiber bioreactor via HBOC supplementation, convection through the ECS, and compares these two different methods of enhancing oxygen transport.
MATERIALS AND METHODS
Hollow Fiber Bioreactor System
Three experimental hollow fiber bioreactor systems were inoculated and maintained in a similar manner as previously described cultures Citation[2]. Briefly, approximately three million C3A hepatoma cells were inoculated into the ECS of each hollow fiber bioreactor system (400-012, Spectrum Laboratories, Rancho Dominguez, CA). All three experimental bioreactors were cultured concurrently within a Heraeus incubator (Kendro Laboratory Products, Hanau, Germany) at 37°C and 5% CO2. For the first week of cell culture, after inoculation, the ECS port valves remained closed in all three systems and no HBOC was supplemented to the media. Thus, all three hollow fiber bioreactor systems were operated initially in the same manner as the control system. This allowed time for the hepatocytes to acclimate to the bioreactor environment, adhere to the outside of the hollow fibers, and proliferate until the cultures achieved confluency. After a week of control system operation, bRBCs (as the HBOC) were added to the media bottle of the bRBC supplemented system and the ECS port valves were opened in the enhanced convection system. Cell filters were placed after the ECS port valves in the convection system to maintain a separate and self-contained cell space, keeping dislodged hepatocytes from being flushed out of the hollow fiber bioreactor ECS and into the media bottle. A schematic of the hollow fiber bioreactor system is shown in .
Figure 1 Schematic of the convection enhanced hollow fiber bioreactor system utilized in the experimental study.
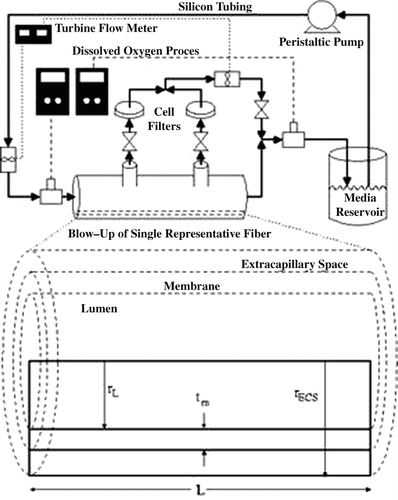
shows the setup of the experimental hollow fiber bioreactor system, where complete media (with or without bRBCs) is pumped from the media reservoir (containing ∼ 250 ml of media), through a length of silicone tubing (∼ 2 m), to facilitate gas transfer with the incubator environment. Circulating media flows through a turbine flow meter, dissolved oxygen probe, and into the hollow fiber bioreactor. Within the bioreactor, the media flows through hollow fibers, exchanging nutrients and waste with the cultured hepatocytes, and exits the bioreactor. In the convection enhanced system, some of the media flows through the membrane, leaks out the ECS ports, through another inline turbine flow meter and recombines with the bioreactor exit flow, . Finally, the complete exiting media flows through another dissolved oxygen probe and returns to the media reservoir bottle.
The internal dimensions of the hollow fiber bioreactors utilized in these experimental trails have been measured, calculated, or are provided by the manufacturer. The single representative fiber presented in has a hollow fiber length (L) of ∼ 10 cm, inner radius (rL) of 165 μ m, and average membrane thickness (tm) of 50 μ m. From the calculated number of fibers and total ECS volume, the radius of the representative ECS (rECS) was calculated to be 278 μ m. This indicates that only approximately three layers of hepatocytes, with an average diameter of 20–25 μ m Citation[9], would be present around each fiber in the representative ECS. The tight hollow fiber spacing of this particular hollow fiber module is a major reason why it was selected for this study. In vivo hepatocyte plates within the liver sinusoids are only one or two cell layers thick Citation[10].
To prevent sedimentation of bRBCs in the media reservoir within the bRBC system, a vortexer (Fischer Scientific, Silver Spring, MD) was set to shake the media bottle hourly in the incubator. Additionally, the hollow fiber bioreactor systems were equipped with inline turbine flow meters and inline dissolved oxygen concentration probes only when measurements were to be taken. Flow rates and dissolved oxygen concentrations were measured at select time points, and then the measurement instruments were removed from the bioreactor system. In the convection system, when the ECS port valves were opened, the total flow rate entering the hollow fiber bioreactor and permeate flow rate exiting via the ECS (as seen in ) were recorded simultaneously, allowing fractional permeate flow through the ECS to be calculated.
Cell Line and Cell Culture Media
The hepatocytes cultured in this study were comprised of the C3A hepatoma cell line (CRL-10741, ATCC, Manassas, VA), an immortalized human hybridoma derived from HepG2 cells. C3A cells were chosen for this study, since they exhibit most functional characteristics of human hepatocytes and maintain a rapid preconfluent doubling time (∼ 1 day) with contact inhibition Citation[11], Citation[12]. Additionally, primary hepatocytes did not maintain high enough viability or cell density throughout our previous experimental studies Citation[8], and this issue can be corrected with the use of an immortalized cell line, such as the C3A hepatocyte.
The circulating culture media utilized in all hollow fiber bioreactor systems within this study was formulated similarly as described previous in the literature Citation[1]. Briefly, complete media was composed of 90% Dulbecco's Modified Eagle's medium, 10% fetal bovine serum, and 200 U/ml of Penicillin and 0.2 mg/ml of Streptomycin (Sigma, St Louis, MO). For the case of the bRBC supplemented system, sterile bRBCs suspended in 0.85% saline solution (Quad 5, Ryegate, MT) were washed twice with complete media and added to the media reservoir bottle at ∼ 10% of the human in vivo RBC concentration. Fresh media make-up and daily media changes were performed at the same time every morning for all three experimental systems. Daily media samples, taken directly from changed media bottles, were immediately frozen and stored for analysis. In the case of the bRBC supplemented system, media samples were spun down (4°C and 4000 rpm for 15 minutes) and only the supernatant was utilized to measure concentrations of analytes. Separating the bRBCs from the media in the bRBC supplemented system's daily sample also gave an indication of bRBC concentration via fractional volume, supporting the measured bRBC counts.
Media Flow Rate and Hollow Fiber Membrane Permeability
Determining specific parameters and dimensions for the experimental setup was very important in calculating oxygen consumption by hepatocytes. Flow rates within the experimental systems were measured via inline turbine flow meters (DFS-2W, DigiFlow Systems, Lucas, OH) connected to a KrosFlo® pressure and flow meter (Spectrum Laboratories). In addition to flow rates through closed ECS cartridges, the flow rates with the ECS ports open were also measured. Inline turbine flow meters were placed at the inlet of the hollow fiber bioreactor (to measure total flow) and where the ECS ports were combined (to measure permeate flow), as seen in . Flow rates for closed and open ECS port systems were taken several times for each system, from which averaged flow rates and fractional permeate flow rates were calculated.
Inline Dissolved Oxygen Probes
Increasing oxygen transport to hepatocytes cultured within hollow fiber bioreactors is a primary aim of this study. In order to get quantitative measurements of the oxygen consumed by hepatocytes, inline dissolved oxygen probes (Lazar Research Labs, Los Angeles, CA) were inserted into the hollow fiber bioreactor systems, as previously described Citation[1], Citation[8]. Briefly, inline dissolved oxygen concentration probes were calibrated by a two point calibration method, and then placed into the system immediately before and after the hollow fiber bioreactor. For the convection system, the outlet oxygen probe was placed after the permeate and outlet flows recombined, as shown in . Once the oxygen probes were set in place and dissolved oxygen readings reached steady state (∼ 1 hour), data points were recorded for various inlet and exit pO2 values. This procedure was repeated at several flow rates for each experimental hollow fiber bioreactor system.
Cell Density
After the experimental study was concluded, the hollow fiber bioreactors (lumen and ECS) were filled with AccuMax (Innovative Cell Technologies, San Diego, CA) to loosen adherent hepatocytes, and dissociate them into a single cell suspension. Accumax is a replacement for trypsin (which had proved ineffective in previous trials) that has protease, collegenolytic, and DNase activity. Cell counts of the removed ECS media were performed with a hemacytometer, and viability determined via the Trypan Blue exclusion method. Initial cell counts were lower than expected, so a second soak with Accumax solution was performed. After a day in the AccuMax solution, the remaining hepatocytes were removed, and another set of cell counts performed. These, and further cell counts, indicated significant proliferation of the hepatocytes within the ECS of all systems and a reasonably high viability, which supported the initial selection of C3A hepatocytes for the experimental trial.
Key Hepatocyte Functional Markers
Maintaining hepatocyte functionality within a BLAD is vital for its clinical success. Therefore, four different hepatocyte functional markers were tracked to examine the impact of increased oxygenation on the function of hepatic hollow fiber bioreactors. Hepatocytes are multifunctional cells, hence tracking metabolic (glucose consumption and lactate production), synthetic (albumin production), and detoxification functions (ammonia removal) are standard metrics of proper differentiated function. The metabolic, synthetic, and detoxification profiles of hepatocytes within each of the experimental bioreactors were determined via assaying daily media samples for all three experimental systems.
As mentioned in the literature, glucose is the primary metabolite of C3A hepatocytes Citation[13]. Previous studies indicated that bRBCs consume insignificant amounts of glucose compared to hepatocyte glucose consumption levels Citation[14], and therefore this small amount of glucose consumption was not considered. Glucose concentration in daily media samples was quantified via a colorimetric assay (Quantichrom DIGL-200, BioAssay Systems, Hayward, CA). A small aliquot from a daily media sample was diluted 50:50 with water, 5 μ l of the diluted sample was added to 500 μ l of reagent, mixed, and placed in a boiling water bath for 8 minutes. Then, samples were placed in a cool water bath for 4 minutes, 200 μ l of room temperature sample was placed into a 96-well plate in duplicate, and the optical density was read at 630 nm on a plate reader (Bio-tek, Winooski, VT). The absorbance was converted to concentration via a calibration curve created from a set of standard glucose solutions (0–300 mg/dl) for each set of samples.
Glucose is primarily metabolized by hepatocytes via the citric acid (Kreb's) cycle, where it can be fully broken down via glycolysis into two pyruvate molecules, and then into carbon dioxide and water via the oxidative phosphorylation pathway. However, in low oxygen environments, pyruvate can be converted into lactate, which is a less oxygen intensive (and lower energy) dead-end reaction product Citation[10]. Therefore, increased lactate production is a marker of hypoxia within the hepatocyte culture. Lactate concentration levels alone do not provide enough information about cellular oxygenation; instead a ratio of lactate production to glucose consumption is a better measure of cellular metabolism.
The lactate concentration within the daily media samples was determined via a lactate assay kit (A-108S, Biomedical Research Service Center, University of Buffalo, NY). A small aliquot from a daily media sample was diluted 50-fold with water, 20 μ l of the diluted sample was combined with 50 μ l of lactate assay solution, mixed, and incubated in a humidified 37°C environment for an hour. After incubation, the reaction was quenched by the addition of 50 μ l of 3% (0.5 M) acetic acid and the optical density was read at 492 nm on a plate reader. The measured absorbance was converted into lactate concentration via a standard lactate concentration curve (0–250 μ M) freshly generated for each set of samples. The ratio of glucose consumed to lactate produced is a standard metric of indirect hepatocyte oxygenation Citation[14], Citation[15]. Under hypoxic conditions, a single mole of glucose is broken down into two moles of lactate; a lower ratio of lactate production to glucose consumption indicates better oxygenation of the hepatocyte culture.
Synthesis of albumin is a standard marker of cell health and functionality within a hepatocyte culture, and is an important function for hepatocytes to maintain in vitro. C3A hepatocytes are a favored cell line for BLAD usage due to their maintenance of albumin synthesis Citation[16]. Albumin is particularly important due to its role in regulation and transport activities within the liver. Additionally, previous studies have indicated that albumin synthesis is an indicator of hepatocyte cell culture confluency/maturation Citation[12]. Production of albumin by hepatocytes is a highly oxygen intensive synthetic pathway, where 75 moles of glucose and 450 moles of oxygen are consumed for each mole of albumin synthesized Citation[17]. Albumin concentration of daily media samples was quantified via the BCG albumin assay kit (DIAG-250, BioAssay Systems). Five μ l of an undiluted media sample was added to 200 μ l of working albumin reagent in a 96-well plate. The plate was tapped lightly to mix, incubated for five minutes at room temperature, and the optical density was read at 620 nm on a plate reader. The measured absorbance was converted to albumin concentration via an eight point calibration curve (0–5 mg/dl) consisting of bovine serum albumin.
Retaining detoxification capability is another necessary function of a viable BLAD. In vivo, ammonia removal is primarily facilitated by the urea cycle, which only occurs in the liver Citation[17], and this is one of the only ways the body can get rid of ammonia. Build-up of ammonia within the blood can lead to, among other things, hepatic encephalopathy, a serious brain condition that can cause a decreased level of consciousness, including coma and, ultimately, death Citation[18]. Additionally, retention of a functioning urea cycle indicates a healthy and viable culture. The concentration of ammonia within the daily media samples was determined via an ammonia assay kit (200-02, Diagnostic Chemicals Ltd, Oxford, CT). A small aliquot from a daily media sample was diluted 50:50 with water, 20 μ l of diluted sample was added to 300 μ l of ammonia reagent, mixed, and incubated for one minute at room temperature. The optical density was read at 340 nm in a plate reader. Then, 2 μ l of glutamate dehydrogenase (GLDH) was added into each well and incubated for six minutes, and the optical density at 340 nm was read again. The measured absorbance, with the initial absorbance and provided standard absorbance, allowed calculation of the ammonia concentration (0.17–0.80 μ g/ml) within the media sample. It is important to note that the maintenance of all these important functions is necessary for the proper function of a BLAD.
RESULTS AND DISCUSSION
The three different experimental hollow fiber bioreactor systems housing C3A hepatocytes were maintained for a period of over three weeks in this experimental study. Samples taken during the daily media changes were frozen, and these were later thawed and assayed to track the metabolic, synthetic, and detoxification functions of the hepatocyte cultures. Additionally, dissolved oxygen measurements were recorded at several flow rates for all three of the experimental hollow fiber bioreactor systems. The dissolved oxygen measurements yield a quantitative measure of oxygen utilization by the hepatocytes, and the functional markers provide information on the retention of hepatocyte differentiated function.
Cell density within the three hollow fiber bioreactors was determined via cell count and viability analysis, as previously described. After the experiment was terminated the bioreactors were determined to house 0.8–1.9 × 109 hepatocytes per bioreactor ECS. This indicated approximately six doublings of the initially inoculated hepatocytes. Since hepatoma cell line populations have been shown to double in 20 ± 3 hours during the early phase of cell growth Citation[13], we can assume that the cartridges reached confluency after the initial week of bioreactor operation.
The total flow rate for the hollow fiber bioreactor system was measured and ranged between 5–23 ml/min for all pump settings utilized in these experimental trials. The fractional permeate flow rate in the convection system was determined to be ∼ 10% of the measured inlet flow rate. Previous values found in the literature have measured fractional permeate flows ranging from 20% to 30% in similar convection systems Citation[19], Citation[20], which indirectly indicated that high cell density obstructed fluid flow through the hollow fiber membrane. These previous studies were conducted on primary hepatocytes, which do not proliferate, usually leading to a reduced cell density within the ECS of the hollow fiber bioreactor. Therefore, the lower values for fractional permeate flow determined for this experiment, compared to the literature, was expected.
Oxygen Consumption Rate
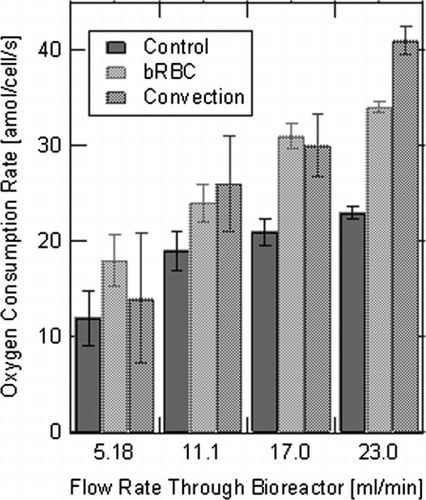
Oxygen provision within the hollow fiber bioreactor and hepatocyte health was measured via several different methods in this experimental study. During the first week of hepatocyte culture, all three systems operated like the control system until confluency was achieved. After a second week of operation, the effects of the experimental operating conditions (HBOC supplementation and enhanced convection) were assumed to be appreciable within the hollow fiber bioreactor systems. Only then were the inline dissolved oxygen probes inserted into each of the experimental systems, as described above. Dissolved oxygen measurements were taken on four separate days for each of the experimental hollow fiber bioreactor systems. From the recorded inlet and outlet dissolved oxygen concentration measurements, the total change in oxygen concentration across the bioreactors was calculated. Utilizing the difference in oxygen concentrations, along with the flow rates, volume of the ECS, and hepatocyte volume fraction, the oxygen consumption rate (OCR) for the bioreactor was calculated via a simple mass balance on the oxygen across the bioreactor, OCR = (CO2,in−CO2,out) Q/V, where CO2 is the total concentration of oxygen (dissolved and bound to the oxygen carrier, Q is the flow rate through the bioreactor, and V is the volume of the bioreactor's ECS.
presents measured OCRs for all three experimental bioreactor systems at four different measured flow rates. The overall media flow rates through the hollow fiber bioreactor systems utilized to measure oxygen consumption were: Q1 = 5.18, Q2 = 11.1, Q3 = 17.0 and Q4 = 23.0 ml/min. In , the control case exhibited the lowest measured oxygen consumption rate, and the bRBC supplemented and convection cases improved oxygen transport to the cultured hepatocytes for all measured flow rates. This effect is even more pronounced at higher flow rates for each bioreactor system. The increase in oxygen transport is significantly greater at the two highest flow rates (Q3 and Q4) and the convection enhanced system had significantly more oxygen transport compared to the bRBC supplemented system at the highest flow rate (Q4). The range of values in the experimental measurements reported in compare well to previously reported literature values Citation[13], Citation[16]. As expected, increasing the media flow rate through the bioreactor increases the OCR for all of the experimental hollow fiber bioreactor systems. Additionally, comparing the bRBC supplemented system to the convection system; it was observed that the measured OCR for the bRBC system is higher compared to the convection system at lower flow rates (Q1 − 3). At the highest flow rate (Q4), the convection system was found to transport more oxygen to hepatocytes within the hollow fiber bioreactor system, . This is hypothesized to happen, because at higher overall flow rates, there is proportionally more permeate flow through the ECS and the effect of convective mass transport becomes more pronounced.
Key Hepatocyte Functional Markers
After the conclusion of the experiment, media samples collected during daily media changes were assayed for key metabolic, synthetic, and detoxification functions. These key functional markers offer more evidence on oxygen availability to the hepatocyte cultures, providing an indication of cell health and proper functionality. The first assays to be performed were the metabolic assays, which measured the production of lactate and the consumption of glucose. As previously discussed, glucose is the primary metabolite in C3A hepatocyte culture, and lactate is generally produced in anaerobic environments. Therefore, the ratio of lactate produced to glucose consumed was calculated to indirectly demonstrate improvement of bioreactor oxygenation.
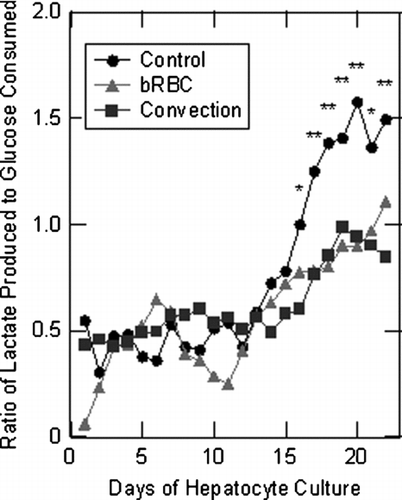
As discussed above, the ratio of lactate production to glucose consumption gives an indication of how well the citric acid cycle is performing Citation[14]. Under well oxygenated conditions glucose can be converted into water and carbon dioxide; however, under hypoxic conditions glucose dead ends within the citric acid cycle producing two molecules of lactic acid. An average of the ratio of lactate produced to glucose consumed is presented as . It is observed that during the beginning of the experiment, the ratios are similar for all systems and that in the latter days of hepatocyte culture, the control case has the highest ratio (indicating lower oxygen provision). The similarity in the average ratios for the first week is expected since all three bioreactors were operated in the same manner (ECS port valves closed and no oxygen carrier present). Next, a lag period was observed for the bioreactor systems during part of the second week, while the hepatocyte cultures adjusted to altered bioreactor operating conditions. During the second week of culture, average ratios begin to diverge: control system ∼ 0.96, bRBC supplemented system ∼ 0.72, and convection system ∼ 0.67 moles of lactate produced to moles of glucose consumed. For the final week of the experiment, diverging ratios were calculated: 1.5, 1.1, and 0.84 for the three systems, respectively. The overall lactate production to glucose consumption ratios for the entire experiment were 0.87 ± 0.6 for the control case, 0.67 ± 0.4 for the bRBC supplemented case, and 0.65 ± 0.3 for the convection case. In , both bRBC supplemented and convection enhanced systems exhibited significantly smaller overall ratios compared to the control, indicating that both the bRBC supplemented and convection enhanced systems were performing better than the control system. Additionally, the ratios seem to indicate that the convection enhanced system is slightly better oxygenated than the bRBC supplemented system, . However, no significant difference between the bRBC supplemented system and the convection system was found for these data.
Figure 3 Averaged values of the ratio of lactate produced to glucose consumed for each of the three experimental hollow fiber bioreactor systems over the course of the entire experiment. Significant differences between the control case compared to the bRBC supplemented and convection enhanced cases are indicted by asterisks over the time point *(p < 0.025) and ** (p < 0.005).
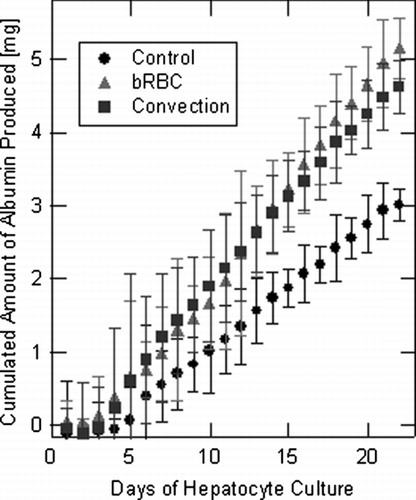
The synthesis of albumin is a highly oxygen intensive process, making it another metric of increased ECS oxygenation. Additionally, albumin synthesis is an important function to maintain in designing a BLAD and an indicator of hepatocyte culture confluency/maturation. In previous studies, hollow fiber membranes with a smaller pore size were investigated, and the ECS media had to be flushed for albumin concentrations to be assayed Citation[14]. In this study, albumin was able to permeate through the membrane, escaping the ECS, and daily media change samples were able to be assayed, in triplicate, to determine albumin concentrations. The accumulated albumin production for all three experimental hollow fiber bioreactor systems is presented in . An initial lag phase albumin production was easily observed during the first week of this experiment, . This was an indication that the hepatocyte cultures achieved confluency, as was previously reported Citation[14]. Mature hepatocytes in this experiment exhibited an average albumin production rate in each system of 7.6, 12.3, and 27.8 μ g/day/106 cells for the control, bRBC supplemented, and convection enhanced systems, respectively. These reported albumin synthesis values compare well to previously published literature values ranging from 5–30 μ g/day/106 cells Citation[21], Citation[22]. More albumin molecules are produced per cell in the convection enhanced system; however, the total albumin production displayed in was utilized as the best measure of total BLAD function. After the first week of culture, the bRBC supplemented and the convection enhanced systems produced significantly more albumin compared to the control system, as shown in . Increased albumin production is primarily attributed to improved oxygen transport as well as higher energy availability from more efficient glucose usage that was observed in the previous section. Again, albumin synthesis data presented in does not show significant differences between the bRBC supplemented case and convection enhanced case for any of the data points throughout the experiment.
Figure 4 Averaged values of accumulated albumin produced by each of the three experimental hollow fiber bioreactor systems over the course of the entire experiment. Error bars were determined from multiple assays of each sample.
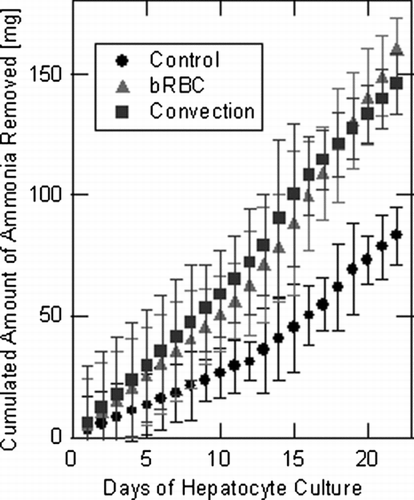
As previously mentioned, proper functioning of the urea cycle (chemical conversion of ammonia into urea) is an important detoxification function to recapitulate within a BLAD. Therefore, ammonia removal from the circulating media stream for all three hollow fiber bioreactor systems was monitored throughout the experimental study. Ammonia levels within daily media samples were monitored via colorimetric assay, in triplicate, where initial ammonia concentrations were subtracted from final ammonia concentrations to calculate daily ammonia removal. presents the accumulated ammonia removal from each of the experimental hollow fiber bioreactor systems. After the first week of bioreactor operation, bRBC supplemented and convection enhanced systems displayed higher ammonia removal rates, and thus were functioning better than the control case, . Increased functionality over the control case is an important measure of BLAD efficacy. On a per cell basis the control system was found to remove from 0.51–1.8 μ g/(hr· 106 cells) with an average of 0.88 μ g/(hr· 106 cells), the bRBC supplemented system removed 1.1–2.6 μ g/(hr· 106 cells) with an average of 1.6 μ g/(hr· 106 cells), and the convection enhanced system removed 1.6–3.0 μ g/(hr· 106 cells) of ammonia with an average removal of 1.8 μ g/(hr· 106 cells). The per cell ammonia removal rates compare well with previously reported values ranging from 0.68–2.2 μ g/(hr· 106 cells) for similar BLAD systems Citation[23], Citation[24], Citation[25]. Additionally, the percentage of total ammonia removal was calculated for all daily media samples. On average, the control system removed 21% of ammonia available within the circulating media, the bRBC supplemented system removed 41%, and the convection system removed 37%. These values are all in relative agreement with previously reported values of percent ammonia removal by hepatoma cells Citation[23]. The removal of ammonia is statistically different between the bRBC supplemented and convection enhanced cases versus the control case, after the first week of the experimental hepatocyte culture, . Again, no significant difference between the bRBC supplemented and convection enhanced case was determined for this detoxification study.
Figure 5 Averaged values of total ammonia removal in each of the three experimental hollow fiber bioreactor systems over the course of the entire experiment. Error bars were determined from multiple assays of each sample.
Assay results for the final days of steady state hepatocyte culture provide some of the most informative data. Therefore, a table was made that reported the metabolic, synthetic, and detoxification activities in the final few days of culture of the three experimental hepatic hollow fiber bioreactor systems, . For the glucose and lactate data, the final four days of operation were averaged to yield the presented data in . The glucose production at the conclusion of the experimental study compares well to previously published glucose consumption rates within a hollow fiber bioreactor, reported as ranging between 0.04–0.8 μ g/(106 cell· hr) by Nyberg et al. Citation[26]. The calculated rate of lactate production by hepatocytes also indicated good agreement between literature values and the reported data Citation[27], Citation[28].
Table 1 Key metabolic markers of metabolism, synthesis, and detoxification, for the last few days of the experimental study. All units are in μ g/(106 cell · hr), except the unitless lactate to glucose ratio
CONCLUSIONS
Oxygen provision is limiting in hepatic hollow fiber bioreactors, as has been previously reported in the literature and demonstrated in the experimental studies described in this paper. In order to remedy this problem, two strategies for increasing oxygen delivery to hepatocytes were investigated and compared. First, supplementation of bRBCs to the circulating media feed stream can increase the oxygen carrying capacity of the media, allowing more oxygen to be available to cultured hepatocytes. This has been demonstrated in our previous work Citation[1], Citation[2], Citation[14]. Second, an altered flow path strategy to increase oxygen transport was explored. This approach introduced permeate flow through the ECS to allow for enhanced convective oxygen mass transport to cultured hepatocytes Citation[19], Citation[20]. In this study, convection and oxygen carrier supplementation were shown to be able to increase oxygen transport to hepatocytes cultured within a hollow fiber bioreactor, compared to a control system with no oxygen carrier supplementation or convective flow through the ECS. Additionally, both oxygenation strategies increased key metabolic, synthetic, and detoxification functions of cultured hepatocytes, compared to the control case. For the fractional permeate flow rate and oxygen carrier (bRBC) concentration utilized in the two altered experimental systems within this study, no significant differences were observed. The implementation of both oxygen carrier supplementation and a convection flow path should further increase oxygen availability to hepatocyte cultures.
REFERENCES
- Sullivan J. P., Gordon J. E., Palmer A. F. Simulation of oxygen carrier mediated oxygen transport to C3A hepatoma cells housed within a hollow fiber bioreactor. Biotechnol Bioeng 2006; 93: 306–317
- Sullivan J. P., Palmer A. F. Targeted oxygen delivery within hepatic hollow fiber bioreactors via supplementation of hemoglobin-based oxygen carriers. Biotechnol Prog 2006; 22: 1374–1387
- Hay P. D., Veitch A. R., Smith M. D., Cousins R. B., Gaylor J. D. S. Oxygen transfer in a diffusion-limited hollow fiber bioartificial liver. Artif Organs 2000; 24: 278–288
- Willaert R., Smets A., De Vuyst L. Mass transfer limitations in diffusion-limited isotropic hollow fiber bioreactors. Biotechnol Techs 1999; 13: 317–323, (1999)
- Patzer J. F. Advances in bioartificial liver assist devices. Bioartificial Organs III: Tissue Sourcing, Immunoisolation and Clinical Trials. New York Academy of Sciences, New York, NY 2001; vol 944: 320–333
- Piret J. M., Cooney C. L. Model of oxygen-transport limitations in hollow fiber bioreactors. Biotechnol Bioeng 1991; 37: 80–92
- Allen J. W., Bhatia S. N. Impr ving the next generation of bioartificial liver devices. Sem Cell & Develop Bio 2002; 13: 447–454
- Sullivan J. P., Gordon J. E., Bou-Akl T., Matthew H. W. T., Palmer A. F. Enhanced oxygen delivery to primary rat hepatocytes within a hollow fiber bioreactor facilitated via hemoglobin-based oxygen carriers. Artif Cell Blood Substit Immobil Biotechnol 2007; 35: 585–606
- Katayama S., Tateno C., Asahara T., Yoshizato K. Size-dependent in-vivo growth potential of adult rat hepatocytes. Amer Jour Path 2001; 158: 97–105
- Van De Graff M. K. Human Anatomy. McGraw-Hill, New York 1998; vol 10
- Hui T., Rozga J., Demetriou A. Bioartificial liver support. J Hepatobiliary Pancreat Surg 2001; 8: 1–15
- Kelly J. H.. Permanent human hepatocyte cell line and its use in a liver assist device (LAD). United States Patent No. 5, 290, 684, 1994
- Nyberg S. L., Remmel R. P., Mann H. J., Peshwa M. V., Hu W. S., Cerra F. B. Primary hepatocytes outperform Hep G2 cells as the source of biotransformation functions in a bioartificial liver. Annals Surg 1994; 220: 59–67
- Gordon J. E., Palmer A. F. Impact of increased oxygen delivery via bovine red blood cell supplementation of culturing media on select metabolic and synthetic functions of C3A hepatocytes maintained within a hollow fiber bioreactor. Artif Cell Blood Subst Immobil Biotechnol 2005; 33: 297–306
- Samsel R. W., Cherqui D., Pietrabissa A., Sanders W. M., Roncella M., Emond J. C., Schumacker P. T. Hepatic oxygen and lactate extraction during stagnant hypoxia. J Appl Physiol 1991; 70: 186–193
- Patzer J. F. Oxygen consumption in a hollow fiber bioartificial liver-revisited. Artif Organs 2004; 28: 83–98
- Arias I. M. The Liver Biology and Pathobiology. Raven Press, New York 1994
- Walker C. O., Schenker S. Pathogenesis of hepatic encephalopathy with special reference to role of ammonia. Amer Jour Clin Nutr 1970; 23: 619–632
- Hay P. D., Veitch A. R., Gaylor J. D. S. Oxygen transfer in a convection-enhanced hollow-fiber bioartificial liver. Artif Organs 2001; 25: 119–130
- Giorgio T., Moscioni A., Rozga J., Demetriou A. Mass transfer in a hollow fiber device used as a bioartificial liver. ASAIO Journal 1993; 39: 886–892
- Khalil M., Shariat-Panahi A., Tootle R., Ryder T., McCloskey P., Roberts E., Hodgson H., Selden C. Human hepatocyte cell lines proliferating as cohesive spheroid colonies in alginate markedly upregulate both synthetic and detoxificatory liver function. J Hepatol 2001; 34: 68–77
- Yu S. H., Buchholz R., Kim S. K. Encapsulation of rat hepatocyte spheroids for the development of artificial liver. Biotechnol Techs 1999; 13: 609–614
- Choi Y. S., Lee D. Y., Kim I. Y., Kang S., Ahn K., Kim H. J., Jeong Y. H., Chun G. T., Park J. H., Kim I. H. Ammonia removal using hepatoma cells in mammalian cell cultures. Biotechnol Prog 2000; 16: 760–768
- Funatsu K., Ijima H., Nakazawa K., Yamashita Y., Shimada M., Sugimachi K. Hybrid artificial liver using hepatocyte organoid culture. Artif Organs 2001; 25: 194–200
- Takai M., Fukuda N., Yoshida T. Comparison of different hepatocyte cell lines for use in a hybrid artificial liver model. Cytotechnology 1997; 24: 39–45
- Nyberg S. L., Shatford R. A., Peshwa M. V., White J. G., Cerra F. B., Hu W. S. Evaluation of a hepatocyte-entrapment hollow fiber bioreactor: A potential bioartificial liver. Biotechnol Bioeng 1993; 41: 194–203
- Phillips J. W., Clark D. G., Henly D. C., Berry M. N. The contribution of glucose cycling to the maintenance of steady-state levels of lactate by hepatocytes during glycolysis and glucogenesis. FEBS Journal (Euro Jour Biochem) 1995; 227: 352–358
- Katz J., Wals P. A., Golden S., Rognstad R. Recycling of glucose by rat hepatocytes. FEBS Journal (Euro Jour Biochem) 1975; 60: 91–101