Abstract
In addition to transfusion alternatives, artificial oxygen carriers are a benefit in ischemia disorders. This study aimed at evaluating the possible effects of PEG-conjugated hemoglobin (PEG-Hb) plus cisplatin on tumor hypoxia and neovasculature. Methods: HeLa cells were injected into submucosa of golden hamster cheek pouch to build tumor model. Animals were randomly assigned to 4 groups (n=10) and treated respectively: group 1, saline; group 2, cisplatin (5mg/kg); group 3, cisplatin (5mg/kg) plus PEG-Hb (0.3g/kg); group 4, cisplatin (5mg/kg) plus PEG-Hb (0.6g/kg). Tumor neovascularization morphological variation and tissue hypoxia were detected by intravital microscopy and immunostaining, respectively. Results: Microvessel tortuosity and area capillary density in peritumoral areas were notably depressed in group 4 compared with group 2 (p<0.05). Hypoxia markers pimonidazole and HIF-1α expression were decreased significantly in group 4. Conclusion: PEG-Hb in high concentration can notably improve tumor tissue oxygenation and normalize neovasculature; it may be a potential adjuvant to chemotherapy in cancer.
INTRODUCTION
It is now accepted that intratumoral hypoixa affects every major aspect of cancer biology. Severe hypoxia is correlating with progression, angiogenesis, invation, metastasis, and therapy resistance, etc. Citation[12]. Thus, tumor hypoxia might represent a novel potential target for therapeutic intervention in solid tumors Citation[9].
A variety of experimental and clinical studies have demonstrated that the structural and functional abnormalities of the vascular microenvironment such as tortuosity, dilation, sacculation and permeability are the special characteristics of cancer newly formed vessels Citation[1], Citation[2], Citation[6]. The microvascular network appears highly chaotic Citation[22]; accordingly the irregular and less effective mircovessels result in a small amount of anticancer drugs reaching the mass and aggravation of tissue hypoxia. These changes make the situation more complex. Microcirculation detection is a valuable parameter in the assessment of the anticancer agent's action on tumor vessels Citation[16]. Previously we have utilized a non-invasive computer-assisted intravital microscopy system to observe and quantitatively analyze the vascular microenvironment repeatedly, which enabled us to evaluate the influence of the drug on tumor microcirculation Citation[24], Citation[25]. So we utilize the model in the artificial oxygen carrier experiment with emphasis on its possible chemo-sensitization effect in cancer treatment.
The development of an artificial oxygen carrier is associated with the known and potential infective agents, ambulance service, and the shortage of donor blood. To date, only nanotechnology based polyhemoglobin (PolyHb) and conjugated Hb continue to show promise in clinical trials Citation[5]. In addition to transfusion alternatives, the artificial oxygen carrier is a benefit in ischemia disorders Citation[3], Citation[4], Citation[7]. The application of it in tumor tissue hypoxia was carried out decades ago. Owing to the difference of tested samples and doses, some controversial data exist, which indicate that there still will be a long way to go.
MATERIALS AND METHODS
Polyethyleneglycol-conjugated Hemoglobin (PEG-Hb)
The PEG-Hb used in this study was a Chinese domestic sample. Its preparation processes and physiochemical properties belong to the manufacturer's proprietary information and will not be discussed in this paper. The sample was kept at 4°C in the dark until use.
Tumor Cell Line
HeLa cells were obtained from the Cell Bank of the Chinese Academy of Medical Sciences, Beijing, China, were grown in RPMI-1640 (Gibco, Life Technologies, Vienna, Austria) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100U/mL penicillin G, 0.1mg/ml streptomycin, termed the growth medium, and maintained in a humidified atmosphere with 5% CO2 at 37°C.
Animal Preparation and Drug Administration
Forty male Syrian hamsters (Mesocricetus auratus), 8 weeks old with an initial average body weight of 85g, were used throughout the following experiments performed in compliance with the guidelines of authorities. The tumor model was built in the cheek pouch of the hamster as we described previously Citation[24], Citation[26]. Briefly, after acclimation, 40 animals were anesthetized with pentobarbital sodium (30mg/kg b.w. i.p., sigma), and 5×106 HeLa cell suspension (100µL) was injected into the submucosa cheek pouch of hamsters. Two days after tumor-cell inoculation, animals all had an observable tumor xenograft in inoculated point. The animals were then randomly assigned to 4 groups (n=10) and treated respectively: group 1 was given saline via a jugular catheter; group 2, cisplatin (5mg/kg in 0.5mL of saline, Mayne Pharma Pty Ltd, Mulgrave, Australia); group 3, cisplatin (5mg/kg) combined with PEG-Hb (0.3g/kg); group 4, cisplatin (5mg/kg) combined with PEG-Hb (0.6g/kg). Five days after tumor-cell inoculation, hamsters in each group received repeated treatment, respectively.
Intravital Microscopy
The development of the newly formed vessels was observed on days 0, 3, 5, and 7 after tumor implantation with an intravital microscope (ACM, Zeiss, Germany). Simultaneously, tumor volume was measured by sliding caliper. During preparation and observation, the hamster's cheek pouch was continuously superfused with warmed physiological solution composed of (in mM) 131.9 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, and 18 NaHCO3, PH 7.4 at 37°C, and equilibrated with oxygen-containing gas. Then the recorded microscopic images were analyzed off-line by a professional staff in a blinded fashion. A computer-assisted digital image processing system (Institute of Microcirculation, PUMC&CAMS, China) was used to quantitatively analyze the changes of the microvessel tortuosity and area functional capillary density.
Detection of Tumor Hypoxia
Animals were sacrificed after a week of respective treatment, intraperitoneally injected hamsters with pimonidazol hydrochloride (60mg/kg) (Hypoxyprobe-1, Chemicon, USA) 1hr before being sacrificed. A tumor mass of appropriate size was fixed in neutral-buffered formalin (10%) following paraffin imbedding. Immunostaining for pimonidazole and HIF-1α was carried out on contiguous sections using procedures described previously Citation[11], Citation[15]. The primary antibodies were polyclonal anti-HIF-1α (1:25 dilution) from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Hypoxyprobe-1Mab1 (1:50 dilution) from Chemicon (California, USA). The resultant immunocomplexes were detected with horseradish peroxidase-conjugated antirabbit IgG (Santa Cruz Biotechnology). Eosin (HIF-1a sections) or hematoxlin (pimonidazole sections) were used as counterstains.
Statistical Analysis
All data were presented as mean±S.E.M. Statistical analysis was performed using the statistical program SPSS 10.0 for Windows (SPSS Inc., Chicago, IL, USA) and differences were analyzed by one-way ANOVA. The level of significance was taken as P < 0.05.
RESULTS
1. Tumor Growth and Development of Neovasculature
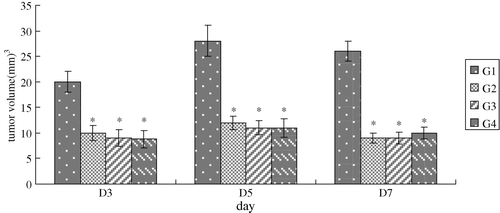
On the 2nd day after implantation, neoplasma were visible in all hamsters; after respective drugs administration, tumor volume was decreased sharply in groups 2, 3 and 4 compared with group 1 (P < 0.01). There was no significant difference in tumor volume when cisplatin combined with or without artificial oxygen carrier in a short time period ().
Figure 1. volume was decreased sharply in G2,G3 and G4 after chemotherapy alone or/and combined with artificial oxygen carrier. *p < 0.01 vs.saline.
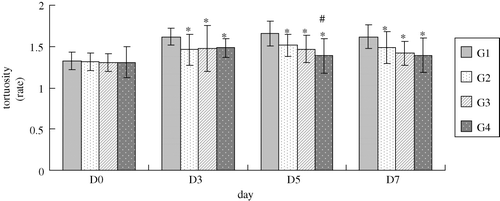
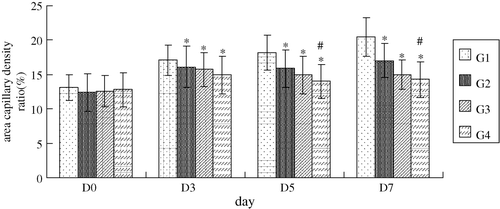
Compared with contralateral normal cheek pouch mucosa microvessels, chaotic and heterogeneous microvasculature was presented in tumor side characterized by dilation, tortuosity, sacculation and permeability profiles. Quantitatively evaluation neoplasma neovascular network revealed that increasing microvessel tortuosity and area capillary density were obvious in peritumoral areas after tumor cell inoculation in group 1. However, these changes were notably depressed after cisplatin combined with or without the oxygen carrier. The interesting thing is that group 4, combined with a higher dose of oxygen carrier, had a further inhibiting effect on these changes compared with group 2 (cisplatin alone) on the fifth day (tortuosity) and fifth and seventh day (capillary density), respectively ( and 3). But in group 3, combined with a low dose of oxygen carrier, this effect was not significant.
2. Expression of Exogenous and Endogenous Hypoxia Marker in Tumor Tissue
To evaluate the degree of tumor hypoxia environment and the changes of oxygenation after respective drugs administration, we carried out immunohistochemical analysis to detect pimonidazole and HIF-1α expression in tumor sections (). In group 1 the tissue was severe hypoxia with pimonidazole (27.6±4.7) and HIF-1α (29.2±5.5). After cisplatin combined with oxygen carrier treatment (groups 3 and 4), the two hypoxia markers both were significantly decreased compared with the control group (p < 0.05). A different concentration of oxygen carrier agent demonstrated capability in improving the tumor hypoxia microenvironment. A high dose of oxygen carrier (0.6g/kg) could markedly increase tumor oxygenation.
Table 1. Pimonidazole and HIF-1α expression in neoplasma (mean±S.E.M.)
DISCUSSION
More attention has recently focused on tumor hypoxia in tumor treatment. Severe hypoxia is correlating with progression, angiogenesis, metastasis and therapy resistance. Previous studies documented that in hypoxia condition tumor cells were susceptible to chemoresistance, with the possible reasons including poor drug distribution, quiencent tumor cell existence, acquired drug resistance feature, and loss of apoptotic potential Citation[23], Citation[27]. Many attempts have been performed based on these experimental and preclinical observations targeting tumor hypoxia.
Artificial oxygen carrier is a perfect mediator in oxygen transmission and offloading. One type of oxygen carrier perfluorocarbon (PFC) emulsions plus carbogen breathing have definite adjuvant effects in radiotherapy as well as chemotherapy in tumor treatment Citation[13], Citation[20], Citation[21]. Teicher further explored hemoglobin preparation on the response of tumor models to radiotherapy and chemotherapy Citation[17–19]. Contrary data have also been reported Citation[10], Citation[14]. The reported results indicate the oxygen carrier's adjuvant effect in tumor therapy may depend on the tested sample type and dose. In this experiment, we have designed two different concentration samples to test the possible effect of PEG-Hb on chemo-sensitization. Although the low concentration of PEG-Hb had some influence, it was not a statistical significance. Herein, PEG-Hb had notable influence on tumor neovasculature and tissue oxygenation induced by PEG-Hb in high concentration; this effect was based on lable tumor hypoxia and microvessels morphological observations.
As we mentioned above, tumor neovascularization has its special charicteristics. In our established tumor model chaotic and heterogeneous microvasculature was presented. A similar phenomenon existed in Gimbrone's tumor model Citation[8]. Tissue hypoxia induce angiogenesis; conversely, the irregular and less effective microvessels result in a small amount of anticancer drugs reaching the mass and aggravation of tissue hypoxia. Therefore, these changes make the situation more complex. Microcirculation detection is a valuable parameter in assessing the anticancer agent's action on tumor vessels Citation[16]. Previously, we have utilized a non-invasive computer-assisted intravital microscopy system to observe and quantitatively analyze the vascular microenvironment repeatedly, which enables us to evaluate the influence of the drug on tumor microcirculation Citation[24], Citation[25]. Herein, we also measured the tumor volume. PEG-Hb had no marked effect on it compared with cisplatin alone. It might be due to the relatively short period of drug administration. We have tested PEG-Hb in another tumor model on BALB/C nude mice with conventional therapy time and a better effect on tumor volume was observed (in press), whereas the microvessels structure normalization was obvious. We are the first to show a direct relationship of oxygen carrier to tumor neovascularization, although this study has some shortcomings.
In summary, tumor angiogenesis can be directly observed in our tumor model, which is a non-invasive ideal platform to quantitatively analyze the microvasculature repeatedly and assess the drugs’ action on it. PEG-Hb in high concentration can notably improve tumor tissue oxygenation and normalize neovasculature. It is indicated that the evaluated artificial oxygen carrier (PEG-Hb) may be a potential adjuvant to chemotherapy in cancer.
Acknowledgements
We thank Prof. T.M.S. Chang, McGill University, Canada, for serious review of this manuscript. This work was supported by Prof. Ruijuan Xiu's UNESCO Award for Women in Science 2000 and the grant of “Knowledge Innovation Project,” Academy of Science, China (No.KJCX1-SW-07).
References
- Bullitt E., Lin N. U., Ewend M. G., Zeng D., Winer E. P., Carey L. A., Smith J. K. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv 2006; 9: 561–568
- Bullitt E., Lin N. U., Smith J. K., Zeng D., Winer E. P., Carey L. A., Lin W., Ewend M. G. Radiology 2007; 245: 824–830
- Burkhoff D., Lefer D. J. Am Heart J 2005; 149: 573–579
- Caswell J. E., Strange M. B., Rimmer D. M., 3rd, Gibson M. F., Cole P., Lefer D. J. Am J Physiol Heart Circ Physiol 2005; 288: H1796–1801
- Chang T. M. Trends Biotechnol 2006; 24: 372–377
- De Felice C., Latini G., Bianciardi G., Parrini S., Fadda G. M., Marini M., Laurini R. N., Kopotic R. J. Gut 2003; 52: 1764–1767
- George I., Yi G. H., Schulman A. R., Morrow B. T., Cheng Y., Gu A., Zhang G., Oz M. C., Burkhoff D., Wang J. Am J Physiol Heart Circ Physiol 2006; 291: H1126–1137
- Gimbrone M. A., Jr, Gullino P. M. Cancer Res 1976; 36: 2611–2620
- Gordan J. D., Simon M. C. Curr Opin Genet Dev 2007; 17: 71–77
- Gottschalk A., Raabe A., Hommel M., Rempf C., Freitag M., Standl T. Artif Cells Blood Substit Immobil Biotechnol 2005; 33: 379–389
- Jankovic B., Aquino-Parsons C., Raleigh J. A., Stanbridge E. J., Durand R. E., Banath J. P., MacPhail S. H., Olive P. L. Cytometry B Clin Cytom 2006; 70: 45–55
- Kim J. W., Gao P., Dang C. V. Cancer Metastasis Rev 2007; 26: 291–298
- Martin D. F., Kimler B. F., Evans R. G., Morantz R. A., Vats T. S. NCI Monogr. 1988; 119–122
- Raabe A., Gottschalk A., Hommel M., Dubben H. H., Strandl T. Strahlenther Onkol 2005; 181: 730–737
- Raleigh J. A., Calkins-Adams D. P., Rinker L. H., Ballenger C. A., Weissler M. C., Fowler W. C., Jr, Novotny D. B., Varia M. A. Cancer Res 1998; 58: 3765–3768
- Read T. A., Farhadi M., Bjerkvig R., Olsen B. R., Rokstad A. M., Huszthy P. C., Vajkoczy P. Cancer Res 2001; 61: 6830–6837
- Teicher B. A., Dupuis N. P., Emi Y., Ikebe M., Kakeji Y., Menon K. In Vivo 1995; 9: 11–18
- Teicher B. A., Holden S. A., Ara G., Herman T. S., Hopkins R. E., Menon K. Biomater Artif Cells Immobilization Biotechnol 1992; 20: 657–660
- Teicher B. A., Holden S. A., Menon K., Hopkins R. E., Gawryl M. S. Cancer Chemother Pharmacol 1993; 33: 57–62
- Teicher B. A., Lazo J. S., Merrill W. W., Filderman A. E., Rose C. M. Cancer Chemother Pharmacol 1986; 18: 213–218
- Teicher B. A., McIntosh-Lowe N. L., Rose C. M. Biomater Artif Cells Artif Organs 1988; 16: 533–546
- Vicaut E. Therapie 2001; 56: 483–494
- Wartenberg M., Ling F. C., Muschen M., Klein F., Acker H., Gassmann M., Petrat K., Putz V., Hescheler J., Sauer H. Faseb J 2003; 17: 503–505
- Xiu R. J., Duan C. G., Mu G. F. Zhonghua Zhong Liu Za Zhi 1987; 9: 95–98
- Xiu R. J., Duan C. G., Mu G. F. Proc Chin Acad Med Sci Peking Union Med Coll 1988; 3: 26–32
- Yu M., Han J., Cui P., Dai M., Li H., Zhang J., Xiu R. Cancer Sci 2008; 99: 391–397
- Zhang W., Zhang H. J Huazhong Univ Sci Technolog Med Sci 2006; 26: 520–523