Abstract
A dynamic heterogeneous phase polymerization reaction is found to be efficient for controllable cross-link of hemoglobin with glutaraldehyde. The selective absorption of the immobile phase and asymmetry of protein concentration leads to narrowness of the molecular weight distribution and lowness of the average molecular weight. Using this method, 53% of hemoglobin obtained is intermolecular cross-linked with 12 molecular equivalents of glutaraldehyde. The majority of poly-hemoglobins is in the range of 128 kD to 258 kD.
INTRODUCTION
Poly-hemoglobin (PolyHb) has been studied as a potential oxygen carrier for a variety of clinical applications. Glutaraldehyde (GDA) is a very common protein cross-linking reagent, which is used to polymerize hemoglobin Citation[1–6]. Despite the commercial development of PolyHb dispersions, it seems that the GDA molecule has some disadvantages for a number of reasons Citation[7]: it is too reactive and lacks any intrinsic or functional specificity, leading to uncontrolled polymerization and wide molecular weight distribution. That leads to low yield of the hemoglobin oligomer (500 kD>Mw>64 kD) suitable for use as potential oxygen carriers, and to a product with high average molecular weight Citation[8], Citation[9]. In fact, the ideal polyHb solution should have low average molecular weight for low viscosity and low vascular resistance. Much effort has been made to prepare polyHb with narrow molecular weight distribution and low average molecular weight in the homogeneous phase, either by adding modifiers such as lysine, working with low hemoglobin (Hb) concentrations, and/or using physically separate compartments (dialysis cartridges) to slow the reaction between Hb and GDA Citation[7]. Another method, primarily reported by Su Zhi-Guo Citation[10], has been developed recently. The results show when GDA (200∼500:1 GDA-Hb molar ratios) reacts with Hb statically adsorbed on anion exchanger, homogeneous polymerized hemoglobin with two tetramers may be prepared; however, the Hb or polyHb will be modified excessively in this way. Herein, we disclose a novel method that it is efficient for controllable cross-link of hemoglobin with GDA. This is based on a concept that an asymmetric dynamic system is created via the advantage of the immobile phase's selective absorption; the Hb or polyHb with low modification (polymerization) are enriched and have more chances to react with each other in the column. We named this method polymerization reaction in cation exchange chromatography (PRCEC) because the cation exchanger is selected as matrix. In this way, 53% Hb is cross-linked by 12 molecular equivalents GDA. The most molar weight of poly-hemoglobins is between 128 kD to 258 kD.
RESULTS AND DISCUSSION
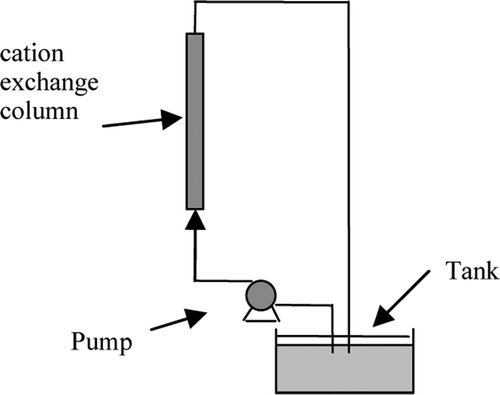
In this work, a set of equipment is installed according to . Then, 5 g Hb in 10 mM PBS (pH 6.3) was deoxygenated by passing a stream of humidified N2 for hours at 4°C in tank. 3.12 ml 2% (W/V) GDA was added to the deoxygenated Hb solution for pre-polymerization. The reaction, according to Scheme 1, First reference to proceeded for 3 h at 4°C. At a 8:1 molar ratio, intra-molecular cross-link is the main reaction. The solution was then pumped into a 1.6×30 cm CM-52 column and circulated for half an hour to form the asymmetric system. The volume of solution in tank was adjusted to 50 ml by adding 10 mM PBS (pH 6.3). 390 µ l 2% GDA is added to the tank every half hour for 8 times. The mixture is circulated in the system at a flow rate 2 ml/min. So, the solution with higher concentration GDA pumps into the column and reacts with Hb. The samples were reduced immediately with NaBH4 and then dialyzed.
The HPLC assay was carried out to determine the molecular weight distribution of PolyHb. The analysis was conducted with an instrument equipped with Superdex G-75 column, TSK G3000 PWXL column and HPLC system. The mobile phase was composed of PBS (66 mM sodium, 50 mM Na2SO4 pH 7.4), flow rate was 0.5 ml/min and the detection wavelength was 280 nm.
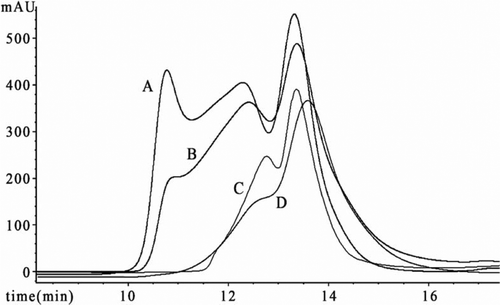
Four representative HPLC spectra are shown in . For these spectrums, the first peak (13.4 min) is the mixture of Hb tetramer and polyHb cross-linked with two Hb. Higher-order cross-linked Hb species (1000 kD > Mw > 128 kD) elutes at 12.3 min in a broad peak. The third sharp peak corresponds to the exclusion limit (Mw > 1000 kD). The area percentage of peaks in curve A is 35.6%, 43.7%, 20.7%, and 46.3%, 45.4%, 8.3% in curve B correspondingly. It shows that the PolyHb polymerized in homogeneous phase (Hb:GDA molar ratio1:16) has larger polymerization than the control in heterogeneous phase. The sharper size of the second peak in curve B indicates there are more polyHb with low molecular weight in solution B.
Figure 2. Gel filtration profile on TSK3000 PWXL column. The curve A, B, C and D is the profile of PolyHb polymerized in homogeneous phase (Hb:GDA molar ratio1:16), Poly-Hb polymerized by PRCEC (Hb:GDA molar ratio1:16), Poly-Hb polymerized by PRCEC (Hb:GDA molar ratio1:12) and a product of pre-polymerization, respectively.
We believe that selective absorption of the column's matrix and the asymmetry of protein concentration in the system contribute to the change of molecular weight distribution. Firstly, according to the theory of acid and base, the reaction between stronger acid and base produces weaker acid and base. Therefore, the value of pK of amino group on Hb is higher than the corresponding Schiff's base. In the solution with appropriate pH, the polymerized hemoglobin and modified hemoglobin have less positive charge than that of native Hb. Furthermore, some basic groups on the surface of polyHb can't approach the acidic groups on the fiber after intermolecular cross-link. So, the native Hb and polyHb with low modification (polymerization) is enriched in the column. Secondly, the concentration of GDA in the system is the same, while the protein concentration is higher in the column. So the reaction takes place mainly in the column. For these two reasons, the native Hb and polyHb with lower modification (polymerization) have more reactive odds with each other. As a final result, the polyHb with narrow molecular weight distribution and low average molecular weight is formed.
The PolyHb with optimized condition, which is synthesized using a 12:1 GDA-Hb molar ratio by PRCEC, is shown in curve C. There are only two peaks on it. The third peak (Mw > 1000 kD) disappears. The poly-dispersity result of two regions measured by multi-angle laser light scattering (MALLS) is shown in and . It shows that most of the molecules in region A are the polyHb with four Hb (256 kD). And, the solution in region B is the mixture of Hb and polyHb with two tetramers. Because the first peak of curve C is the largest in three curves, it indicates that Hb tetramer and polyHb cross-linked with two Hb tetramers are the main body of this solution. The Gel filtration profile of PolyHb on Superdex G-75 column is shown in . The result shows that approximately 53% of total protein is intermolecular cross-linked.
Figure 3. MALLS spectrum of Poly-Hb prepared by PRCEC (Hb:GDA molar ratio = 1:12). The LS, AUX1 and AUX2 were the signals of laser light scattering, refractive index, and UV absorbance, respectively.
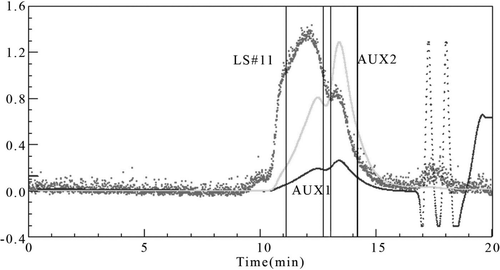
Figure 4. Gel filtration profile on Superdex G-75 column. The second peak (17.4min) is the Hb tetramer. The first peak (13.9min) is the polyHb. The percentage of area in this peak is 53%.
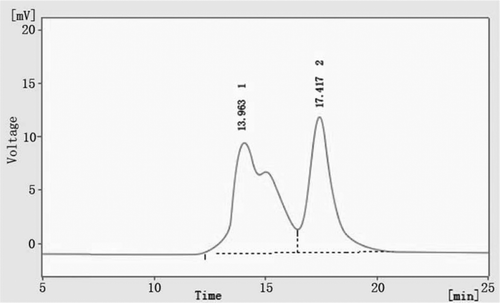
Table 1. Poly-dispersity of Poly-Hb polymerized on a cation exchange column (Hb:GDA = 1:12). Mw, Mn and Mz are weight-average molecular mass, number-average molecular mass and z-average molecular mass, respectively.
CONCLUSION
In summary, a novel method of hemoglobin polymerization (PRCEC) is developed. A well-defined polyHb (mainly 128 kD to 256 kD) was prepared with high yield as a potential oxygen carrier. Using the new PRCEC method, the polyHb with narrow molecular weight distribution and low average molecular weigh can be obtained. It can be foreseen that the mode is fit for all the reactions with the following condition: the interaction between one substrate and matrix is strongest in the system; the chromatograph column is low-grade overload.
References
- Squires J.E. Artificial blood. Science 2002; 295: 1002–1005, Review
- Chang T.M.S. Stabilisation of enzymes by microencapsulation with a concentrated protein solution or by microencapsulation followed by cross-linking with glutaraldehyde. Biochem. Biophys. Res. Commun. 1971; 44(6)1531–1536
- Lanzkron S., Moliterno A.R., Norris E.J., et al. Polymerized human Hb use in acute chest syndrome: a case report. Transfusion 2002; 42: 1422–1427
- Chang T.M.S. Future generations of red blood cell substitutes. J. Intern. Med. 2003; 253: 527–535
- Winslow R.M. Current status of blood substitute research: towards a new paradigm. J. Intern. Med. 2003; 253: 508–517
- Moore E.E., Johnson J.L., Cheng A.M., et al. Insights from studies of blood substitutes in trauma. Shock 2005; 24: 197–205
- MacDonald S.L, Pepper D.S. Hemoglobin polymerization. Methods Enzymol. 1994; 231: 287–308
- Eike J.H., Palmer A.F. Effect of glutaraldehyde concentration on the physical properties of polymerized hemoglobin-based oxygen carriers. Biotechnol. Prog. 2004; 20: 1225–1232
- Eike H.E., Palmer A.F. Effect of NaBH4 concentration and reaction time on physical properties of glutaraldehyde-polymerized hemoglobin. Biotechnol. Prog. 2004; 20: 946–952
- Tao, Hu and Zhiguo, Su. 2003. C.N. Patent 01136190.5.