Abstract
Angiogenesis, an essential event involved in a tumor's progression and metastasis, is regulated by hypoxia. Hypoxia widely exists in solid tumors due to the abnormal vasculature of tumor tissue and insufficiency of tissue oxygenation. We speculate that hemoglobin-based oxygen carriers (HBOCs) can attenuate tissue hypoxia, thereby suppressingthe angiogenesis in solid tumor in the context that HBOCs have the ability to increase tissue oxygenation. In the present study, PEG-conjugated hemoglobin solution (0.3 g/kg i.v. or 0.6 g/kg i.v.) was intravenously administrated to BALB/c nude mice bearing the cervical tumor twice a week with or without the treatment of cisplatin (5mg/kg i.p.) to investigate whether PEG-conjugated hemoglobin has a chemo-sensitization effect though anti-angiogenesis pathway. Tumor volume was measured every three days and tumor hypoxia was detected by immunohistochemistry for Hypoxyprobe™-1. Anti-angiogenic effect was accessed by detection of mRNA and protein levels of vascular endothelial growth factor (VEGF), the most important angiogenic factor. Our results showed that high concentration of PEG-conjugated hemoglobin solution significantly impeded the growth of tumor when compared with the control group. Moreover, VEGF expression was declined when treated with PEG-conjugated hemoglobin, possibly through the HIF regulation system. Collectively, treatment of PEG-conjugated hemoglobin combination with cisplatin has an antiangiogeic effect, but the underlying mechanism should be further studied.
INTRODUCTION
Solid tumors frequently contain a hypoxic microenvironment, which is uniquely different from that of normal tissues. The hypoxic microenvironment induces adaptive changes to tumor cell metabolism, and this alteration can further distort the local microenvironment Citation[1]. As a result, the abnormal microenvironment inhibits many standard cytotoxic anticancer therapies and predicts for a poor clinical outcome. Angiogenesis, an essential event involved in tumor's progression and metastasis, is also induced by tumor hypoxia Citation[2], Citation[3]. But unlike normal blood vessels, newly formed tumor vasculature has abnormal organization, structure, and function Citation[4], Citation[5]. Vascular hyper permeability caused by the leaky vessels and the lack of functional lymphatic vessels inside tumors causes elevation of interstitial fluid pressure in solid tumors. The abnormal tumor environment makes it difficult to deliver therapeutic agents to tumors Citation[6] and makes tumor cells more resistant to chemotherapy Citation[7]. Furthermore, elevated tumor interstitial fluid pressure increases fluid flow from the tumor margin into the peri-tumor area and may facilitate tumor metastasis Citation[1], Citation[8]. Anti-angiogenic therapy is considered to be a very promising strategy because it can “normalize” the tumor vascular net to limit the growth of tumor and enforce the efficacy of chemotherapy Citation[9], Citation[10].
A hemoglobin-based oxygen carrier (HBOC), designed with a purpose to substitute blood transfusions Citation[11], shows the potential to be used to cure some hypoxic ischemic diseases as a kind of oxygen therapy in recent years. One of the most important applications is that it can be used in cancer treatment as a radio- or chemotherapy sensitizer Citation[12–15]. It is speculated that HBOC may have a chemo-sensitivity effect when combined with chemo-therapy in cancer treatment because it is possible to ameliorate tumor hypoxia microenvironment, which is a characteristic of solid tumor. Some studies have reported that intravenous administration of hemoglobin solution was effective in ameliorating tumor hypoxia conditions Citation[12], Citation[15], Citation[16] and improving the efficacy of both irradiation Citation[12], Citation[14], Citation[17], Citation[18] and chemotherapeutic agents Citation[14], Citation[18–20]. Until now there has been no study to investigate the effect of hemoglobin solution on tumor angiogenesis. The present study was performed to investigate the influence of PEG-HB, a new kind of PEG-Hb solution, on the hypoxia microenvironment and tumor angiogenesis.
MATERIALS AND METHODS
Polyethyleneglycol-conjugated Hemoglobin (PEG-Hb)
The PEG-Hb used in this study was a Chinese domestic sample. Its preparation processes and physiochemical properties belong to the manufacturer's proprietary information and will not be discussed in this paper. The sample was kept at 4oC in the dark until use.
Cell Line
HeLa cells were obtained from the cell biology center of the Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC). Cells were cultured in RPMI-1640 (Gibco, Life Technologies, Vienna, Austria), supplemented with 10% heat-inactivated FBS (Gibco), 4 mM glutamine, 100 nM Na-pyruvate, 25 mM Hepes, 100 u/ml penicillin and 100 u/ml streptomycin, and incubated at 37°C in a humidified atmosphere containing 5% CO2 atmosphere.
Xenograft Tumor Model and Drug Administration
We subcutaneously injected 0.2ml 5×106 HeLa cells into the armpits of 5-week-old female BALB/c nude mice. We measured tumors with calipers twice a week and calculated tumor volume as (length×width2). After two weeks of innoculation, we select the mouse whose tumor volume was around 60-80mm3. The nude mice were separated into five groups (n = 10) randomly and treated differently as follows: physiological saline (group 1), cisplatin (5mg/kg, group 2), cisplatin with PEG-HB at dose levels of 0.3 g/kg (group 3), 0.6 g/kg (group 4), or PEG-HB alone at dose level of 0.45 g/kg (group 5) was administered to each group intravenously, twice a week, for four weeks. The general health of mice was monitored daily. Tumor dimensions and body weights were recorded two to three times a week starting with the first day of treatment.
Hypoxia Detection
Hypoxyprobe™-1 kit (Chemicon, CA, USA) was used to detect the tumor hypoxia in vivo. Hypoxyprobe-1(pimonidazol hydrochloride) is a chemical component that specifically binds to proteins in hypoxic cells at an oxygen pressure equal to or lower than 10 mmHg Citation[21]. The high water solubility of Hypoxyprobe™-1 permits small volume injections to be made, which is convenient for studies with small animals. The formed protein adducts are detected by staining with specific monoclonal antibodies and the amount of adducts formed is proportional to the level of hypoxia.
To assess hypoxia regions, we intraperitoneally injected mice with 60 mg/kg Hypoxyprobe-1, 1h before sacrificed. After the mice were sacrificed, tumors were rapidly removed and fixed in 10% formalin, dehydrated and embedded in paraffin. Immunohistochemical studies were performed on deparaffinized and rehydrated sections, according to the manufacturing instructions. The slides were exposed to hypoxyprobe-1 Mab1 (diluted 1:50) for 40 min at RT, rinsed in phosphate-buffered saline and 0.2% Brij 35 for seven times at 0°C, and incubated with biotinylated secondary antibody. Finally, the DAB were performed according to the manufacturer's protocol (DAB Kit, Promega, Madison, WI, USA). Controls were performed by processing slides in absence of the primary antibody. Each tumor was scored semi-quantitatively section by section in all cases using a scoring system where hypoxia in the ranges of 0; >0–5%; >5–15%; >15–30%; >30% were assigned scores of 0, +1, +2, +3 and +4. The cumulative scores of each group were calculated and averaged for use as descriptors.
Western Blot Analysis
For HIF-1α expression analysis, tumors were grinded in liquid nitrogen and suspended in 100–200µl lysis buffer (50mmol/L Tris, 150mmol/L NaCl, 5mmol/LEDTA, 5mmol/L EGTA, 1%SDS, pH7.5), then ultrasonicated on ice until the solution became clear. The total protein concentrations were measured with the Bradfold method. Samples were heated at 100°C for 5min with 2×SDS loading buffer and briefly cooled on ice. 50µg total proteins from each sample were subjected to 8% or 12%SDS-PAGE to detect HIF-1α (120KD) and β-actin (43KD), respectively. Proteins were electrophoretically transferred to PVDF membranes (Millipore Corp., Bedford, MA, USA) in transfer buffer (25 mmol/L Tris, 200mmol/L glycerin, 20% methanol, pH8.5) at 100V for 3 hours, and then membranes were blocked with 5% skim milk in PBS for 1h at room temperature. Specific immunodetections were carried out by incubation with primary antibodies (rabbit polyclonal antibody to HIF-1α, sc-10790, Santa Cruz; goat polyclonal antibody to β-Actin, sc-1616, Santa Cruz) diluted 1:500 in skim milk overnight at 4°C. After three washes with PBS, the membranes were incubated for another 1h with horseradish peroxidase-conjugated goat anti-rabbit or rabbit anti-goat IgG (BeiJing ZhongShan Goldbridge Biotechnology Co. Ltd.) and diluted 1:10000 in PBS at room temperature. Antigens were revealed using a chemiluminescence assay (Western Blotting Luminol Reagent, sc-2048, Santa Cruz).
RT-PCR Analysis
Tumors were grinded in liquid nitrogen, and then total RNA was extracted separately from tumor tissues of each group with TRIZOL® reagent following the manufacturer's instructions. A 2µg (treated in 8 µL DEPC water in an Ep tube) sample of total RNA (added 1µl 10mM dNTPmix and 1µl 0.5µg/µL Oligo (dt) 12–18) was denatured by incubating at 65°C for 5 min, and the tube was placed on ice for 2 min, and then reverse-transcribed into complementary DNA (cDNA) using the following procedures: briefly, the denatured RNA was incubated for 42°C for 2 min with 2µl 10×RT buffer, 4µl 25mM MgCl2, 2µl 0.1M DTT and 1µl RNaseOUTTM Recombinant Rnase inhibitor (50u/µL), then we added 1µl (50units) SuperScriptTM II RT, and incubated it at 42°C for 50min and terminated at 70°C for 15min in a total volume of 20µl; finally, samples were chilled on ice, 1µl Rnase H was added and incubated for 20 min at 37°C before PCR. For PCR, 2µl of the resulting cDNA, 36.75µl of tripled-distilled H2O, 5µl of 10×PCR buffer, 3µl of MgCl2 (25 mmol/L), 1µl of dNTPs (10mmol/L), 1µl of each of sense and antisense primers (10 µmol/L), and 0.25µl Taq DNA polymerase (5 u/ul) in a total volume of 50µl were added. Amplifications for VEGF were performed for 30 cycles and β-actin was amplified for 21 cycles. Each amplification consisting of denaturation at 94°C for 45s, primer annealing at 54°C for 45s and extension at 72°C for 1 min. Cycles were preceded by incubation at 94°C for 5 min to ensure full denaturation of the target gene, followed by an extra incubation at 72°C for 7min to ensure full extension of the products. PCR products were analyzed on 1% agarose gel containing ethidium bromide. The sequences of the primers for VEGF165 were sense 5′-GGGCAGAATCATCACGAAGT-3′ and antisense 5′-AAATGCTTTCTCCGCTCTGA-3′ (359 bp) and for β-actin, sense 5′-GTGCGTGACATTAAGGAG-3′ and antisense 5′-CTAAGTCATAGTCCGCCT-3′ (520 bp).
VEGF ELISA Assay
VEGF protein levels in blood were quantified by ELISA methods. The blood wasw collected before the mice were sacrificed and centrifuged at 12,000 rpm at 4°C for 15 min, and then VEGF concentration in serum was accessed by ELISA according to the manufacturer's instructions (VEGF ELISA kit, Promega, Madison, WI, USA). The values of OD (A450 values) were measured at 450 nm. The standard curve was worked out by the SPSS statistical software. Serum was harvested with 6 replicated and the experiment was performed three times.
STATISTICAL ANALYSIS
Statistical analysis was performed using the statistical program SPSS 10.0 for windows (SPSS Inc., Chicago, IL, USA). All data are presented as mean±S.E.M and are analyzed by One-Way ANOVA. P values < 0.05 were considered as statistically significant.
RESULT
Influence of PEG-HB on Tumor Growth
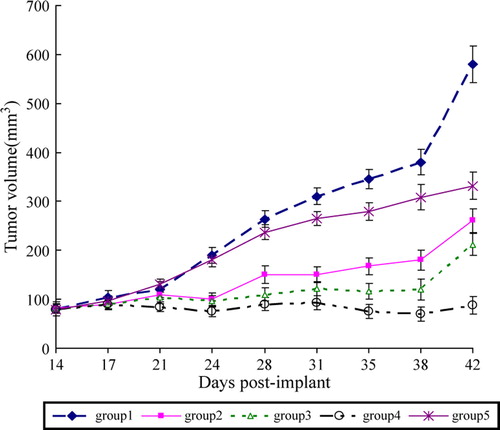
The result showed that administration PEG-HB combined with cisplatin caused a inhibition of tumor growth and resulted in a reduction of tumor volume in comparison with administration cisplatin only (group 2) ( ). While co-administration of cisplatin and lower dose (0.3 g/kg) of PEG-HB (group 3) showed no significant gained anti-tumor efficacy as compared with cisplatin, co-administration of cisplatin and lower dose (0.6 g/kg) of PEG-HB (group 4) has a significant difference with group 1 (P < 0.01). It is surprising that administration with PEG-HB alone to the tumor-burden mice in group 5 also exhibited slight anti-tumor efficacy compared with the control group (group 1).
Influence of PEG-HB on Tumor Hypoxia
To access hypoxia in tumor tissues of the five groups, animals were intravenously injected with hypoxyprobe-1 (pimonidazole hydrochloride) 1 hour before the animals were sacrificed, then the tissues were processed according to Hypoxyprobe™-1 Kit protocol. The hypoxyprobe-1 immunohistochemistry staining of hypoxic tumor cells is scored for quantitative assessment (A). The results showed that there exist more binding of pimonidazole hypoxyprobe to tumors harvested from animals that were administered with PEG-HB (group 3, 4, 5), which means PEG-HB enhanced the oxygenation in tumor tissues.
Figure 2. Tumor hypoxia reflected by (A) images of immunocytochemical staining of Hypoxyprobe-1 in tumor tissue, (B) Pimonidazole binding scores, and (C) expression of HIF-1α. *P < 0.05, **P < 0.05.
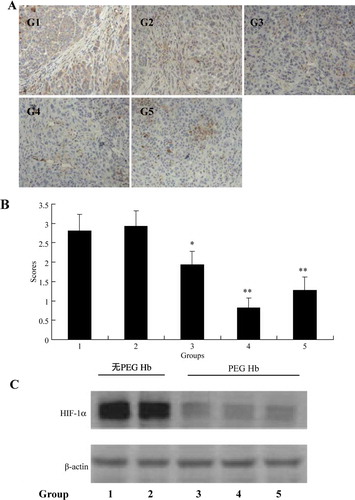
We also chose HIF-1α as another indicator to reflect the influence of PEG-HB on tumor oxygenation. HIF-1α, the heart role of hypoxia regulation system, exists only in hypoxia tissues. The western blot of HIF-1α showed the similar results of the immunostaining of hypoxyprobe-1. Administration of PEG-HB significant reduced the expression of HIF-1α in tumor tissues (B).
Influence of PEG-HB on Expression of Angiogenic Factor VEGF
VEGF, the most active endogenous pro-angiogenic factor and the specific endothelial cell mitogen, was selected to be a reliable index to investigate the antiangiogenesis effect of PEG-HB. VEGF mRNA levels in tumor tissues were detected by RT-PCR method. The results showed that 0.6 g/kg PEG-HB downregulated the expression of VEGF mRNA compared with other groups (A).
Figure 3. VEGF mRNA levels in nude mice tumor tissues and VEGF protein concentrations in the blood serum of the nude mice were assessed by RT-PCR (A) and ELISA (B), respectively. Data shown are representative of three independent experiments. *P < 0.05.
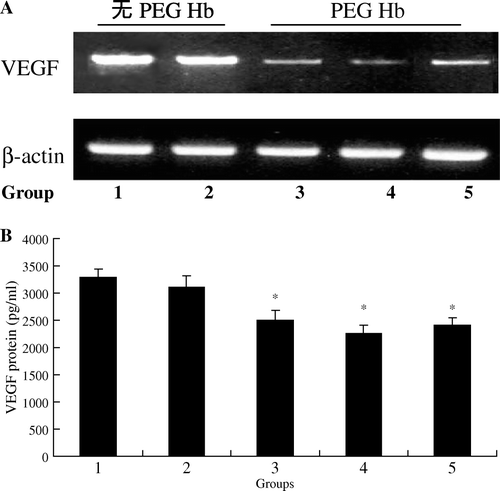
We next assessed whether the influence of VEGF mRNA by melatonin resulted in a decreased production of VEGF protein by ELISA method. The results (B) showed that levels of VEGF protein were notably decreased in the serum of mice administrated with PEG-HB (group 3, 4, 5) (P < 0.05). But it seems there is no significant difference between these three groups treated with different concentrations of PEG-HB.
DISCUSSION
HBOCs have been tried to elevate the efficacy of radio- and chemotherapy though incensement of oxygenation in virtue of its potent oxygen carry and delivery capability Citation[22]. Besides, it is regarded that HBOCs are easier to get into the abnormal microvessels of tumors compared with red blood cells because of their smaller diameter Citation[23]. Teicher et al. reported that ultrapurified polymerized bovine hemoglobin solution could result in decreased tumor hypoxia Citation[12], Citation[15], Citation[16] and increased both irradiation Citation[12–14], Citation[17] and chemotherapeutic response Citation[14], Citation[15], Citation[19], Citation[20], which were confirmed by the results of our present study. Administration of high concentration (0.6 g/kg) of PEG-HB, which is derived from PEG-conjugated hemoglobin, can effectively increase the anti-tumor effect of cisplatin, one of the most important chemotherapeutic drugs in clinical use. The chemo sensitize effect is attributed to its amelioration of tumor hypoxia.
As we know, widespread hypoxia within solid tumors is one of the most potent stimuli of angiogenesis, which is essential for tumor's growth, development and metastasis Citation[24], Citation[25]. Increased angiogenesis has been shown to be associated with the tumor development of metastases, poor prognosis and reduced survival Citation[26]. Tumor hypoxia will induce the expression of angiogenic factors such as VEGF, SDF and PDGF, hence broke the balance between angiogenic factors and antiangiogenic factors Citation[27]. VEGF, the most important mediator of tumor angiogenesis, is crucially concerned with cancer development and high vascularization among these angiogenic factors Citation[28]. Tumor hypoxia has been a therapeutic target to overcome tumor angiogenesis. Ultrapurified polymerized bovine hemoglobin solutions have improved their capability to overcome tumor hypoxia. Until now, there has been no data documenting the possible effect of hemoglobin solution on tumor angiogenesis. We are the first to show that HBOC can suppress the tumor angiogenesis through downregulation of VEGF. Administration of PEG-HB was found to suppress VEGF mRNA expression in tumor tissues. And the VEGF protein levels also decreased after treatment with PEG-HB. Moreover, the decrease of VEGF seems to have a positive correlation of the concentration of PEG-HB.
We speculated that downregulation of VEGF might be associated with decreased HIF-1α protein levels. It is well established that HIF-1, the key mediator of the hypoxia response, is the most important regulator of VEGF Citation[29], Citation[30]. HIF-1 is a ubiquitous transcription factor consisting of HIF-1α and HIF-1β subunits Citation[31]. Under normoxic conditions, HIF-1α is rapidly degraded through the ubiquitin-proteasome system, whereas HIF-1β is constitutively expressed. When the oxygen is insufficient, HIF-1α is released from the von Hippel-Lindau tumor suppressor protein and translocates into the nucleus, where it heterodimerizes with HIF-1β and binds on the hypoxia responsive element (HRE) to regulate hypoxia-driven gene expression Citation[30]. In our experiment, HIF-1α protein levels were decreased in PEG-HB administration groups, which indicated that PEG-HB may suppress the VEGF expression through the inhibition of the accumulation of HIF-1α.
In summary, our present study shows that PEG-HB inhibits the expression of endogenous HIF and VEGF in a rodent tumor model, which may be a very innovative and challenging method of cancer anti-angiogenesis therapeutics.
Acknowledgements
We thank Prof. TMS Chang, McGill University, Canada, for serious review of this manuscript. This work was supported by Prof. Ruijuan Xiu's UNESCO Award for Women in Science 2000 and the grant of “Knowledge Innovation Project” Academy of Science, China (No.KJCX1-SW-07).
References
- Fukumura D., Jain R. K. Microvasc Res 2007; 74: 72–84
- Carmeliet P. Nature 2005; 438: 932–936
- Gruber M., Simon M. C. Curr Opin Hematol 2006; 13: 169–174
- Ferrara N., Kerbel R. S. Nature 2005; 438: 967–974
- Manegold P. C., Hutter J., Pahernik S. A., Messmer K., Dellian M. Blood 1970; 101: 1976(2003
- Tredan O., Galmarini C. M., Patel K., Tannock I. F. J Natl Cancer Inst 2007; 99: 1441–1454
- Brown J. M. J Natl Cancer Inst 1990; 82: 338–339
- Jain R. K., di Tomaso E., Duda D. G., Loeffler J. S., Sorensen A. G., Batchelor T. T. Nat Rev Neurosci 2007; 8: 610–622
- Hellmann, K. Nat Med, 10: 329; author reply 329–330(2004).
- Jain R. K. Science 2005; 307: 58–62
- Winslow R. M. Vox Sang 2006; 91: 102–110
- Robinson M. F., Dupuis N. P., Kusumoto T., Liu F., Menon K., Teicher B. A. Artif Cells Blood Substit Immobil Biotechnol 1995; 23: 431–438
- Teicher B. A., Ara G., Herbst R., Takeuchi H., Keyes S., Northey D. In Vivo 1997; 11: 301–311
- Teicher B. A., Dupuis N. P., Emi Y., Ikebe M., Kakeji Y., Menon K. In Vivo 1995; 9: 11–18
- Teicher B. A., Holden S. A., Dupuis N. P., Kusomoto T., Liu M., Liu F., Menon K. Artif Cells Blood Substit Immobil Biotechnol 1994; 22: 827–833
- Teicher B. A., Schwartz G. N., Alvarez Sotomayor E., Robinson M. F., Dupuis N. P., Menon K. J Cancer Res Clin Oncol 1993b; 120: 85–90
- Tanaka J., Holden S. A., Herman T. S., Teicher B. A. Anticancer Res 1992; 12: 1029–1033
- Teicher B. A., Holden S. A., Ara G., Herman T. S., Hopkins R. E., Menon K. Biomater Artif Cells Immobilization Biotechnol 1992; 20: 657–660
- Teicher B. A., Herman T. S., Hopkins R. E., Menon K. Int J Radiat Oncol Biol Phys 1991; 21: 969–974
- Teicher B. A., Holden S. A., Menon K., Hopkins R. E., Gawryl M. S. Cancer Chemother Pharmacol 1993a; 33: 57–62
- Hofer S. O., Mitchell G. M., Penington A. J., Morrison W. A., RomeoMeeuw R., Keramidaris E., Palmer J., Knight K. R. Br J Plast Surg 2005; 58: 1104–1114
- Yu M., Dai M., Liu Q., Xiu R. Cancer Treat Rev 2007; 33: 757–761
- Gottschalk A., Raabe A., Hommel M., Rempf C., Freitag M., Standl T. Artif Cells Blood Substit Immobil Biotechnol 2005; 33: 379–389
- Folkman J. Nat Med 1995; 1: 27–31
- Folkman J., Shing Y. J Biol Chem 1992; 267: 10931–10934
- Weidner N., Carroll P. R., Flax J., Blumenfeld W., Folkman J. Am J Pathol 1993; 143: 401–409
- Liao D., Johnson R. S. Cancer Metastasis Rev 2007; 26: 281–290
- Coultas L., Chawengsaksophak K., Rossant J. Nature 2005; 438: 937–945
- Ferrara N., Gerber H. P., LeCouter J. Nat Med 2003; 9: 669–676
- Pugh C. W., Ratcliffe P. J. Nat Med 2003; 9: 677–684
- Bruick R. K., McKnight S. L. Science 2001; 294: 1337–1340