Abstract
One of the most important challenges in bioartificial liver designed for patients suffering from acute liver failure is oxygenation of cells within the bioreactor. The aim of this study was to evaluate the impact of oxygenation of bioartificial liver using perfluorocarbon (PFC) emulsion on the metabolic activity of hepatic cells in vitro. Mineral fibers coated with collagen type I were used as scaffolds for hepatic cells. Significantly higher total protein synthesis by hepatic C3A cells cultured in the bioreactor for 24 hours, in the group treated with medium supplemented with PFC emulsion, was observed in comparison with medium without PFC. Albumin production increased in the group treated with PFC after 1 hour of perfusion and was continuously, statistically, significantly higher during perfusion then the control group. In conclusion, the use of oxygen carriers, such as the PFC emulsion, can significantly improve synthetic performance of the bioreactor. Mineral fibers coated with extracellular proteins may serve as support for hepatic cells in the bioreactor.
INTRODUCTION
Bioartificial liver (BAL) devices have been designed to support patients with acute or acute on chronic liver failure. The BAL treatment should provide synthetic and detoxification function of the patients’ liver by incorporating hepatocytes, which are the biological component of the BAL device until the liver becomes available for allotransplantation or to support the liver function to regeneration of his own one. The availability of human livers for orthotopic transplantation is limited and only a small percentage of acute liver failure patients can be treated with an expensive liver transplantation procedure. Temporary liver support was investigated to develop various extracorporeal BAL systems. Various types of BAL devices differing in geometry and employed blood perfusion systems have been designed, including a hollow fiber bioreactor, fluidized bioreactors in which cells are encapsulated in alginate beads and suspended in fluidic conditions, as well as devices where cells are seeded on synthetic scaffolds and patients plasma contacts directly with them Citation[1], Citation[2].
One of the most important challenges in the BAL functions is oxygenation of cells within the bioreactor. The liver, a highly anaerobic organ, is dependent on adequate oxygenation. The O2 supply is provided to the liver in vivo via the highly oxygenated hepatic artery, which mixes with the nutrient rich portal vein. Additionally, the hepatic sinusoid (the primary functional unit of the liver) is arranged in such a way that O2 diffusion distances are minimized Citation[3]. To improve oxygenation of tissues, different oxygen carriers have been used, including artificial blood cells with hemoglobin or emulsion of water insoluble perfluorocarbons Citation[4]. Perfluorochemicals are chemically inert synthetic molecules that consist primarily of carbon and fluorine atoms, and are transparent, colorless liquids. They have the ability to physically dissolve significant quantities of many various gases including oxygen and carbon dioxide. Perfluorocarbon emulsions are characterized by a linear relationship between oxygen partial pressure and oxygen content, different from those of hemoglobin, which exhibits a sigmoidal oxygen dissociation curve. Oxygen carried by the perfluorocarbon is in the dissolved state, resulting in higher oxygen partial pressures in the microcirculation and thereby augmenting the driving pressure for the diffusion of dissolved oxygen into the tissue Citation[5]. For our experiments a bioartificial liver device, in which human hepatic cells (C3A, derived from hepatoma) were seeded on the mineral wood fibers coated with collagen type I and perfused using culture medium with addition of perfluorocarbon emulsion, has been designed. Our previous studies indicated that coating of the human hepatoma cell support with collagen type I significantly improves cell adhesion Citation[6]. The aim of this study was to evaluate the impact of oxygenation of bioartificial liver using a commercially available perfluorocarbon emulsion (Perftoran) on the metabolic activity of hepatic cells in vitro.
MATERIALS AND METHODS
Cells and Culture Media
Hepatic C3A cells derived from human hepatoma cells line HepG2 were purchased from ATCC (LGCPromochem) and cultured in DMEM medium. The cells were maintained in the culture medium at 37°C in a humidified atmosphere of 5% CO2. C3A cells were seeded at a density of 2×105 cells/cm2 in a standard culture flask of 25cm5. The cell number in the suspension was estimated by a hemacytometer under the microscope. The culture medium was changed three times per week. DMEM culture medium supplemented with non-essential amino-acids, 10% of foetal calf serum, streptomycin (0,1mg/ml), penicillin (100 U/ml) HEPES buffer (1 mM) was used (all from Gibco, USA). DAPI was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Bioreactor Experiments
Bioreactors designed for our experiments consisted of a cylindrical polycarbonate housing filled with commercially available microfibers of mineral wool (Isover, Poland) equipped with inlet and outlet. Bioreactors were autoclaved and subsequently the fibers were coated with the rat tail tendon collagen type I at concentration of 0,5 mg/mL in acetic acid (BDsciences, USA). C3A cells were seeded on the fibers and incubated in static conditions for subsequent 30 minutes to allow cell adhesion. After cell attachment the flow of the medium in the bioreactor was adjusted to 3 mL/min. The following media were used in the bioreactor experiments: DMEM with addition of Perftoran emulsion (5%) or DMEM with addition of all chemicals as in Perftoran but without perfluorochemicals. Experimental set-up consisted of the bioreactor, peristaltic pump and medium reservoir in which circulating fluid was constantly oxygenated. DMEM medium without serum was used to assess total protein content secreted by cells to the medium during the perfusion experiment. After 1, 6 and 24 hours of the circulation experiment, medium samples were taken and stored in −20°C until assayed.
Microscopic Analysis of Cells
Samples of fibers with the cells after bioreactor experiments were taken and fixed in 4% paraformaldehyde and subsequently permeabilized with 0,1% Triton X-100 in PBS then washed. The cell nuclei stained with 4,6- diamidino-2-phenylindole (DAPI, 10 µM) were observed under the fluorescence microscope (Olympus IX71) equipped with CCD camera (DP70) and the pictures were taken. For scanning electron microscopy the cells were fixed and subsequently dehydrated in ethanol gradient and covered with gold. A Jeol microscope was used for the SEM observations.
Assessment of Glucose Concentration
For glucose determination in the medium samples a clinical analyzer was used, Envag 2300 Stat.
Analysis of Albumin Synthesis
Albumin secreted by the cells to the medium was measured using a commercially available indirect sandwich enzyme-linked immunosorbent assay (ELISA) kit. Human specific primary antibodies (produced in goat) and secondary detection antibody (produced in goat), linked to horseradish peroxidase, were from Bethyl (USA). Quantization was achieved by comparison with a standard curve for human albumin in the culture medium performed on each ELISA plate. The kit performance was optimized and standard dilutions from 400 to 6.25 ng/ml of human albumin. Detection was carried out spectrophotometrically at 450 nm using a microplate reader (Biotek, USA).
Analysis of Total Protein Synthesis
The total protein synthesized by C3A cells during the culture in the bioreactor perfused with the control DMEM medium and the DMEM medium with addition of Perftoran (5%) was assayed. The media were used without addition of fetal calf serum to analyze only proteins produced by the cells. We used the Bradford method for protein determination (Sigma-Aldrich, USA), subsequently read in the microplate spectrophotometer (Biotek), and concentrations were calculated from the standard curve of albumin (Sigma-Aldrich, USA).
RESULTS
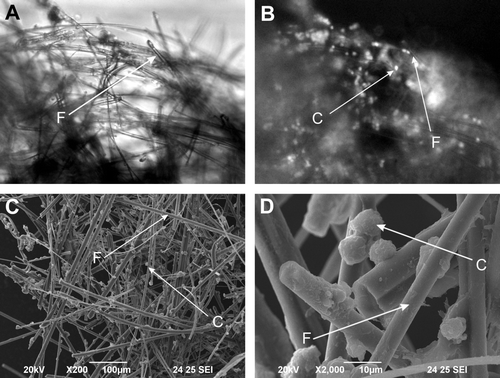
Human hepatoma C3A cells seeded on the collagen type I coated mineral fibers were observed (A-D). Scanning electron microscopy as well as fluorescence and light microscopy revealed that the human hepatoma C3A cells attached very easily to the fibers and survived on the modified surface. This kind of cell seeding allows direct perfusion and contact of the cells with the fluid without semipermeable membrane and is more physiological. Using the DAPI cell neclei staining the attached C3A cells were easily visible, when compared with light microscopy (C, 1D). Covering of mineral fibers with collagen type I isolated from rat tail tendon was also visible on the pictures taken using the scanning electron microscope (A, 1B).
Figure 1. Hepatic cells cultured on mineral fibers. A – Light microscopic picture of mineral fibers with hepatoma C3A cells. B – corresponding fluorescence picture with DAPI stained nuclei of human hepatoma C3A cells. C, D – Scanning electron microscopy pictures taken from human hepatoma C3A cells cultured on mineral fibers. C – human hepatoma C3A cell; F – mineral fiber.
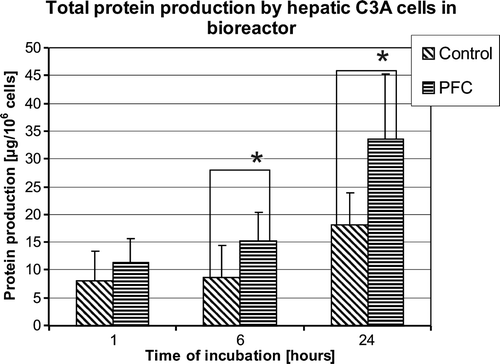
Analyzing total protein synthesis by the hepatic C3A cells cultured in the bioreactor for 24 hours in groups treated and non-treated with PFC, significant increase in the group treated with medium supplemented with perfluorocarbon emulsion was observed (). After 1 hour of perfusion no statistically significant differences between the groups were observed. Total protein synthesized by C3A cells in the bioreactor during 6 and 24 hours of the culture was significantly higher for PFC treated than culture group (8,62±5,86 vs. 15,15±5,26 µg/106 of cells after 6 hours, 18,02±5,90 vs. 33,61±11,65 µg/106 of cells, p < 0,05 after 24 hours, control vs. PFC treated, respectively).
Figure 2. Total protein synthesis by human hepatoma C3A cells in bioreactor after 1, 6, and 24 hours of perfusion. Control – boioreactor perfused with control DMEM medium; PFC – bioreactor perfused with DMEM medium supplemented with 5% of PFC emulsion. (n = 6, *p < 0.05).
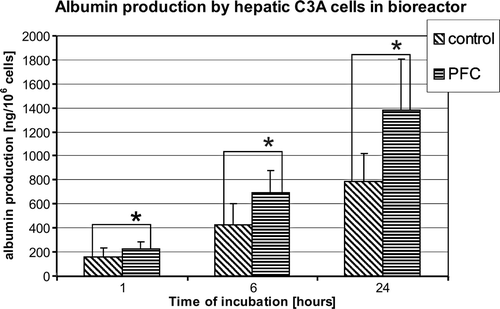
Similar effect was observed in albumin production by the hepatic C3A cells when cultured in the bioreactor perfused using the medium containing 5% of PFC emulsion. Albumin production increased during the experiment in both the groups (). Albumin production was higher in the group treated with PFC after 1 hour of perfusion and continuingly statistically significant higher during the experiment than the control group (79,37±30,09 vs. 113,12±37,68 ng/106 cells after 1 hour, 211,58±90,0 vs. 347,89±88,05 ng/106 cells after 6 hours and 394,48±215,16 vs. 690±114,89 ng/106 of cells, control vs. PFC treated, p < 0.05, respectively).
Figure 3. Albumin production by human hepatoma C3A in bioreactor after 1, 6, and 24 hours of perfusion. Control – bioreactors perfused with control DMEM medium; PFC – bioreactors perfused with DMEM medium supplemented with 5% of PFC emulsion. (n = 6, * p < 0.05).
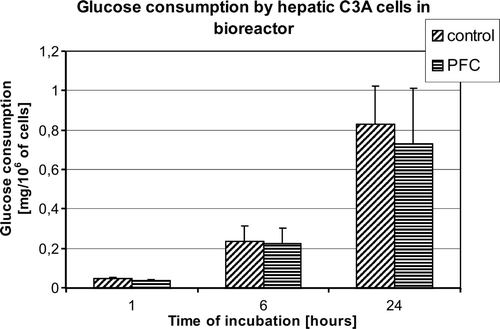
To assess metabolic state of the cells in the bioreactor glucose consumption was analyzed. No significant differences in the glucose consumption were observed between the examined groups, but mean glucose consumption was observed for the group treated with PFC (.).
DISCUSSION
Oxygen plays an important role in the metabolism of hepatocytes Citation[7], Citation[8]. Design of the bioartificial liver device has to take into account the system for oxygen delivery to the entrapped cells. The mass transfer between cells and the surrounding fluid should be maximally increased. The direct perfused bioreactor enables free exchange of any metabolites between the cells and the perfusion fluid. Cells in the direct perfused bioreactor have unlimited contact with oxygen dissolved in the medium. Some of the bioartificial liver bioreactors have gas-permeable hydrophobic membranes, which allow one to increase oxygen concentration in the medium Citation[9], Citation[10]. Perfluorocarbons are chemically and biologically inert artificial fluorinated organic fluids that are immiscible in water and have a high solubility for oxygen. The amount of dissolved oxygen in PFC is directly related to the ambient oxygen tension Citation[4]. Gas molecules are not chemically bound to PFC but absorbed and released by simple diffusion. Oxygen solubility in the liquid PFCs used for blood substitutes is large when compared, for example, to the same gas in water. Oxygen solubility higher than 10–20 times that of pure water is found in several commercially available PFCs. PFCs are stable and virtually chemically inert due to the presence of very strong carbon-fluorine bonds. PFCs are used in cold preservation of organs during storage before transplantation Citation[11], Citation[12]. Its properties significantly improved islets’ isolation after extended cold storage Citation[13], Citation[14]. Studies of Nieuwoudt Citation[15] carried out on pig hepatocytes entrapped in a bioreactor indicated that addition of the PFCs’ emulsion significantly improves liver cell detoxificatory functions as measured in lidocaine clearence.
In these studies, a commercially available PFC emulsion, Perftoran, was used for the bioreactor oxygenation. Human hepatoma cells, C3A line, were examined in our experimental bioartificial liver. The collagen coated mineral fibers were used as support for the cells in the direct perfused bioreactor. Significant increase in protein synthesis, including albumin, the main protein produced by hepatocytes in the liver, was observed when the cells in the bioreactor were perfused with the medium enriched in PFCs. Its beneficial effect is connected with the increased oxygen content available for the cells. In studies of Nieuwouldt et al. Citation[15], no differences in albumin production between the bioreactor with or without PFCs were observed. In the mentioned studies, the concentration of PFCs in the emulsion reached 20% and the experiments were carried out on pig hepatic cells Citation[15]. Significant differences in metabolism of primary cells and cell lines derived from cancers (e.g. hepatoma) could be explained by different cell synthetic properties Citation[16–18]. Human hepatoma cells reveal very low detoxificatory functions whereas they are able to produce significant amount of liver proteins (albumin) in bioreactor Citation[16]. Protein synthesis is a very energy-consuming process in living cells. The anaerobic glycolysis takes place when hepatic cells receive insufficient amount of oxygen and the final product is lactate. The conversion of glucose to lactate leads to generation of 2 mol ATP per mol of glucose, whereas during oxidative phosphorylation one mol of glucose generates 38 mol of ATP Citation[19]. In present experiments the glucose consumption was lower in the PFC treated group than in the control, but no statistically significant differences were observed. PFC addition to the culture lowers lactate production by hepatoma cells Citation[20]. This fact indicates that low energy generating anaerobic glycolysis, which was characteristic for the control group, decreased when PFC was added to the perfusion medium. The energy that was saved during aerobic glycolysis in hepatocytes in higher oxygen supply using PFC was used by the cells for protein synthesis.
The mineral fibers that were used in our experiments may serve as a cell support in the bioreactor. Its extended surface significantly increases the area available for the cells. The cells attached to the matrix are directly perfused by the medium containing PFC with dissolved oxygen. Such configuration enables higher mass transfer between the cells and the surrounding fluid. Modification of the fiber coating with other extracellular matrix elements is possible to assess its influence on the cell metabolism in the perfused conditions.
In conclusion, oxygenation of the bioreactor and the oxygen supply of hepatic cells is one of the most important challenges in bioartificial liver development. The use of oxygen carriers, such as the PFC emulsion, can significantly improve synthetic performance of the bioreactor. Mineral fibers coated with extracellular proteins may serve as support for hepatic cells in the bioreactor.
References
- Allen J.W., Hassanein T., Bhatia S.N. Advances in bioartificial liver devices. Hepatology 2001; 34: 447–455
- Park J.K., Lee D.H. Bioartificial liver systems: current status and future perspective. J Biosci Bioeng 2005; 99: 311–319
- Sullivan J.P., Gordon J.E., Palmer A.F. Simulation of oxygen carrier mediated oxygen transport to C3A hepatoma cells housed within a hollow fiber bioreactor. Biotechnol Bioeng 2006; 93: 306–317
- Ness P.M., Cushing M.M. Oxygen therapeutics: pursuit of an alternative to the donor red blood cell. Arch Pathol Lab Med 2007; 131: 734–741
- Spahn D.R. Blood substitutes. Artificial oxygen carriers: perfluorocarbon emulsions. Crit Care 1999; 3: R93–97
- Kinasiewicz A., Werynski A. C3A hepatocytes adhesion to culture surface modified by collagen and heparin. Lekar a Technika 2006; 36: 127–131
- Catapano G., De Bartolo L., Lombardi C.P., et al. The effect of oxygen transport resistances on the viability and functions of isolated rat hepatocytes. Int J Artif Organs 1996; 19: 61–71
- Catapano G., De B.L. Combined effect of oxygen and ammonia on the kinetics of ammonia elimination and oxygen consumption of adherent rat liver cells. Int J Artif Organs 2002; 25: 151–157
- Flendrig L.M., la Soe J.W., Jorning G.G., et al. In vitro evaluation of a novel bioreactor based on an integral oxygenator and a spirally wound nonwoven polyester matrix for hepatocyte culture as small aggregates. J Hepatol 1997; 26: 1379–1392
- Gerlach J.C., Encke J., Hole O., et al. Bioreactor for a larger scale hepatocyte in vitro perfusion. Transplantation 1994; 58: 984–988
- Salehi P., Mirbolooki M., Kin T., et al. Ameliorating injury during preservation and isolation of human islets using the two-layer method with perfluorocarbon and UW solution. Cell Transplant 2006; 15: 187–194
- Yamamoto N., Konishi Y., Wakashiro S., et al. Seventy-two-hour preservation of porcine liver by continuous hypothermic perfusion with UW solution in comparison with simple cold storage. J Surg Res 1991; 51: 288–292
- Brandhorst D., Iken M., Brendel M.D., et al. Successful pancreas preservation by a perfluorocarbon-based one-layer method for subsequent pig islet isolation. Transplantation 2005; 79: 433–437
- Noguchi H., Ueda M., Nakai Y., et al. Modified two-layer preservation method (M-Kyoto/PFC) improves islet yields in islet isolation. Am J Transplant 2006; 6: 496–504
- Nieuwoudt M.J., Moolman S.F., Van Wyk K.J., et al. Hepatocyte function in a radial-flow bioreactor using a perfluorocarbon oxygen carrier. Artif Organs 2005; 29: 915–918
- Nyberg S.L., Remmel R.P., Mann H.J., et al. Primary hepatocytes outperform Hep G2 cells as the source of biotransformation functions in a bioartificial liver. Ann Surg 1994; 220: 59–67
- Pickering C.S., Watkins R.H., Dickson A.J. Rat primary hepatocytes and H4 hepatoma cells display differential sensitivity to cyclic AMP at the level of expression of tyrosine aminotransferase. Biochem Biophys Res Commun 1998; 252: 764–769
- Wilkening S., Stahl F., Bader A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab Dispos 2003; 31: 1035–1042
- Michal, G. (1999). Carbohydrate metabolism and citrate cycle. In Biochemical Pathways: An Atlas of Biochemistry and Molecular Biology. John Wiley & Sons Inc, pp. 27–45. Spektrum Akademischer Verlag.
- Khattak S.F., Chin K.S., Bhatia S.R., et al. Enhancing oxygen tension and cellular function in alginate cell encapsulation devices through the use of perfluorocarbons. Biotechnol Bioeng 2007; 96: 156–166