Abstract
In this work, pectinesterase isolated from Malatya apricot was immobilized onto acid-treated bentonite surface by simple adsorption at pH 9.0. The properties of free and immobilized enzyme were defined. The effect of various factors such as pH, temperature, heat, and storage stability on immobilized enzyme were investigated. Optimum pH and temperature were determined to be 9.0 and 50°C, respectively. Kinetic parameters of the immobilized enzyme (Km and Vmax values) were also determined as 0.51 mM of the Km and 14.6 µmol min−1 mg−1 of the Vmax. No drastic change was observed in the Km value after immobilization. The Vmax value of immobilized enzyme was 8.4-fold bigger than those of free enzyme. Thermal and storage stability experiments were carried out. The patterns of heat stability indicated that the immobilization process tends to stabilize the enzyme. The properties of the immobilized enzyme were compared to those of the free enzyme.
INTRODUCTION
Immobilized enzymes are currently the subject of considerable research because of their advantages over soluble enzymes, especially in industrial preparations. Because free enzymes have limited industrial utilization, reusability and low recovery yield, it has been great attention for enzyme immobilization on solid supports Citation[1], Citation[2]. The other advantages of immobilized enzymes over free enzymes were the possibility of running enzymatic reactions continuously, rapid termination of reactions, controlled product formation, ease of enzyme removal from the reaction mixture (thus saving the time of a purification step for removing the enzyme from the product system], adaptability to various engineering designs, prolonged activity, and obtainability of a concerted or sequential reaction of several enzymes by the use of mixed or stratified beds Citation[3–5].
Recently, because of their practical applications, there has been renewed interest in immobilization by non-covalent methods, and some enzymes have been immobilized non-covalently on different supports, including bentonit Citation[6], Citation[7], polypropylene powder, amberlite XAD-7, controlled pore glass Citation[8], anion exchange resin Citation[9], porous chitoxan beads Citation[10], Citation[11], and CaCO3 Citation[12]. For preparing the bioreactor, immobilization of enzymes by physical interaction is considered to be the easiest way. The major objectives of immobilization are the economic application of enzyme systems in various industrial and technological processes Citation[6].
There are various methods for immobilization of enzymes Citation[13], Citation[14] and these may be divided into physical methods based on molecular interactions between the enzyme and carrier, and chemical methods based on formation of covalent bonds. Although polymeric systems have largely been employed as carriers of immobilized enzymes Citation[15–17], a great deal of work has also been done on the enzyme-soil interaction, which is also an important natural phenomenon in terrestrial and ecosystems Citation[17]. Thus, the adsorption of proteins and enzymes onto mineral surfaces is a subject of great significance and, therefore, deserves more attention. Realizing the need for a careful investigation on enzyme-mineral interaction, the proposed study undertakes the immobilization of pectin esterase onto bentonite mineral surfaces.
Bentonites possess important and unique properties that give them great commercial value in decolorizing oils, in the manufacture of catalysts, in bonding of molding sands, in the preparation of oil-well drilling muds, and in many other relatively minor uses. It also has widespread use for clarification of edible and mineral oils, paints, cosmetics, and pharmaceuticals Citation[16]. It has been considered a good candidate matrix for enzyme immobilization since it exists naturally in an immobilized form in soil and participates in humification processes Citation[18]. In addition, it has the highest absorbent capacity of any mineral clay, as well as a high adsorptive capacity because it has high surface area.
The enzyme pectinesterase (PE, EC 3.1.1.11) has been found in plants as well as in pathogenic fungi and bacteria and catalyzes the hydrolytic cleavage of the methylester moieties on pectin molecules, resulting in the release of methanol and partially de-esterified pectin.
PE is of significance to the citrus industry since it has been definitively established as the causative agent of juice clarification and gelation of frozen concentrates Citation[19]. Pectinesterase (PE) is well described in higher plants. It has been extracted and/or purified from different sources including acerola Citation[19], pear Citation[20], soursop fruits Citation[21], Citation[22], lemon Citation[23], tomatoes Citation[24–33], oranges and grapefruits Citation[34–37], apples Citation[38–40], and bananas Citation[41], Citation[42]. The properties of PE obtained from different fruits vary; therefore, it is advisable to determine the optimum conditions for the processing of different fruits Citation[43].
The bentonite used was obtained from the west of Turkey (Kütahya). The purpose of this study was to immobilize PE isolated from Malatya apricot fruit pulp onto bentonite by the adsorption method and determine some of its enzymatic properties. The immobilized enzyme was characterized by determining kinetic constants, optimum pH and temperature, thermal and storage stability, and reusability. The properties of the bentonite-immobilized PE enzyme were compared to those of free enzyme.
EXPERIMENTAL
Materials
Apricots used in this study were harvested from the Eastern Anatolia Region region in Malatya, the most important apricot production region in Turkey and in the world, producing 7–10% of the world's table apricots and 80–85% of dried apricots, and purchased at commercial maturity from a local market and stored at −20°C. Bentonite used in this study was obtained from MTA (Ankara, Turkey). Dialysis tubes (12000 Dalton) used for dialysis, pectin, KH2PO4 (%99,0], DEAE-Sephadex were purchased from Sigma Chem. Co. (St. Louis, MO), Pectin, (NH4]2 SO4, Na2HPO4 and H2SO4 were purchased from Fluka (Riedel-de Haën, Sigma-Aldrich Laborchemikalien, Seelze, Germany), Sodium azyde, potassium tartarate, sodium tungstate, sodium molybdate, CuSO4 5H2O, Li2SO4, NaOH, HCI, NaCI were purchased from Merck (Schuchardt OHG, Hohenbrunn, Germany). All chemicals used in this study were of analytical grade and were used without further purification. The absorbance measurements were carried out with Shimadzu UV-Vis-1601 spectrophotometer. pH measurements were carried out with Orion 720A model pH-meter. In this study, we also used Beckman Optima XL-100K model ultracentrifuge, mixed and thermostated water bath (Thermolyne Cimarca) and vortex (Nüve N 110).
Methods
Extraction and Partial Purification of Malatya Apricot Pectinesterase
For the apricot PE extraction initially, 500 g Malatya apricots were stored at −20°C during one week and kept at +4°C for dissolving the frosted fruit. Then, the apricots were halved, cored and weighed (465 g.). Apricot tissue was homogenized with 931 mL of 2N NaCI by using a Waring blender for 5 min and homogenate was filtered with cheese cloth. The homogenate, called crude enzyme extract, was centrifuged at 24.000×g for 30 min at 4°C. The enzyme solution from the supernatant was fractionated with solid ammonium sulfate and the precipitate of 40- 80% saturation was collected by centrifugation at 24.000×g for 30 min at 4°C. In the ammonium sulfate precipitation, the addition of solid ammonium sulfate was carried out slowly by completely dissolving it after each addition during one hour. The pellet was redissolved in 120 ml homogenization buffer (0.2 M sodium phosphate buffer, pH 7.5) and dialyzed at 4°C against the same buffer in cellulose dialysis tubing (mol. wt. cut off 12000–14000 Da). Dialysis buffer was changed five times with 8 hrs intervals. The dialyzed solution was kept in stoppered test tubes at −20°C. The partially purified enzyme was used for the characterization of apricot pectinesterase enzyme.
Immobilization Procedure
Preparation of Acid-treated Bentonite
The chemical composition of bentonite is given in . Before bentonite was used to immobilize pectinesterase enzyme, it was mixed in 50 ml 3 M H2SO4 for 2 h, filtered and washed several times with distilled water, then dried at room temperature for a week.
Table 1. Chemical composition of bentonite
Immobilization of Enzyme
For the immobilization of pectinesterase enzyme isolated from Malatya apricot on bentonite, 5.0 g of acid-treated bentonite was suspended in 15 ml of pectinesterase isolated from Malatya apricot (Prunus armeniaca L.) after it was diluted at 1:1 ratio with 0.2 N sodium phosphate buffer (pH 7.5) solution. After mildly stirring for 90 min at 4°C, the suspension was centrifuged at 12,000 rpm at room temperature for 15 min and precipitate was washed three times with 0.2 N sodium phosphate buffer solution (pH 7.5). For each centrifugation procedure, the supernatant was analyzed for remaining pectinesterase activity by using our modified method proposed by Versteeg et al. Citation[44] as described above. The protein assay of the each supernatant was carried out by the Lowry method Citation[45]. The amount of adsorbed protein was calculated from the difference between the amount of protein introduced into the reaction mixture and the amount of protein in the filtrate and washing solutions after immobilization.
Protein Determination
Protein concentrations were determined by the method of Lowry et al. Citation[45] by using bovine serum albumin as standard. The amount of bound protein was calculated as mentioned above.
Assay of Enzyme Activity
Pectinesterase activity was determined manually by using our modified method proposed by Versteeg et al. Citation[44]. Concisely, the method involves the measurement of the releasing rate of carboxyl groups in a pectin solution in 0.1 N NaCl (1% w/v) at room temperature. Pectin (%1) in 0.1 N NaCl (1% w/v) was prepared and stored according to the procedure described by Rouse and Atkins Citation[46]. The reaction was started by the addition of 0.05 g bentonite-immobilized pectinesterase dissolved in 0.5 mL sodium phosphate buffer (pH 7.5) enzyme to 4 ml of%1 pectin solution in 0.1 N NaCl (1% w/v) and the reaction pH was adjusted and maintained manually at desirable pH of the enzyme by the addition of 0.1 and/or 0,01N NaOH solution. After the pH was fixed, 0.2 ml 0.01N NaOH was added and provided a sudden increase in pH. The returning “time” to adjusted pH from suddenly increased pH is recorded. PE activity was calculated by the following formula Citation[19], Citation[47], Citation[48]:
The method was carried out at various temperatures and pH values with pectin substrate. One unit of PE activity was defined as the amount of enzyme that released 1 µmol of carboxyl group produced in min. at room temperature. Experiments were triplicated.
Effect of pH on Immobilized Enzyme Activity
The free and immobilized immobilized pectinesterase activities as a function of pH were determined. 0.05 g enzyme dissolved with 0.5 mL 0.2 N phosphate buffer (pH 7.5) was added into 4 mL 1% pectin in 0.2 N NaCl. The pH influence was studied manually with 0.2 mL 0.01N NaOH after adjusting the pH of the reaction solution to one of the pH values [4–9.5) tested. The optimum pH value obtained from these assays was used in all the other experiments.
Effect of Temprature on PE Activity
The temperature effect on the activities of free and bentonite-adsorbed enzyme was tested manually at 1% pectin concentration at pH 9.0 and PE activity as a function of temperature under standard assay conditions were determined by using temperatures from 10 to 90°C. The temperatures were controlled by means of a circulating water bath. At a specific temperature, for determining relative activity of both PEs, 0.05 g enzyme dissolved with 0.5 mL 0.2 N phosphate buffer (pH 7.5) was incubated for 15 min at different temperatures in circulating water bath without substrate. The pectin (1%) was added after cooling.
Thermal Stability of Apricot PE
Thermal stability of free and immobilized pectinesterase was performed between 60 and 90°C temperatures manually under standard assay conditions by measuring the residual activity of the enzyme in 0.2M phosphate buffer (pH 7.5). 0.05 g enzyme dissolved with 0.5 mL 0.2 N phosphate buffer (pH 7.5) in three different test tubes was incubated during 45 min. in a circulating water bath at the specified temperature. After each of the sample portions was withdrawn at various time intervals during 45 min, and cooled, it was added into 1% pectin residual activity assayed for various time intervals (15–45 min). The stability of the enzyme was expressed as remaining activity. The data obtained from the thermal stability profile were used to analyze some thermodynamic parameters related to apricot PE activity.
Determination of Activation Energy (Ea)
For free and immobilized Malatya apricot PE, activation energy (Ea) was calculated from the slope of the Arrhenius plot obtained by plotting the logarithm of reaction rate constants (ln k) vs. the reciprocal of the absolute temperature (1/T). Thermal inactivation rate constants were calculated by comparing the activity changes upon heat treatment with the unheated enzyme extracts as reported by Amiza and Apenten Citation[49].
Kinetic Properties
To determine Km and Vmax constants of free and immobilized pectinesterases, the activities were measured for both enzymes in the presence of various substrate concentrations (0.05, 0.075, 0.125, 0.25, 0.5, 0.75, 1 mM). Michael-Menten constant (Km) values and the maximum velocities (Vmax) were determined using the Lineweaver- Burk double reciprocal plot Citation[50], in which the reciprocals of the initial velocities of the pectinesterase activity were plotted against the reciprocals of the concentration of pectin used.
RESULTS AND DISCUSSION
Extraction and Partial Purification of PE
In this study, initially, pectinesterase enzyme was isolated and partially purified pectinesterase from Malatya apricot pulp by using 2M NaCl prepared with 0.2M sodium phosphate buffer (pH 7.5). In the isolation and partially purification procedure, it was carried out homogenization with NaCl, ultrafiltration at 4°C, saturation with ammonium sulphate and dialysis, respectively. For adjusting ionic strength of the solution, the optimum concentration of NaCl used in this study was similar to that found by us in extracting pectinesterase from apricot pulp Citation[51]. The precipitate obtained from 40–80% ammonium sulphate fractionations of crude extract of 420 g of apricot pulp was dissolved and dialysed with 0.2 M sodium phosphate buffer (pH 7.5).
The activity and protein amounts obtained after purification procedure are seen in . 5.2 and 12.2-fold purification were obtained with 80% saturation of solid ammonium sulphate and dialysis, respectively. Dialysis membrane is used to remove salts coming from ammonium sulphate saturation, organic solvents and inhibitor of low molecular weight used in purification procedures from enzyme solution. Enzyme activity, in general, affects some factors such as temperature, pH, enzyme and substrate amounts. The effect of these parameters on the enzyme was investigated by determining of enzyme stability and its optimum conditions in our study. The specific activity and protein concentration of the free enzyme are 62.06 IU/mg protein and 23.5 mg/ml, respectively, because the highest enzyme activity was obtained with dialysed enzyme. Enzyme immobilization was performed with the dialyzed enzyme solution obtained from ammonium sulfate fractionation.
Table 2. Purification of pectinesterase enzyme isolated from Malatya apricot
Properties of Bentonite Support
Bentonite is a readily, abundantly available, inexpensive clay mineral in Turkey. It is a very suitable enzyme immobilization matrix because of existing as an immobilized form in soil, its wide surface area and its highest adsorptive capacity Citation[18]. For a long time, its low density and high specific surface area advantages over other minerals have been accepted. In the literature, several enzymes are immobilized on bentonite. Catalase Citation[52], polyphenol oxidase isolated from quince Citation[18], diastase Citation[7], aspartase to determine aspartic acid in various media Citation[53] immobilizations onto bentonite clay surfaces were carried out. Dong et al. Citation[54] made a kinetic analysis of clays modified with enzymes. Futhermore, Ito et al. Citation[55] studied the adsorption of certain proteins, obtained by fermentation, on silica gels.
Two of the most attractive properties are the simplicity of the method and the retention of activity during immobilization. Furthermore, this method is a very economical procedure for the immobilization of enzymes. The stability of the adsorbed enzyme derivative will depend on the strength of the noncovalent bonds formed between the support and the amino acid residues on the surface of the protein. Two principal types of bonds can be formed between the enzyme and the inorganic support: electrostatic bonds and hydrogen bonds. The main disadvantage of this method is the weak binding of enzymes.
In the present study, partially purified PE enzyme isolated from Malatya apricot pulp was immobilized on bentonite by adsorption. Increasing the amount of enzyme caused a decrease in activity. This has been explained by “crowding” of the enzyme on the surface of bentonite. At higher enzyme concentrations, the enzyme activity after immobilization was increased about 7-fold. The immobilized enzyme used in the present work had immobilization yield of 90.2% ().
Table 3. Immobilization of PE from Malatya apricot on bentonite
Effect of pH and Temperature on PE Activity
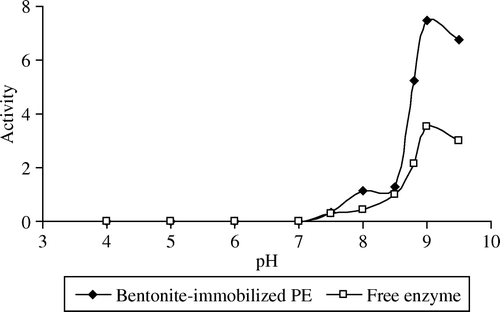
The activities of free and immobilized pectinesterase were determined at different pH values between 1 and 10 by using 1% pectin in 0.1 N NaCl (w/v). The optimum pH values of free and immobilized pectinesterase were determined from the graph of pH plotted against activity (). As seen in , the optimum pH of bentonite-immobilized PE was 9.0. On both sides of the optimum pH, the amounts of free and immobilized pectinesterase activities decrease. There is no shift from the optimum pH of the free enzyme. It has been reported that soil clay systems such as bentonite have ion-exchange properties because of their permanent and pH dependent surface area. Permanent charge is negative due to isomorphous cation substitution in the lattice structure of bentonite. The pH dependent charge is directly or indirectly dependent on the reaction of the bentonite to the presence of amorphous colloids such as SiO2 and Fe2O3 oxides Citation[56].
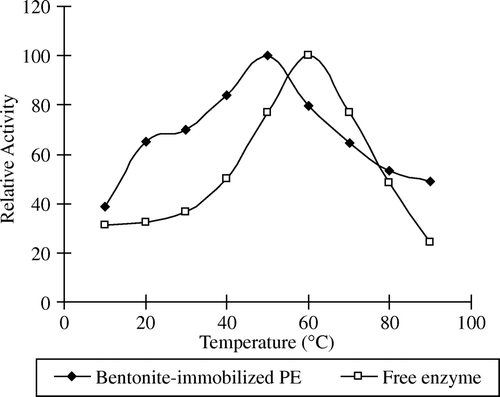
The optimum temperature for maximum free and immobilized PE activity was determined by using 1% pectin substrate in 0.1N NaCl (w/v) at nine different temperatures between 10 and 90°C. The maximum catalytic activity at 60°C for free enzyme and 50°C for immobilized enzyme was obtained (). The stability of free enzyme reduces more rapidly compared to those of the immobilized form against the temperature increases. The free and immobilized pectinesterases lost 65% and 75% of their optimum activities at 80°C. Above optimum temperature of both enzymes, the activity remained 25% and 50% of free and immobilized enzyme at 90°C, respectively.
Although the optimum temperature of the free enzyme is higher than that of the immobilized enzyme, the immobilized enzyme is importantly more thermostable than the free one. The immobilization procedure probably helps to maintain the structure of the free pectinesterase enzyme. The stability of immobilized pectinesterase is significantly improved over those of the free form at lower and higher temperatures. The mobility of the pectinesterase molecules probably increases with increasing temperature since the immobilization is normally a diffusion controlled process. This situation can cause increased immobilization efficiency. At higher temperature, the electrostatic attractions are normally weakened and, therefore, a lower immobilization should be expected Citation[7].
Thermal Inactivation of Free and Immobilized Apricot PE
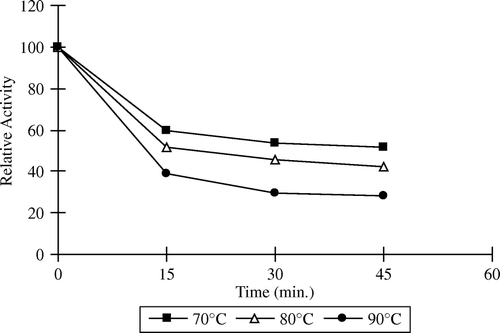
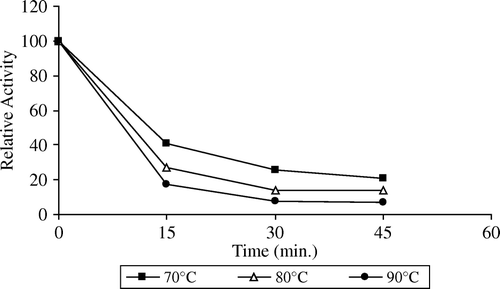
Thermal inactivation profiles of free and immobilized pectinesterase between 70 and 90°C are shown in and . As seen in and , apricot PE enzyme activity slightly changed at 70°C after 15 min. Increasing the temperature from 70°C to 90°C showed little decrease in activity after 15 min. The free and immobilized enzyme retained 25% and 60% activity, respectively, at 70°C after 45 min incubation period. Free and immobilized pectinesterase enzyme retained 8% and 30% the original activity at 90°C for 45 min, respectively. At all temperatures, the immobilized enzyme was inactivated at a much slower rate than the free form. The free enzyme lost on a large scale its initial activity at 90°C after 45 min. From these results, it was expressed that the structure of enzyme is protected by immobilizing onto support against thermal treatment. The resistance of immobilized pectinesterase against temperature is an important potential advantage for practical applications of this enzyme. It was observed that the immobilized enzyme had storage stability for a period of one year but had no reusability in the batch process.
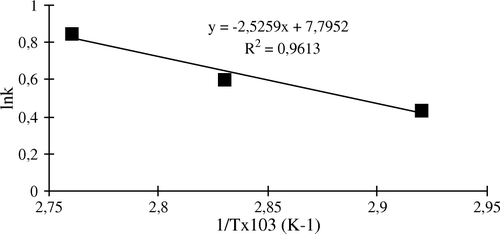
At each temperature, the rate constants for thermal inactivation k were calculated from the slope of the lineer part of the curve. The temperature dependence of k was evaluated by using the Arrhenius equation. From a plot of ln k-1/T (), Ea (activation energy) was calculated from the slope of the straight line and found to be 5.03 kcal mol−1.
The inactivation rate constant and the half-lives (t1/2) of free and immobilized apricot PE were calculated and represented in . As seen in , the inactivation rate constant of the free enzyme is bigger than those of the immobilized one while the half-life of the immobilized PE is longer than the free ones. If the inactivation rate constant is higher, an enzyme is less thermostable Citation[57]. From this reason, as seen in the table, the enzyme is less stable at higher temperature. It is seen in that the increase in the temperature causes the decrease in t1/2 values.
Table 4. Inactivation rate constant and half-lives (t1/2) of free and immobilized apricot PE
Kinetic Parameters of PE
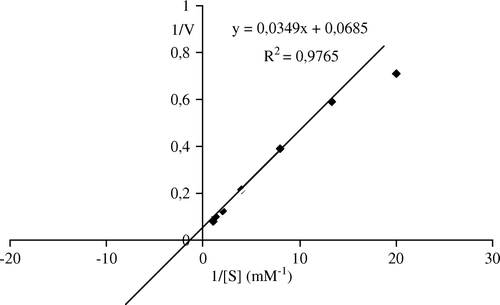
Kinetics of the immobilized pectinesterase isolated from Malatya apricot were calculated from Lineweaver–Burk plots ( and ). The Km was 0.51 mM and Vmax was 14.6 µmol min−1 mg−1 while the Km and Vmax values of free enzyme were 0.77 and 1.75, respectively. The Km values for both free and immobilized pectinesterase is nearly the same, but Vmax value of immobilized enzyme is importantly increased. The results indicated that the adsorption of pectinesterase enzyme provides it to react more quickly. The Km of 4×10−2 mM and Vmax of 15 mmol h−1 mg−1 for bentonite-immobilized lipase for olive oil was reported Citation[6]. Alkan et al. Citation[52] calculated the value of Km of 13.90 mM and the Vmax of 65.78 µM/min for free catalase; the Km of 13.22 mM and the Vmax of 55.86 µM/min were stated for the bentonite-immobilized catalase.
Figure 6. Lineweaver-Burk plots of apricot PE activity as a function of substrate concentration. Reaction mixtures contained varying levels of pectin (0.05–1 mM) in 0.1M NaCl (w/v) and 0.4 ml of partially purified enzyme. The pH of the reaction was 9.0 and the temperature was 30°C.
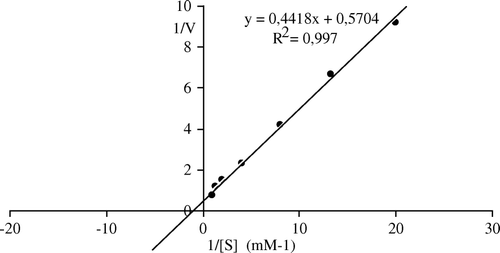
Figure 7. Lineweaver-Burk plots of bentonite-immobilized Malatya apricot PE activity as a function of substrate concentration. Reaction mixtures contained varying levels of pectin (0.05–1 mM) in 0.1M NaCl (w/v) and 0.05 g of patially purified enzyme. The pH of the reaction was 9.0 and the temperature was 30°C.
Storage Stability Studies
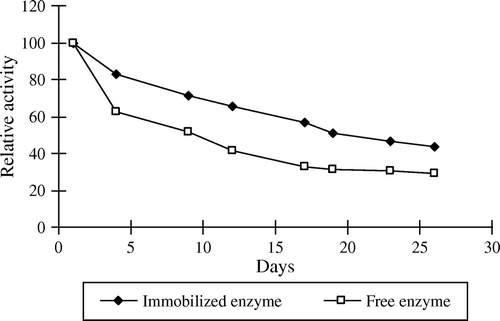
The free and immobilized pectinesterase was stored at 4°C and the enzyme activity was measured for a period of 30 days. Results are shown in . In the first four days, the 65% and 85% of enzyme activity were retained for free and immobilized enzymes, respectively. The free enzyme lost 60% of its initial activity and immobilized enzyme retained 50% of its initial activity within 30 days.
CONCLUSION
In this study, acid-treated bentonite was prepared and used as a support for the immobilization of pectinesterase isolated from Malatya apricot (Prunus armeniaca L.). The proposed immobilization procedure is very simple to carry out. The immobilized enzyme also showed good properties that are very important parameters in applications. The pH value for optimum activity of pectinesterase was not affected by the immobilization reaction. The maximum enzyme activity as a function of temperature for the immobilized PE was shown at 50°C and the thermal stability of the enzyme was increased by immobilization. The bentonite showed the desired properties for immobilization of bioactive macromolecules. The cheap and abundant bentonite support material could also be considered for large-scale immobilization of enzymes.
References
- Maechiling-Strasser C., Dejardin P., Galin J.C., Schmitt A. J. Biomed Mater Res. 1989; 23: 1385
- Kondo A., Oku S., Murakami F., Higashitani K. Colloids Surf. 1993; 5: 197
- Zaborsky, O.O., Weast, R.C (1973). Immobilized Enzyme. CRC Press, Cleveland, OH, p.1.
- Axen, R. (1974). In Salmona, M., Soronio, C., Garattini, S Immobilized Enzyme, 9.Raven Press, NewYork.
- Katchalski, E.K. (1982). In Weetall, H.H., Royer, G.P Enzyme Engineering. Plenum Press, NewYork, p.12.
- Yeşiloğlu Y. Process Biochemistry 2005; 40: 2155–2159
- Bajpai A.K., Sachdeva R. Colloid Polym Sci. 2002; 280: 892–899
- Haas M.J., Scott K., Jun W., Janssen G. J Am Oil Chem Soc. 1991; 71: 483–90
- Yesiloglu Y. J Am Oil Chem Soc. 2004; 81: 157–60
- Magnin D., Dumitriu S., Magny P., Chornet E. Biotechnol Prog. 2001; 17: 734–41
- Pereira E.B., Castro H.F., Moraes F.F, Zanin G.M. Appl. Biochem. Biotechnol. 2001; 91: 739–52
- Rosu R., Uozaki Y., Iwasaki Y., Yamane T. J Am Oil Chem Soc 1997; 74: 445–50
- Chibata I. Immobilized Enzyme. Kodansha Ltd, London 1978, Halsted Press
- Bunga H.R. Immobized Bas, Basic Biochemical Engineering. Bilin Associate, New Delhi 1989
- Moustafa A.B., Kahil T., Faizalla A. J. Appl. Polym Sci. 2000; 76: 594
- Takami H., Abika T., Horikoshi K. Appl. Microbiol Biotechnol. 1989; 30: 120–124
- Quiquampoix H., Chassin P., Ratcliffe R.G. Progr Colloid Polym Sci. 1989; 79: 59
- Yağar H., Sağiroğlu A. Turk J Chem. 2002; 26: 751–758
- Assis S.A., Martins A.B.G., Oliveira O.M.M. Journal of the Science of Food and Agriculture 2007; 87: 1845–1849
- Contreras-Esquive J.C., Correa-Robles C., Aguilar C.N., Rodriguez J., Romeroc J., Hoursd R.A. Food Chemistry 1999; 65: 153–156
- Arbaisah S.M., Asbi B.A., Junainab A.H., Jamilah B. Food Chemistry 1996; 55: 289–292
- Arbaisah S.M., Asbi B.A., Junainab A.H., Jamilah B. Food Chemistry 1997; 59: 3340
- MacDonald H. M, Evans R.E., Spencer W.J. J. Sci Food Agric. 1993; 62: 163–168
- Whitaker J.R. Enzyme Microb Technol. 1984; 6: 341–349
- Giovane A.L., Quagliuolo L., Servillo C., Balestrieri B., Laratta R., Loiudice D., et al. J Food Biochem. 1994; 17: 339–349
- Tijskens L.M.M., Rodis P.S., Hertog M.L.A.T.M., Proxenia N., Van Dijk C. J Food Eng. 1999; 39: 167–177
- Delince H., Radola B.J. Biochem Biophys Acta. 1970; 214: 178–189
- Gaffe J., Tieman D.M., Handa A.K. Plant Physiol. 1994; 105: 199–203
- Markovic O., Jornvall O.H. Eur Biochem. 1986; 158: 455–462
- Pressey R., Woods F.M. Phytochemistry 1992; 31: 1139–1142
- Tucker G.A., Robertson N.G., Grierson D. J Sci Food Agric. 1982; 33: 396–400
- Warrilow A.G., Turner R.J., Jones M.G. Phytochemistry 1994; 35: 863–868
- Warrilow A.G., Jones M.G. Phytochemistry 1995; 39: 277–282
- Seymour T.A., Preston J.F., Wicker L., Lindsay J.A., Marshall M.R. J Agric Food Chem. 1991; 39: 1080–1085
- Ingallinera B., Barbagallo R.N., Spagna G., Palmeri R., Todaro A. Enzyme and Microbial Technology 2005; 36: 258–263
- Versteeg C., Rombouts F.M., Pilnik W. Lebensm Wiss Technol 1978; 1: 267–274
- Robertson G.L. J Sci Food Agric 1976; 27: 261–265
- Denes J.M., Baron A.A., Drilleau J.F. J Sci Food Agric. 2000; 80: 1503–1509
- King K. Int J Food Sci Technol. 1990; 25: 188–197
- King K. J Sci Food Agric. 1991; 57: 43–48
- Brady C.J. Aust J Plant Physiol. 1976; 3: 163–172
- Markovic O., Heinrichova K., Lenkey B. Collect Czech Chem Commun. 1975; 40: 769–774
- Fayyaz A, Asbi B.A., Ghazali H.M., Che Man Y.B., Jinap S. Food Chem. 1995a; 53: 125–129
- Versteeg C., Rombouts K.M., Spaansen C.H., Pilnik W. J Food Sci. 1980; 45: 969–971
- Lowry H., Rosebrough N.J., Farr A.L., Randall R.J. J Biol Chem. 1951; 193: 265
- Rouse, A.H. and Atkins, C.D. (1955). Fla. Agr. Expt. Sta. Bull 570.
- Assis S.A., Lima D.C., Oliveira F. Food Chem. 2000; 71: 465–467
- Assis S.A., Martins A.B.G., Quaguianoni D.G., Oliveir O.M.M.F. J Agric Food Chem. 2002; 50: 4103–4107
- Amiza M.A., Apenten R.K.O. Journal of the Science of Food and Agriculture 1994; 66: 389–391
- Lineweaver H., Burk D. J. Am. Chem Soc. 1934; 56: 658
- Fayyaz A., Asbi B.A., Ghazali H.M., Che Man Y.B., Jinap S. Food Chem. 1993; 49: 373–378
- Alkan S., Ceylan H., Arslan O. J. Serb. Chem Soc. 2005; 70(5)721–726
- Naidja A., Huang P.M., Bollog J.M. J Molecular Catalysis 1997; 115: 305–316
- Dong X.D., Lu J., Cha C. J Electroanal Chem. 1995; 2: 195–201
- Ito M., Yamauchi K., Matsuzawa K. Collloids and Surfaces. 1993; 74: 1
- Chichester F. W., Harward M. E., Youngberg C. T. Clays and Clay Minerals 1970; 18: 81–90
- Marangoni A.G. Enzyme Kinetics: A Modern Approach. New Jersey USA: John Wiley & Sons. 2003; 140–157