Abstract
Hypoxic tumors are significantly more malignant, metastatic, radio- and chemoresistant. The use of artificial oxygen carriers represents a new approach to the problem of hypoxia. In the present study, female athymic BALB/c nude mice bearing the cervical carcinoma were untreated or treated with cisplatin to determine whether administration of artificial oxygen carrier (PEG-conjugated Hemoglobin, PEG-Hb) could improve the tumor oxygenation and enhance the anti-tumor efficacy of cisplatin. Pimonidazole staining was employed to detect tumor tissue oxygenation status. We found that the application of a higher dose (0.6 g/kg) PEG-Hb could significantly ameliorate the hypoxic condition in cervical carcinoma xenograft models. Co-administration of PEG-Hb (0.6 g/kg) with cisplatin produced significant tumor growth inhibition and pro-apoptotic and anti-proliferative effects as compared to cisplatin alone. These suggest the evaluated PEG-Hb in this experiment has positive effects on cisplatin or cisplatin-based chemotherapy, and further work to optimize its application is warranted.
INTRODUCTION
The presence of hypoxia in solid tumors has been recognized for more than 50 years. Hypoxic cells are more resistant to standard chemotherapy and radiotherapy, are more invasive and metastatic, resistant to apoptosis, and genetically unstable Citation[19]. Therefore, hypoxia has a key negative role in tumor prognosis, both because it causes resistance to standard therapies and because it promotes a more malignant phenotype. So it is then not surprising that hypoxia has been considered an attractive target for the development of novel anti-cancer therapies, including prodrugs activated by hypoxia, hypoxia-specific gene therapy, targeting the hypoxia-inducible factor-1(HIF-1α) transcription factor, and recombinant anaerobic bacteria Citation[3]. However, to date, most of these strategies are still in preclinical or early clinical development. The potential to improve local control and survival by hypoxia modification was demonstrated by a meta-analysis of 83 clinical trials Citation[21] and a number of therapeutic strategies have also been established to overcome tumor hypoxia by improving oxygen supply either by oxygen or carbogen breathing or by increasing the hemoglobin level and oxygen delivery Citation[13], Citation[15]. The use of artificial oxygen carriers represents a new approach to solving the problem of hypoxia.
Previous studies have demonstrated that intravenous administration of ultrapurified polymerized bovine hemoglobin solution was effective in increasing the oxygenation throughout experimental tumors under normal air breathing conditions Citation[24], Citation[33], Citation[35]. Therefore, an enhancement of tissue oxygenation (tpO2) in rodent tumors associated with an increased tumor growth delay was reported for purified bovine hemoglobin solutions combined with irradiation Citation[24], Citation[28], Citation[30], Citation[32], as well as with chemotherapeutic agents Citation[30–32], Citation[34] such as carmustine (BCNU), cyclophosphamide, ifosfamide, adriamycin, TNP-470, minocycline, melphalan. When carbogen breathing was added to administration of the hemoglobin preparations, further increased therapeutic response and decreased tumor hypoxia were achieved. Various modified hemoglobin prepared from bovine, human, or mouse Hb, for example the polyethyleneglycol-conjugated bovine hemoglobin, could also increase tumor oxygen content Citation[18], Citation[20] and improve the effectiveness of radiotherapy in rodent models Citation[20]. Perhaps spurred by these encouraging results, a clinical phase I/II study on the effect of polyethyleneglycol-conjugated hemoglobin (Enzon Corp., USA) for radiosensitization of tumors has been performed Citation[26].
The present study was designed to determine whether co-administration of polyethyleneglycol-conjugated hemoglobin (PEG-Hb) and cisplatin could alter the extent of oxygenation in cervical carcinoma xenograft model and influence the response to chemotherapy.
MATERIALS AND METHODS
Polyethyleneglycol-conjugated Hemoglobin (PEG-Hb)
PEG-Hb used in this study was a Chinese domestic sample. Its preparation processes and physiochemical properties belong to the manufacturer's proprietary information and will not be discussed in this paper. The sample was kept at 4°C in the dark until use.
Tumor Cell Line
HeLa cells were obtained from the Cell Bank of the Chinese Academy of Medical Sciences, Beijing, China. The cell line was maintained and propagated in RPMI-1640 (Gibco, Life Technologies, Vienna, Austria) supplemented with 10% heat-inactivated fetal bovine serum (Gibco) and 100 U/ml penicillin + 0.1 mg/ml streptomycin in 75 cm2 tissue plastic flasks (Corning, USA). Cells were maintained at 37°C in a humidified 5% CO2 atmosphere.
In Vivo Experiments with Subcutaneous Tumor
Female athymic BALB/c nude mice, 5 weeks old, weighing 19–23 g at the start of the study, were implanted subcutaneously with 0.2 ml 5×106 HeLa cells into right flank. Mice were housed at SPF laboratory in accordance with state guidelines for humane treatment and care of the laboratory animals. Mice received food and water ad libitum. Treatment began when tumors reached a volume of 60–80 mm3. Animals were randomly assigned to five groups (n = 10 per group). Physiological saline (group 1), cisplatin(5mg/kg, group 2), cisplatin with PEG-HB at dose levels of 0.3 g/kg (group 3), 0.6 g/kg (group 4), or PEG-HB alone at dose level of 0.45 g/kg (group 5) was administered to each group intravenously, twice a week, for four weeks. Tumor size was calculated using the equation (l×w2)/2, where l and w refer to the larger and smaller dimensions collected at each measurement. Efficacy was measured as percent tumor growth inhibition (TGI) relative to saline-treated group. TGI is calculated by the equation [1-(T/C)]×100, where T and C represent the mean tumor mass on the last day of therapy in the treatment (T) and saline control (C) groups, respectively. The general health of mice was monitored daily. Tumor dimensions and body weights were recorded two to three times a week starting with the first day of treatment.
Immunohistochemistry
On the last day of therapy, tumors were removed, weighed and fixed in 10% formalin and embedded in paraffin for immunohistochemical (IHC) analysis. 5µm sections were deparaffinized and rehydrated in a graded series of ethanol following standard protocol. Sections were treated with 3% H2O2 for 10 min to eliminate endogenous peroxidase activity, and then immunostaining of tumor sections (n = 10 tumors in each group) was performed with an anti-PCNA antibody (Zymed Laboratories) diluted 1:100 for determination of the proliferation index in HeLa tumors. After incubation for 30 min at room temperature they were exposed to biotinylated secondary antibodies for 10 min. Antigen-antibody complexes were visualized using the substrate-chromogen mixture (Zymed Laboratories Inc., San Francisco, CA) and counterstained with hematoxylin. Omission or substitution of the primary antibody with preimmune serum was used as a negative control. Immunoreactivity was assessed by the intensity and percentage of positive staining. Staining intensity was scored as 0 (negative), 1 (faint), 2 (moderate), and 3 (strong) positive staining in the ranges of ≤25%, 26%∼50%, 51%∼75%, >75% were assigned scores of +1, +2, +3, and +4. The cumulative scores of each group was calculated and averaged for using as descriptors.
The extent of apoptosis in the tumors was measured by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining using the In Situ Cell Death Detection Kit (Roche Applied Science, Penzberg, Germany), following the manufacturer's protocol. This system end-labels the fragmented DNA of apoptotic cells. Omission of the enzyme in the TUNEL reaction was used as a negative control and cells treated with DNase I were used as a positive control. The number of TUNEL-positive cells was counted in five different fields under a light microscope at ×200 magnification.
Tumor hypoxia was determined using Hypoxyprobe™-1 Kit for the Detection of Tissue Hypoxia (Chemicon International, Temecula, CA) in which, 1 h before tumor collection, mice were injected intravenously with 60 mg/kg pimonidazole hydrochloride. Then the formalin-fixed, paraffin-embedded tumor sections were incubated with 150 µl of a 1:50 dilution of Hypoxyprobe™-1 Mab1, and biotin-conjugated F(ab’)2 (1:500) was used to reveal Hypoxyprobe™-1 adducts in the hypoxic tumor tssues. Multiple biopsies were taken per tumor and one section was stained per biopsy. Each tumor was scored semi-quantitatively section by section in all cases, using a scoring system where hypoxia in the ranges of 0, >0–5%, >5–15%, >15–30%, >30% were assigned scores of 0, +1, +2, +3, and +4. The cumulative scores of each group was calculated and averaged for using as descriptors.
DATA ANALYSIS
Data were analyzed statistically with one-way ANOVA or with two-tail student's t test. A value of P < 0.05 was considered significant.
RESULTS
Effect of Cisplatin Alone or in Combination with PEG-Hb on Tumor Growth In Vivo
Co-administration of cisplatin and 0.6 g/kg PEG-Hb (group 4) caused a significant inhibition of tumor growth and resulted in a significant reduction of tumor volume in compared with groups at the end of each treatment (P < 0.01; ).
Figure 1. Representative photographs of the harvested tumors from each group and serial changes in pre-established tumor volume during each treatment in athymic mice.
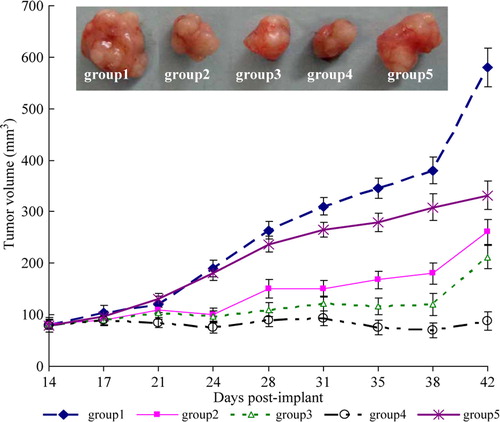
The results in showed that cisplatin alone or in conbination with PEG-Hb exhibited an antitumor effect against the subcutaneous model of human cervical carcinoma when administrated by intravenous administration. The inhibition rate reached 81.1% (group 4) when cisplatin co-administrated with PEG-Hb at a higher dose of 0.6 g/kg, much higher than the anti-tumor activity of cisplatin alone (group 2) as 73.47%. However, co-administration of cisplatin and lower dose (0.3 g/kg) of PEG-Hb (group 3) showed no significant gained anti-tumor efficacy as compared with cisplatin alone. To our surprise, PEG-Hb alone administrated to the tumor-burden mice in group 5 also exhibited slight anti-tumor efficacy at a inhibition rate of 20.54%.
Table 1. Effect of cisplatin, or cisplatin with PEG-Hb, or PEG-Hb alone on HeLa tumor cell growth in nude mice by intravenous administration (twice a week for four weeks)
On the other hand, cisplatin showed considerable side effects as indicated by negative increase of animal's body weight in groups 2, 3, and 4. When administrated in combination with PEG-Hb at the indicated doses, cisplatin led to more pronounced, though not significant, loss in body weight of mice, which indicated that the tested sample PEG-Hb itself may have some side effects.
PEG-Hb Can Ameliorate Tumor Hypoxia
We chose the exogenous hypoxia specific Hypoxyprobe 1 kit that consists of pimonidazole hydrochloride and a mouse monoclonal IgG antibody directed against 2-nitroimidazole reduction products. Pimonidazole immunostaining of hypoxic tumor cells is scored for quantitative assessment and shown in .
Table 2. Pimonidazole binding scores in cervical cancer xenografts
Hypoxia is a very common phenomenon in cervical cancer models. As shown in , tumors harvested from animals that administrated with saline (group 1) or cisplatin (group 2) without the oxygen-carrying agent exhibited more hypoxic as revealed by higher scores of pimonidazole binding. While tumor-burden animals that received oxygen carrier PEG-Hb (groups 3, 4, and 5) demonstrated decreased tumor hypoxia, especially in group 4, which received more PEG-Hb at a higher dose of 0.6 g/kg.
PEG-Hb Can Increase the Therapeutic Response of Cisplatin
Since co-administration of cisplatin and 0.6 g/kg PEG-Hb can cause a significant inhibition of tumor growth and gain an inhibition rate of 81.1%. To further elucidate these performances, a proliferative immunocytochemical marker, the Proliferating Cell Nuclear Antigen (PCNA) and TUNEL assay, were used to determine the proliferative activity and apoptosis status in tumor sections, respectively.
As presented in , the intensity and positive staining of PCNA, a proliferative marker reflect cell proliferative activity in tumors harvested from each group, has significant difference in the five groups. Animals receiving saline or PEG-Hb alone have much more proliferative tumor cells with average PCNA scores over 4, while cisplatin treatment (groups 2, 3, and 4) can significantly down-regulate the expression of PCNA, especially in group 4, which received cisplatin in combination with a higher dose (0.6 g/kg) of PEG-Hb. On the other hand, many more apoptotic cells could be observed in the tumors harvested from cisplatin or cisplatin conbined with PEG-HB treatment groups (P < 0.01). 45.4 percent of nuclei are apoptotic in the case of cisplatin conbined with PEG-Hb treatment in group 4 (P < 0.001).
Table 3. PCNA scores and apoptosis index in tumor sections
DISCUSSION
Cervical cancer is a major health issue worldwide. Each year, approximately 500,000 new cases of cervical cancer are diagnosed, and more than 270,000 deaths result from this disease Citation[22]. In the United States (US), in 2007, an estimated 11,150 cases of cervical cancer are expected to be diagnosed, and every 2.5 h a woman will die of cervical cancer in the US Citation[14]. The treatment of cervical cancer can include surgery, radiation therapy, chemoradiation therapy, or chemotherapy. However, more and more evidence revealed that hypoxia is a common phenomenon in cervical cancers Citation[2], Citation[11], Citation[12], which endows cervical carcinomas significantly more malignant, metastatic, radio- and chemoresistant. To date, a variety of strategies designed to improve tumor oxygenation to overcome resistance to radiation and chemotherapy has been investigated. These efforts, such as hyperoxic gas inhalation Citation[16], VEGF blockade Citation[1], Citation[36], mild hyperthermia Citation[7], respiratory inhibition Citation[27], and Ras inhibition Citation[4], Citation[6], Citation[25] have had only limited success. With the encouraging development of artificial oxygen carriers in recent years, hemoglobin-based oxygen carriers (HBOCs), which initially developed as alternatives for blood transfusion, might be biochemically tailored for specific clinical indications such as cancer chemo- and radiotherapy sensitization Citation[38].
In fact, although Raabe et al. demonstated that low-dose (0.3 g/kg) application of HBOC-201 (a glutaraldehyde-polymerized bovine hemoglobin solution; Biopure, Cambridge, MA) did not improve the response of the rhabdomyosarcoma R1H of the rat to fractionated irradiation Citation[23] since the application of low dose hemoglobin solution HBOC-201 (0.3 g/kg) alone failed to improve tumor tissue or healthy skeletal muscle oxygenation Citation[9], several experimental tumor studies have demonstrated that administration of ultrapurified polymerized bovine hemoglobin solution could result in decreased tumor hypoxia Citation[24], Citation[33], Citation[35] and increased both irradiation Citation[24], Citation[28], Citation[30], Citation[32] and chemotherapeutic response Citation[30–32], Citation[34]. However, several studies have shown that these earlier generations of hemoglobin derivatives induce several undesired effects, including vasoconstriction, hypertension, renal toxicity, and platelet aggregation Citation[5], Citation[37]. Recent development in the field of artificial oxygen carriers based on the modification of hemoglobin have led to the formulation of polyethylene glycol (PEG)-conjugated Hb compounds, which was shown to be free of hypertension and was also shown to release oxygen in an artificial capillary in a manner virtually identical to native RBCs Citation[37]. Some of these kinds of products could also increase tumor oxygen content Citation[18], Citation[20], Citation[29] and improve the effectiveness of radio- and chemotherapy in rodent tumor models Citation[18], Citation[29].
In our present study, the evaluated PEG-Hb, which is also derived from PEG-conjugated hemoglobin, can effectively increase the oxygen-carrying capacity of blood and thus improve tumor oxygenation. Cisplatin is one of the most important chemotherapeutic drugs in clinical use. The US National Cancer Institute alert in February 1999 stated that concomitant cisplatin-based chemotherapy and radiotherapy should be considered for all patients with cervical cancer Citation[10] and it is believed to kill cells through interaction with DNA, mainly by formation of various DNA adducts, which lead to initiation of apoptosis Citation[8]. There is evidence that cisplatin was less effective under hypoxic conditions Citation[17]. Thus, in this study, we also investigated the chemotherapy sensitizing effect of PEG-Hb in vivo. We found that the reduction of tumor volume as well as pro-apoptotic (TUNEL-positive) and anti-proliferative effects in HeLa nude mice xenograft with co-administration of cisplatin and higher dose of PEG-Hb are more pronounced than that produced by cisplatin alone. In addition, no manifestation of the side effects (body weight drop and mortality) associated with the PEG-Hb is observed.
In conclusion, the results of this study suggest that the evaluated PEG-Hb are useful in enhancing the oxygenation of tumor tissue, resulting in more therapeutic response of cisplatin. Application of this type of oxygen carrier may provide a means of increasing the effectiveness of certain chemotherapeutic agents such as cisplatin. However, the tailoring of optimal dosage/schedule is still to be further optimized.
Acknowledgements
We thank Prof. T.M.S. Chang, McGill University, Canada, for serious review of this manuscript. This work was supported by Prof. Ruijuan Xiu's UNESCO Award for Woman in Science 2000 and the grant of “Knowledge Innovation Project” Academy of Science, China (No.KJCX1-SW-07).
References
- Ansiaux R., Baudelet C., Jordan B. F., Beghein N., Sonveaux P., De Wever J., Martinive P., Gregoire V., Feron O., Gallez B. Clin Cancer Res 2005; 11: 743–750
- Birner P., Schindl M., Obermair A., Plank C., Breitenecker G., Oberhuber G. Cancer Res 2000; 60: 4693–4696
- Brown J. M., Wilson W. R. Nat Rev Cancer 2004; 4: 437–447
- Brunner T. B., Gupta A. K., Shi Y., Hahn S. M., Muschel R. J., McKenna W. G., Bernhard E. J. Int J Radiat Biol 2003; 79: 569–576
- Chang T. M. Trends Biotechnol 2006; 24: 372–377
- Delmas C., Heliez C., Cohen-Jonathan E., End D., Bonnet J., Favre G., Toulas C. Int J Cancer 2002; 100: 43–48
- Dewhirst M. W., Vujaskovic Z., Jones E., Thrall D. Int J Hyperthermia 2005; 21: 779–790
- Fuertesa M. A., Castillab J., Alonsoa C., Perez J. M. Curr Med Chem 2003; 10: 257–266
- Gottschalk A., Raabe A., Hommel M., Rempf C., Freitag M., Standl T. Artif Cells Blood Substit Immobil Biotechnol 2005; 33: 379–389
- Green J. A., Kirwan J. M., Tierney J. F., Symonds P., Fresco L., Collingwood M., Williams C. J. Lancet 2001; 358: 781–786
- Haugland H. K., Vukovic V., Pintilie M., Fyles A. W., Milosevic M., Hill R. P., Hedley D. W. Int J Radiat Oncol Biol Phys 2002; 53: 854–861
- Hockel M., Schlenger K., Aral B., Mitze M., Schaffer U., Vaupel P. Cancer Res 1996; 56: 4509–4515
- Hoogsteen I. J., Pop L. A., Marres H. A., Merkx M. A., van den Hoogen F. J., van der Kogel A. J., Kaanders J. H. Int J Radiat Oncol Biol Phys 2006; 64: 83–89
- Jemal A., Siegel R., Ward E., Murray T., Xu J., Thun M. J. CA Cancer J Clin 2007; 57: 43–66
- Kaanders J. H., Bussink J., van der Kogel A. J. Lancet Oncol 2002; 3: 728–737
- Kaanders J. H., Bussink J., van der Kogel A. J. Semin Radiat Oncol 2004; 14: 233–240
- Koch S., Mayer F., Honecker F., Schittenhelm M., Bokemeyer C. Br J Cancer 2003; 89: 2133–2139
- Linberg R., Conover C. D., Shum K. L., Shorr R. G. In Vivo 1998; 12: 167–173
- Melillo G. Cancer Metastasis Rev 2007; 26: 341–352
- Nozue M., Lee I., Manning J. M., Manning L. R., Jain R. K. J Surg Oncol 1996; 62: 109–114
- Overgaard J., Horsman M. R. Semin Radiat Oncol 1996; 6: 10–21
- Parkin D. M., Bray F., Ferlay J., Pisani P. CA Cancer J Clin 2005; 55: 74–108
- Raabe A., Gottschalk A., Hommel M., Dubben H. H., Strandl T. Strahlenther Onkol 2005; 181: 730–737
- Robinson M. F., Dupuis N. P., Kusumoto T., Liu F., Menon K., Teicher B. A. Artif Cells Blood Substit Immobil Biotechnol 1995; 23: 431–438
- Shi Y., Wu J., Mick R., Cerniglia G. J., Cohen-Jonathan E., Rhim J. S., Koch C. J., Bernhard E. J. Prostate 2005; 62: 69–82
- Shorr R. G., Kwong S., Gilbert C., Benesch R. E. Artif Cells Blood Substit Immobil Biotechnol 1999; 27: 185–202
- Snyder S. A., Lanzen J. L., Braun R. D., Rosner G., Secomb T. W., Biaglow J., Brizel D. M., Dewhirst M. W. Int J Radiat Oncol Biol Phys 2001; 51: 494–506
- Tanaka J., Holden S. A., Herman T. S., Teicher B. A. Anticancer Res 1992; 12: 1029–1033
- Teicher B. A., Ara G., Herbst R., Takeuchi H., Keyes S., Northey D. In Vivo 1997; 11: 301–311
- Teicher B. A., Dupuis N. P., Emi Y., Ikebe M., Kakeji Y., Menon K. In Vivo 1995; 9: 11–18
- Teicher B. A., Herman T. S., Hopkins R. E., Menon K. Int J Radiat Oncol Biol Phys 1991; 21: 969–974
- Teicher B. A., Holden S. A., Ara G., Herman T. S., Hopkins R. E., Menon K. Biomater Artif Cells Immobilization Biotechnol 1992; 20: 657–660
- Teicher B. A., Holden S. A., Dupuis N. P., Kusomoto T., Liu M., Liu F., Menon K. Artif Cells Blood Substit Immobil Biotechnol 1994; 22: 827–833
- Teicher B. A., Holden S. A., Menon K., Hopkins R. E., Gawryl M. S. Cancer Chemother Pharmacol 1993a; 33: 57–62
- Teicher B. A., Schwartz G. N., Alvarez Sotomayor E., Robinson M. F., Dupuis N. P., Menon K. J Cancer Res Clin Oncol 1993b; 120: 85–90
- Winkler F., Kozin S. V., Tong R. T., Chae S. S., Booth M. F., Garkavtsev I., Xu L., Hicklin D. J., Fukumura D., di Tomaso E., Munn L. L., Jain R. K. Cancer Cell 2004; 6: 553–563
- Winslow R. M. Semin Hematol 2007; 44: 51–59
- Yu M., Dai M., Liu Q., Xiu R. Cancer Treat Rev 2007; 33: 757–761