Abstract
Twenty-nine modular stent-grafts deployed transrenally to repair AAAs with short necks in dogs were harvested at autopsy of the animals after scheduled durations of implantations of 10 days, one month, three months, and six months. Analyses of the explanted devices included non-destructive techniques such as gross observations, X-rays CT scan, IVUS and angioscopy. Further to appropriate dissection, histological investigations were carried out by means of scanning electron microscopy (SEM) and light microscopy. All the 29 specimens were extensively encapsulated with fibrous tissues but the fibrous capsule was thin in six of them; four capsules were ulcerated. The X-rays confirmed the stability of the devices that were still straight (12), slightly bent (12) or bent (4). The modules were misaligned in only one case. IVUS and angioscopy confirmed the patency of all the stent-grafts with thin internal capsules both proximally and distally with variable capsulation in the mid-section of the grafts. The left renal artery orifices were found to be patent at dissection with no obstruction to flow. The luminal flow surface of the stent-grafts was smooth and glistening proximally and distally containing endothelial like cells and vasa-vasorum. Poor healing was noted in the aneurysm area. Transrenal deployment of this modular stent-graft is feasible and gave excellent results with regard to biofunctionality and biocompatibility. The device proved to be safe and efficient.
INTRODUCTION
The Latecba modular stent-graft proved experimentally and clinically Citation[1], Citation[2] its capacity to be deployed with aortic necks shorter than 15 mm to treat AAAs Citation[3], Citation[4]. The experimental model was developed with a prosthetic aneurysm created by means of a specifically designed graft implanted from below the renal arteries to the trifurcation in 29 dogs for scheduled duration of up to six months. The proximal bare stent of the stent-graft was deployed at the supra-renal level. The biofunctionality of the device was documented by angiography together with Echodoppler in the 29 implants prior to animal sacrifice. There still are issues related to the healing and the long-term biostability of the device. This paper focuses on the analysis of the 29 explanted stent-grafts to highlight their 3Bs features (biocompatibility, biostability and biodurability).
MATERIAL AND METHOD
Harvesting of the Prostheses
The sacrifices of the animals were scheduled as follows: 10 days, acute; 30 days, short-term; 90 days, mid-term; 180 days, long-term. The anaesthetic protocol was similar to the procedure developed for the implantation of the prosthetic aneurysm. The aorta with the endograft was dissected from the celiac trunk to the trifurcation. The dog was administered IV heparin at a dose of 80U/kg. The aorta was clamped over the coeliac trunk and the branches of the trifurcation. The aorta was sectioned at the level of the clamps and the segment with the endoprosthesis was explanted en bloc. It was rinsed in a 2 % physiological heparinized solution. The specimen was fixed in a 10 % solution of formaline. After the explantation, the animal received a lethal injection of 30 meq of potassium chloride in bolus.
NON-DESTRUCTIVE STUDY OF THE EXPLANTED DEVICES
Gross Morphology
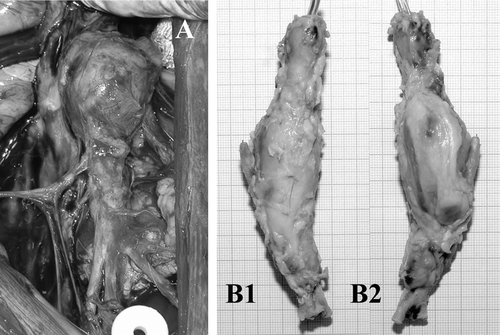
Each explanted device was first photographed using a numerical camera to visualize the details of the encapsulation of the device and the adjacent tissues. Special attention was paid to the presence of ulcerations and visibility of the polyester of the prosthetic aneurysm.
X-rays
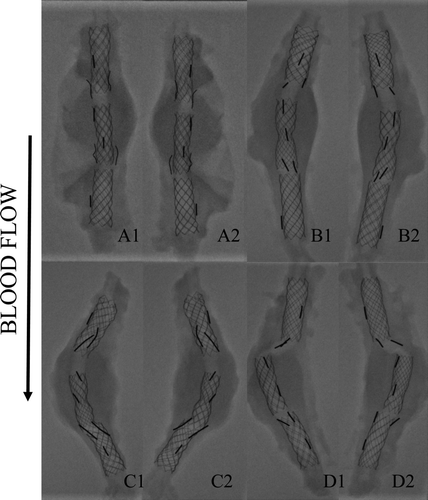
The specimens were placed in an Innova General Electric system (GE Medical Systems, Milwaukee, WI, USA). Precise images of the stents were obtained to evaluate the biostability and the biostability of the metallic structure.
IVUS
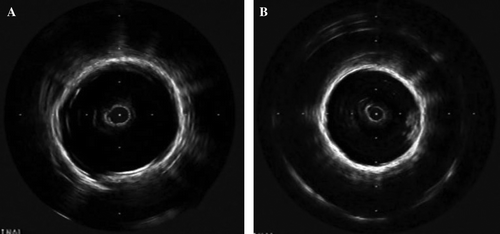
Explanted endovascular devices were visualized by means of a Clearview Ultra machine (Boston Scientific BSC, Natick, MA, USA), fitted with an Atlantis SR Pro 40 MHz coronary imaging catheter.
Angioscopy
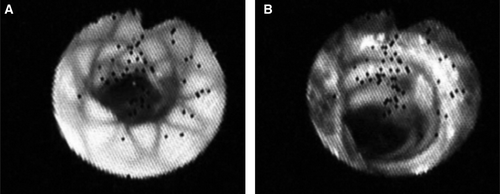
It was performed with a 2.8 mm diameter Olympus angioscope connected to a Storz SCB processor.
PATHOLOGY
Each explanted specimen was cut in 3 sub-specimens: the proximal and distal, respectively, down and up to the area of the prosthetic aneurysm were opened in halves longitudinally and the prosthetic aneurysm zone was cut in several consecutive rings, perpendicular to the axis of the blood flow.
Scanning Electron Microscopy
Representative samples of the sub specimens (0.5×0.5 cm) were selected and post-fixed in osmium tetroxyde. After drying in hexamethyldisilazene, the samples were gold palladium coated and observed in a scanning electron microscope (SEM) at voltages ranging from 15 kv to 30 kv.
Light Microscopy
The selected samples were embedded in polymethylmethacyclate (PMMA) or a similar polymer to hold together the biological tissues the textile knit and the metallic stents. After polymerization of the PMMA, the specimens were trimmed of excess polymer and sectioned on a precision banding saw under coolant. The 300 µm slices obtained were affixed onto a methacrylate slide with a UV-cured adhesive. Guiding and polishing followed to obtain slices 25-30 µm thick Citation[5]. After hematoxylin and eosin staining the slices were observed in light microscopy.
RESULTS
The sacrifices were performed according to the pre-established schedules, and following the as-described protocol. The following durations of implantation were performed:
10 days: four animals were sacrificed after 10 days (one case) to 12 days (three cases) of implantation;
30 days: five animals were sacrificed shortly after one month implantation ranging from 31 days (two cases) to 37 days (one case) and to a maximum of 39 days (two cases);
90 days: five animals were sacrificed after 3 months implantation ranging from 90 days (one case), to 91 days (one case), to 93 days (2 cases), and to 97 days (one case).
190 days: 15 animals were sacrificed after an average of 6 months implantation ranging from 179 days (2 cases), 180 days (4 cases), 181 days (5 cases), 182 days (1 case), 183 days (2 cases), to 186 days (1 case).
NON-DESTRUCTIVE INVESTIGATIONS
Gross Observations of the Explanted “en bloc” Specimens
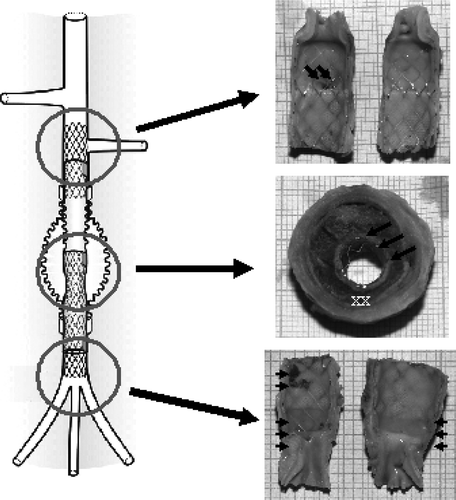
All of them were extensively encapsulated with fibrous tissues that developed externally all over the polyester wall of the prosthetic aneurysm. All the specimens had a very similar morphology whatever the duration of implantation was. The encapsulation was excellent in 25 animals out of 29; however, the polyester wall of the prosthetic aneurysm was visible through fibrous translucide tissue in 6 of those cases (). Ulcerations were noted in 4 cases.
X-rays
The X-rays of the explanted devices confirmed the patency of all the stent-grafts. These devices demonstrated good aneurysm exclusion in all the animals. All the stent-grafts were fully deployed and preserved their stability after the deployment. The devices were still straight (12 cases) or slightly bent, i.e., acute angle between the two modules <15 degrees (12 cases) after harvesting. Some devices were bent (4 cases), i.e., acute angle between the two modules > 15 degrees and the stents of the last one were misaligned. Results are illustrated in . It should also be mentioned that a mild constriction was generally observed in the telescoping area of the Module A and the Module B. This was anticipated as the distal diameter of the Module B was reduced to guarantee a stable assembling of the 2 modules.
IVUS
The ultra-sound observations permitted us to confirm the good deployment of the devices, with the exception of the stent-grafts damaged at harvesting whose proximal or distal extremity was flattened. The materials involved in the construction of the devices did not present any rupture; the shape of the stents were preserved, showing no disruption.
In 18 cases the internal capsule was discrete or absent. Some capsule developments were detected in 7 cases, one short-term, one mid-term, and 5 long-terms. The extension of the short-term one was limited to a few locations in the device. In the long-term stent-grafts the encapsulations were generally not concentric and their extents varied depending on the location. Four devices held mural thrombi, very localized in two cases and concentric with thrombi of different ages in the other two cases. Some illustrations are given in .
Angioscopy
The luminal surface of the endoprosthesis was easily visualized with the endoscope. As the reorganization of the thrombotic matrix was still in progress, the internal capsule was generally thin or absent. The deployment of the stainless steel stents proved to be adequate; all of them were intact. The weft-knit polyester grafts with its crimps was well evidenced downwards the stent in the Module A. The constriction at the telescopic area of this Module A and the Module B was evidenced ().
Figure 4. Illustration in endoscopy of the luminal surface of the stent-grafts. (A) distal end of the transrenal stent constriction in a stent-graft; (B) constriction at the anchorage of Module B in Module A with variable levels of thrombotic anchorages in a stent-graft.
Bare polyester knit and stainless steel stents were uncovered or minimally covered with internal capsule in more than 50% of the length of the device in 2 out of 4 acute cases, and only 1 in the 5 mid-term cases. In 18 out of 27 devices, the thrombotic matrix was poorly organized. It was, however, sufficient to prevent any blood percolation through the polyester wall. The formation of an internal capsule was observed in only 5 long-term implants with anchorage of parietal thrombi in the device whose alignment of the stents was severely impaired.
PATHOLOGY
Gross Observation
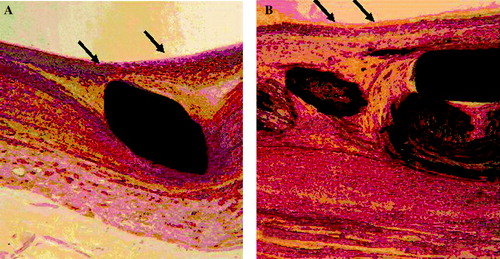
The areas adjacent to the host artery were rapidly healed, whereas those in the mid-section of the devices adjacent to the prosthetic aneurysm held a thrombotic matrix with bare stents exposed to the blood flow. Proximally, the orifice of the left renal artery was always well evidenced and the stent placement inside the polyester graft allowed a complete modeling of the interface of the graft material permitting a complete healing after one month of implantation. The aneurysm sac was filled with a solidified thrombus mostly poorly reorganized, while the stent itself was frequently exposed to the blood flow. Distally, the luminal surface did not differ much from the proximal one, except that some scattered micro thrombi could be observed time to time ().
Figure 5. Gross observation after dissection of a stent-graft implanted for 3 months. The flow surface of the proximal and distal segments, i.e., contact with the host artery, are smooth and glistening. Proximally, the blood supply of the kidney does not suffer impairment as the bare stent is kept open vis-à-vis the ostia of the left kidney artery (double arrows). The luminal surface of the medium segment is immature as bare stent struts and polyester knit are exposed to the blood flow (triple arrows). The stent-graft holds a reorganized sac capsule while the blood thrombus in the aneurismal sac has fully solidified but its reorganization is limited to the area in contact with the polyester of the stent-graft (X). The prosthetic aneurysm is well encapsulated (XX). Distally, the internal capsule encompasses all the bare stent (arrow head) with some minor mural thrombi (double arrow heads) while the distal extremity of the stent causes some overdistension of the aorta (triple arrow heads).
Scanning Electron Microscopy
The most striking observation was related to the anchorage of the stent to the proximal host artery above and vis-à-vis that proved to be the smooth with minimal incrustation in the wall of the artery allowing and enhanced healing of the interface. Endothelial cells were irregularly scattered after 10 days, but covering most of the luminal surface for the devices implanted for longer durations, with eventually the presence of vasa-vasorum vis-à-vis the orifice of the renal arteries; the stents were bare permitting the blood flow supply to the kidneys with a minimum of impairments ( and ). The flow surface of the middle segment of the device was irregular with stents mostly bare or coated with a thrombotic matrix. The polyester wall was lined with poorly organized thrombi (). Distally, the flow surface was also rapidly covered with a smooth and glistening surface, but the stents were more deeply anchored in the host artery, resulting in some thrombotic accumulation in the acute implantation.
Figure 6. Scanning electron microphotography of the proximal section of the Module A in a stent-graft explanted six months after implantation. The struts of the stent are embedded in a very thin fragile capsule whose splitting is probably due to specimen processing (arrow). The flow surface of the artery beneath the stent is smooth and glistening with an intimate contact with the stent (A:×27). The entrance of the left kidney is kept open preventing impairment in the blood supply (B:×27).
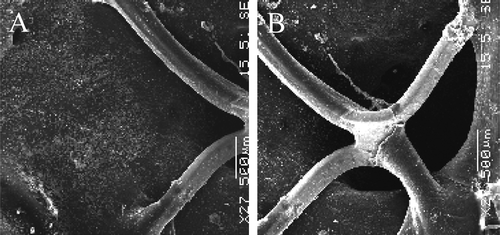
Figure 7. Scanning electron microphotography of the proximal segment of the Module A. This stent-graft was implanted for 6 months. In the areas of contact between the stent and the host artery the struts of the stent are well incorporated (double arrows) and the flow surface is well healed with the presence of a vasa-vasorum (arrows) (A:×22) whose ostia is well defined (arrow heads) and luminal surface covered with a continuous layer of endothelial cells (B:×600).
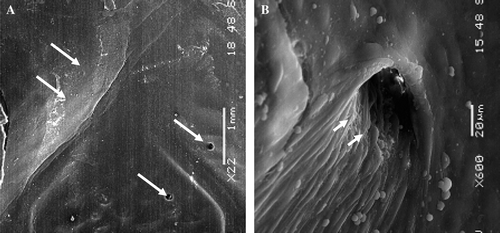
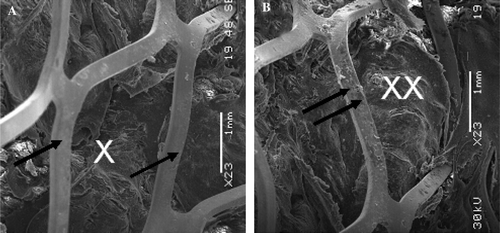
Figure 8. Scanning electron microphotography of the luminal surface of the most proximal segment of the Module B implanted for 12 days. It shows the bare stent (single arrows) and with some minor blood debris (double arrows) above the polyester impregnated with a thrombotic matrix that makes the prosthetic wall impervious to blood percolating (A*;B**) (A:×23) (B:×23).
Light Microscopy
The histology confirmed the SEM observations. The initial thrombi that appeared after implantation were still present in 10 days, but they began to be reorganized and they became fully organized after longer durations of implantation, with smooth muscle cell migration from the media and the ad rentitia and formation for continuous complete cellular neointima (). However, in the aneurysmal sac the healing remained immature. The interstices within the wall of the polyester were sufficiently filled with a thrombotic matrix made of fibrin, blood debris, and immature collagen to prevent any blood percolating through the wall.
Figure 9. Histology of the section at the level the left renal artery in a stent-graft implanted for six months. The internal capsule is well developed with endothelial-like cells on the flow surface (arrows). Such a capsule is made of collagen incorporating numerous cells (fibroblasts and smooth muscle cells) without any necrotic tissue: stent only (A:×200) and stent and polyester (B:×200).
DISCUSSION
The Latecba device was specifically designed to be implanted in the absence of a neck with sufficient length downstream the renal arteries (<15mm). Such a stent-graft whose upstream section is deployed trans-renally proved to be both efficient and safe: the patency was 100% and no endoleak was observed. The modular system can therefore be well anchored and well adapted to any pathological configuration of the arterial tree. Transrenal fixation originally proposed by Lawrence et al. represents the most important evolution in the new generation of the stent graft Citation[6–8]. Most of the commercially available devices privilege the addition of hooks and/or barbs to the proximal stents, to better fix mechanically the device Citation[9]. The hereby described devices offer an additional healing capacity derived from the Palmaz type stent Citation[10]. The selection of a weft-knit polyester graft guarantees an improved conformability of the deployed device, thanks to its superior compliance Citation[11]. Stent wires cross the renal artery ostia and they might impair the blood flow or the cross-sectional area Citation[9]. However, the renal arteries remained patent permitting adequate blood supply to the kidneys Citation[12], Citation[13]. The hereby observed cleanliness of the stent wires that cross the ostia of the renal artery support the biofonctionality, the biocompatibility and the biodurability of this transrenal concept graft. However, the exact characteristics of the interaction between the foreign materials and the host remain to be elucidated Citation[14–16]. The non-destructive analyses confirmed the excellent biofonctionality and the absence of damage to the polyester and the stents. The IVUS and the angioscopy observations confirmed the presence of a thin internal capsulae, proximally and distally. Healing of foreign surfaces has been a topic of discussion for decades. An ideal artificial blood conduit implanted by open surgery Citation[17–19] would become sandwiched between an internal capsule whose luminal surface becomes covered with endothelial cells and whose external capsule incorporates vasa-vasorum that go through the prosthetic wall to guarantee the development of a normal internal capsule. Unfortunately, in humans such blood conduits become rarely endothelialized despite the capacity of the internal capsule to synthetize prostacyclin and thus generate a favourable PGI2/TXA2 balance Citation[20], Citation[21]. The flow surface is becoming less thombogenic and guarantees a lower thrombotic threshold velocity Citation[22–24]. The situation is even more critical in stent-grafts. Clinical observations have documented the lack of healing Citation[15]. Retrieval programs report the same phenomenon Citation[25]. The experimental results are however different, depending upon the protocol. Healing is regularly reported in absence of prosthetic aneurysm, whereas it does not occur in case of models closer to the aneurismal phenomenons Citation[26].
In this regard, the behaviour of the modular Latecba stent-graft represents a great interest as proximally and distally a very complete healing is achieved contributing to guarantee an intimate incorporation with the host artery likely to improve the mechanical and hemodynamical characteristics of the site. The section vis-à-vis the aneurysm still requires additional research to permit the endothelialisation of the flow surface and the complete healing of the aneurismal sac Citation[27].
CONCLUSION
The Latecba stent-graft opens a new avenue in the field of endovascular surgery, thanks to its capacity to be deployed in the presence of aneurysms whose neck is too short and involves the renal arteries. Clinically, it already proved its interest, particularly in patients with short necks Citation[28] or in highly specific situations Citation[29]. With the non-invasive techniques now available, it is feasible to properly and regularly assume the biofunctionality and the biostability of such devices in vivo.
Acknowledgements
This research program was supported by Latecba, Buenos Aires (Argentina). The authors are indebted to Dr. Sergio Ferraris and Dr. Juan Santamaria (veterinarians) for help and guidance. They also thank Dr. Jean-François Tanguy (Pascale Geoffroy) and Dr. Jean-Claude Tardif (Johanne Vincent). The technical support of Marie Bolduc, Véronique Gagné and Richard Janvier was greatly appreciated. The collaboration of Wasatch Histo Consultants Inc. (Cathy Mayton) was of considerable help.
References
- Guidoin, R. Peirano, M.A.M, Barone, HD , et al. Transrenal deployment of a modular stent-graft to repair AAAs with short-necks: experiments in dogs. Art. Cells, Blood Subst. Biotechnol (accepted for publication).
- Bertoni H., Peirano M.A., Fava M., Barone H.D. Results of balloon expandable endoprotheses (SETA) for repair of abdominal aortic aneurysm. 31st Annual Scientific Meeting, SIR Toronto. Canada, 2006; 30/3 – 4/4: 2006
- Dillavou E.D., Muluk S.C., Rhee R.Y., et al. Does hostile neck anatomy preclude successful endovascular aortic aneurysm?. J. Vasc. Surg. 2003; 38: 657–663
- MacLean A.A., Katzen B.T. The short proximal AAA neck. A comparison of EVAR outcomes among groups of patients with different proximal neck lengths. Endovascular Today. 2006; 5(5)79–84
- Guidoin R., Zhang Z., Douville Y., et al. Polymethylmethacrylate (PMMA) as an embedding medium preserving tissues and foreign materials encroaching in endovascular devices. Art. Cells Blood Subst. Immobil. Biotechnol. 2006; 34: 349–366
- Laurence D.D., Charnsangavej C., Wright K.C., et al. Percutaneous endovascular graft: experimental evaluation. Radiology 1987; 163: 357–360
- Sun Z. Transrenal fixation of aortic stent-grafts: current status and future direction. J. Endovasc. Ther. 2004; 11: 539–549
- Sun Z., Mwipatayi B.P., Semmen J.B., Lawrence-Brown M. Short to mid-term outcomes of fenestrated endovascular aortic aneurysm: a systematic review. J. Endovasc Ther. 2006; 13: 747–753
- Greenberg R.K., Chuter T.A., Laurence-Brown M., et al. Analysis of renal function after aneurysm repair with a device using suprarenal fixation (Zenith AAA endovascular graft) in contrast to open surgical repair. J. Vasc Surg. 2004; 39: 1219–1228
- Palmaz J.C., Benson A., Sprague E.A. Influence of surface topography on endothelialisation of intravascular metallic material. J. Vasc. Interv. Rad. 1999; 10: 439–444
- Debille E., Guidoin R., Charara J., . Dilatability and stretching characteristics of the elastic behaviour. Medical Textiles for Implantation, H. Plank, M. Dauner, M. Renardy, et al. Springer-Verlag, Berlin 1990; 137–185
- Teodorescu V.J., Morrissey N.J., Olin J.W. Duplex ultrasonography and its impact on providing endograft surveillance. Mt. Sinai J. Med. 2003; 70: 364–366
- Kalliafas S., Travis S.J., Macierewicz J., et al. Infra-renal color duplex examination of aortic endograft patients with suprarenal stents. J Endovasc. Ther. 2001; 8: 582–596
- Major A., Guidoin R., Soulez G., et al. Implant degradation and poor healing after endovascular repair of abdominal aortic aneurysms: an analysis of explanted stent-grafts. J. Endovasc. Ther. 2006; 13: 457–467
- Malina M., Brunkwall J., Ivancev K., et al. Endovascular healing is inadequate for fixation of Dacron stent-grafts in human aortic vessels. Eur. J. Endovasc. Surg. 2000; 19: 5–11
- Riepe G., Heintz C., Kaiser E., et al. What can we learn from explanted endovascular devices?. Eur. J. Vasc. Endovasc. Surg. 2002; 24: 117–122
- Berger K., Sauvage L.R., Rao A.M., Wood S.J. Healing of arterial prostheses in man: its incompleteness. Ann. Surg. 1972; 175: 118–127
- Shi Q., Wu M.H., Onuki Y., et al. Endothelium on the flow surface of human aortic Dacron vascular grafts. J. Vasc. D Surg. 1997; 25: 736–742
- Onuki Y., Koouchi Y., Yoshida H., et al. Early flow surface endothelialization before microvessel ingrowth in accfelerated graft healing with Brdu identification of cellular proliferation. Ann. Vasc. Surg. 1998; 12: 207–215
- Moncada S., Gryglewski R., Bunting S., Vane J.R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature 1976; 263: 663–665
- Moncada S. Prostaglandin endoperoxides and thromboxanes: formation and effects. Nauyn Schmiedebergs Arch. Pharmacol. 1977; 297(Suppl. 1)S81–S84
- Sinzinger H.M., Silberbauer K., Winter M., Auerswald W. Implanted vascular prostheses generate prostacyclin. Lancet 1978; 2(8094)840
- Matejka M., Feigl W., Sinzinger H., et al. Evidence that thrombus organizing blood derived cells produce prostaglandins: 2. Artery 1980; 8: 43–49
- Merhi Y., Guidoin R., Forest J.C. Fate of polyester arterial prostheses implanted as throracoabdominal by-passes in dogs: haematology, pathology and biochemistry. Clin. Invest. Med. 1988; 11: 403–416
- Guidoin R., Marois Y., Douville Y., et al. First-generation aortic endografts: analysis of explanted Stentor devices from Eurostar Registry. J. Endovasc. Ther. 2000; 7: 105–122
- Guidoin R., Douville Y., Marois Y., et al. La chirurgie vasculaire avec effraction tissulaire minimale pour exclusion d'anévrisme: intérêts et limites des essais chez l'animal. ITBM-RBM 2002; 23: 212–234
- Liu L.S., Wei D.H., Tang C.K., et al. A HUVEC line with a stable expression of the endothelialization of blood conduits. Art. Cells Blood Subst. Biotech. 2007; 35: 319–331
- Marenchino R.G., Zgrablich C., Cesareo V.G. Tratamiento endovascular de patologia aortic. Rev. Arg. Cir. Cardiovasc. 2003; 1: 29–32
- Dorros G., Jaff M.R., Parikh A., et al. In-vivo crushing of an aortic stent enables endovascular repair of a large Infrarenal aortic pseudoaneurysm. J. Endovasc. Surg. 1998; 5: 359–364